 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Rad6 functions as a ubiquitin-conjugating protein that regulates cellular processes in many fungal species. However, its role in filamentous entomopathogenic fungi remains poorly understood. This study characterizes Rad6 in Beauveria bassiana, a filamentous fungus widely employed as a critical fungicide globally. The results demonstrate a significant association between Rad6 and conidial properties, heat shock response, and UV-B tolerance. Concurrently, the mutant strain exhibited heightened sensitivity to oxidative stress, cell wall interfering agents, DNA damage stress, and prolonged heat shock. Furthermore, the absence of Rad6 significantly extended the median lethal time (LT50) of Galleria mellonella infected by B. bassiana. This delay could be attributed to reduced Pr1 proteases and extracellular cuticle-degrading enzymes, diminished dimorphic transition rates, and dysregulated antioxidant enzymes. Additionally, the absence of Rad6 had a more pronounced effect on genetic information processing, metabolism, and cellular processes under normal conditions. However, its impact was limited to metabolism in oxidative stress. This study offers a comprehensive understanding of the pivotal roles of Rad6 in conidial and hyphal stress tolerance, environmental adaptation, and the pathogenesis of Beauveria bassiana.
Introduction
The conidia of Beauveria bassiana, constituting the primary active ingredient in fungal biocontrol agents, are crucial for the field application of these agents due to their high yield and resistance to various adverse factors, such as ultraviolet radiation, oxidative stress and chemical agents [Citation1]. UV rays reaching the surface after penetrating the atmospheric ozone layer primarily consist of medium- and short-wavelength UV-A and UV-B [Citation2]. Excessive UV radiation can induce damage to macromolecules within fungal cells, including DNA, RNA, proteins, ribosomes, and biomembranes [Citation3,Citation4]. Molecular and cellular effects of ultraviolet light-induced genotoxicity have been extensively studied [Citation5,Citation6]. To address this challenge, organisms have evolved DNA damage tolerance (DDT) pathways that enable replication fork bypass to overcome obstacles [Citation7]. Two parallel pathways of DNA damage tolerance, namely error-prone translesion DNA synthesis (TLS) and error-free lesion bypass employing sister-strand recombination [Citation8], are both mediated by Rad6 in Saccharomyces cerevisiae [Citation9].
Rad6, a typical ubiquitin-conjugating protein (E2), typically collaborates with ubiquitin-protein ligases (E3) in the post-translational modification (PTM) of proteins. In Saccharomyces cerevisiae, the Rad6-mediated postreplication repair (PRR) epistasis group plays a pivotal role in DNA damage tolerance (DDT) and functions beyond the S phase [Citation10,Citation11]. The Rad6-Rad18 complex can monoubiquitinate proliferating cell nuclear antigen (PCNA) at the Lys164 residue during translesion synthesis (TLS). Monoubiquitinated PCNA can undergo further polyubiquitination by the Mms2–Ubc13–Rad5 complex at the identical residues, forming a Lys63-linked polyubiquitin chain that facilitates error-free lesion bypass [Citation12]. Ubiquitylation at Lys164 of PCNA in response to UV irradiation triggers translesion synthesis [Citation13], while K63 polyubiquitination modulates the oxidative stress response [Citation14]. Rad6 and Bre1, another E2 and E3, respectively, are known to directly interact with the histone modification domain of Paf1 complex subunit Rtf1 [Citation15–17]. Stimulating H2B ubiquitylation, Rad6 undertakes the second major function by mono-ubiquitinating H2B at Lys123 (H2BK123ub), which is responsible for telomere length, recombination, and the DNA damage checkpoint response [Citation18,Citation19]. This monoubiquitination is promoted by Large 1 (Lge1), a protein associated with Bre1, during the early stages of transcription elongation [Citation20]. Additionally, methylations of H3K4 and H3 lysine79 require the preceding monoubiquitination of H2B histone H2BK123ub [Citation21], linking the cell cycle, cell ageing, defects in mitotic cell growth, and meiosis in vivo [Citation22–24]. Moreover, the signal from chromatin to kinetochores is subject to the transregulation of Dam1 methylation by H2BK123ub [Citation25]. The third major role of Rad6 is reflected in an E3 enzyme, Ubr1, which serves as the central recognition component of the N-end rule-dependent protein degradation pathway [Citation26]. Other functions include lowering the mating efficiency of MATa strains, sensitivity to toxic AICAR, and starvation-induced reverse mutation [Citation26–28].
Beauveria bassiana is a filamentous fungal insect pathogen representing the primary fungal insecticide source [Citation29]. It has evolved complex machinery to respond to and tolerate various stresses associated with host immunity defences and host habitats [Citation1,Citation30,Citation31]. Recently, research of B. bassiana from various origins has been widely reported. For example, B. bassiana SAN01 isolated from onion leaves can proliferate at different plant biomass levels and secrete high levels of xylanase and endoglucanase outside the cells [Citation32]. B. bassiana HQ917687 isolated from soil exhibits higher Pr1 activity and virulence in a medium with a low C/N ratio (10:1), as well as higher conidial production [Citation33]. Two B. bassiana AAUB03 and AAUB2 strains obtained from Ethiopian soil have stronger pathogenicity against Tuta absoluta [Citation34]. The B. bassiana Mn1 isolated from Malacosoma neustria has positive biological control potential and high pathogenicity to the host [Citation35].
Putative Rad6 homologs exist in filamentous fungi and the function of some Rad6 homologs have been explored. In Magnaporthe oryzae, the MoRad6-mediated ubiquitination pathways are essential for its infection-related development and pathogenicity [Citation36]. Recently, a Rad6 homolog in B. bassiana was characterized as an important protein required for asexual and insect-pathogenic lifecycles, solar UV damage repair, and genomic expression. The subcellular localization of Rad6 in B. bassiana is in both the cytoplasm and the nuclei, but it is rarely present in vacuoles. In addition, Rad6 interacts with Rad18 in B. bassiana, shows a weak interaction with Rad1 and Rad10, and is associated with the photolyase regulators WC1 and WC2 [Citation37]. Although previous studies have highlighted the considerable potential of Rad6 for DNA repair, histone ubiquitination, and protein degradation [Citation10,Citation38,Citation39], no effort has been made to explore the potential roles of various Rad6 proteins in stress tolerance and other cellular events in fungal insect pathogens. Rad6 proteins may have unrecognized roles associated with the biological control potential of fungal insect pathogens or important cellular processes/events in B. bassiana, given that Rad6 can stimulate intracellular proteasome activity, DNA repair activity, and protein degradation through Ubr1 in other filamentous fungi. This study aims to test the hypothesis by characterizing the Rad6 ortholog in B. bassiana.
Results
Rad6 is involved in maintaining fungal growth in normal or compound-stressed conditions
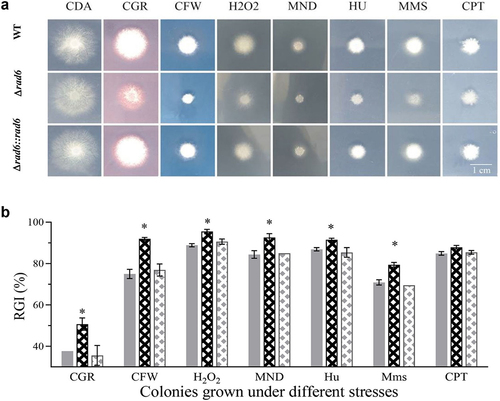
The wild-type (WT) strain, as well as the deletion and complemented mutants of rad6, exhibited similar growth in terms of diameter on standard CDA plates. However, they demonstrated significantly stronger inhibition when exposed to various chemical stresses (). The rad6 disruption strains exhibited higher sensitivity to cell wall stress agents such as Congo red (CGR) and calcofluor white (CFW), with a relative inhibition rate (RGI) of 50.7% and 91.9%, compared to 37.7% and 75.0% in the WT strains, respectively. In the oxidative stress groups, the RGI of rad6 mutant strains was significantly 6.6% and 8.23% higher than that of the control strains when exposed to H2O2 and menadione (MND), respectively. Interestingly, only hydroxyurea (HU) and methane sulphonate (MMS) had a significant effect on the rad6 mutants, whereas camptothecin (CPT) did not ().
Figure 1. Colonies grown under the normal of compound-stress conditions. (a) Photographic depiction of strains exposed to diverse chemical stress agents; (b) Relative Growth Inhibition (RGI) of strains in response to cell wall interfering agents (CGR and CFW), oxidation stress (H2O2 and MND), and DNA damaging agents (HU, Mms, and CPT); Asterisked bars in each group significantly differ from unmarked bars (Tukey’s HSD, p < 0.05). Error bar: SD from three replicates.
Essential role of Rad6 in conidial properties and stress tolerance
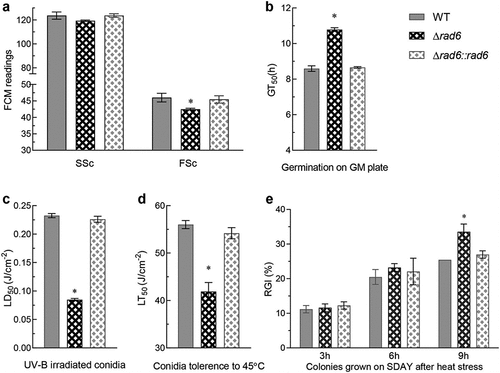
To assess the conidial size and density of WT and rad6 disruption strains, the readings of forward (Fsc) and side scatter (Ssc) detectors were obtained from the flow cytometry of three samples. The conidial size among the three groups showed no significant difference, but the conidial density of the mutant strain decreased significantly by 8% (). The germination rate of conidia after 24 hours on the germination plates represents the conidial activity in some aspects. Compared to the median germination time (GT50) of 8.59 hours of WT, the GT50 of rad6 mutants was 10.78 hours, indicating a 26.2% slower germination time (). The median lethal dose (LD50) of mutant strain was 0.08 J/cm2, dramatically decreasing by 63% compared to the 0.23 J/cm2 in control groups (). The median lethal time (LT50) of the WT and rad6 disruption strains were 56.0 and 41.9 minutes, respectively, indicating a 25.2% decrease in conidial thermal tolerance in mutant strain (). When the colonies were subjected to heat stress, the mutant and control strains did not exhibit significant differences in short- and mid-term stimulation. However, the mutant strain showed a significantly higher RGI of 33.55%, while the WT strains were at 25.5% after 9 hours of incubation ().
Figure 2. Roles of Rad6 in regulating conidial property and stress tolerance. (a,b) Conidial size (measured by SSc), density (measured by FSc), and germination rate; (c,d) Conidial tolerance to UV-B irradiation and heat shock at 45°C. (e) Strain tolerance to 42°C heat shock. Asterisked bars in each group significantly differ from unmarked bars (Tukey’s HSD, p < 0.05). Error bar: SD from three replicates.
Impact of Rad6 in virulence
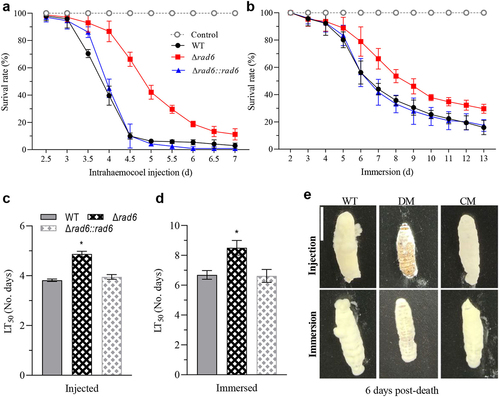
In the bioassay of two infection models, all the WT and complemental strains showed a similar steep declining curve compared to the smooth descending curves in the rad6 disruption strain (). The LT50 of Δrad6 strain was 4.87 days post-injection, 27.5% slower than the 3.82 days in the WT strain (). Meanwhile, the LT50 of Δrad6 strain and WT strains were 8.51 and 6.69 days post-immersion, respectively (). After 6 days of incubation, the fungal outgrowths on cadaver surfaces killed by mutant strain were much thinner than those in the control group in both infection modes ().
Figure 3. The vital role of Rad6 in the pathogenetic of Beauveria bassiana. (a,b) Survival rates of insects in injection and immersion Modes; (c,d) Median lethal time (LT50) of insects in injection and immersion modes. (e) Images of fungal outgrowth in two infection modes. Asterisked bars in each group significantly differ from unmarked bars (Tukey’s HSD, p < 0.05). Error bar: SD from three replicates.
Contribution of Rad6 to virulence-related events
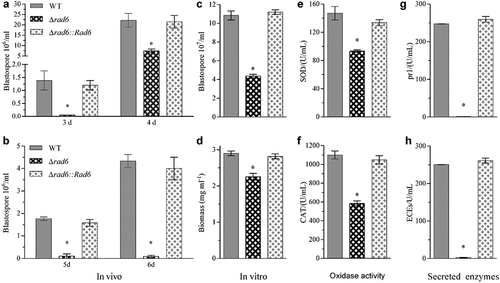
The hyphal body concentration in the haemolymph of larvae infected by Δrad6 conidia was only 3.6% and 33.8% of those in control groups 3- and 4-days post injection (). In the immersion infection method, the hyphal body concentration in the experiment group was 0.1 × 106/mL 5- and 6-day post immersion, while it was to 1.8 and 4.3 × 106/mL in the WT infected larvae, respectively. The hyphal body concentrations in control groups were 18 and 43-fold than those in Δrad6 conidia infected larvae (). Additionally, the number of blastospores was found to be lower in the haemocoel of G. mellonella infected by the Δrad6 strain when compared to that infected by WT or the complementary strains both via injection or immersion (Figure S1). In the TPB incubated cultures, the blastospore production of Δrad6 strain was 40.2% of that in the WT strain, the hyphal biomass was 77.9% of that in the WT strain (). The rad6 disruption mutant had only 63.5% of the SOD enzymes activity compared to the WT strain (). Similarly, the CAT enzymes activity decreased from the 1100 U/mL in the WT strain to 585 U/mL in the Δrad6 strain (). Pr1 proteases and extracellular (cuticle-degrading) enzymes (ECEs) activities dramatically decreased by more than 99% in the five days’ old cultures of Δrad6 strain compared to those in the WT strain ().
Figure 4. Cellular events related to virulence. (a,b) In vivo hyphal body concentration of two infection modes; (c,d) In vitro blastospore production and biomass; (e,f) Activities of SOD and CAT enzymes; (g,h) Activities of Pr1 proteases and extracellular (cuticle-degrading) enzymes (ECEs). Asterisked bars in each group significantly differ from unmarked bars (Tukey’s HSD, p < 0.05). Error bar: SD from three replicates.
Vital roles of Rad6 in transcriptional regulation
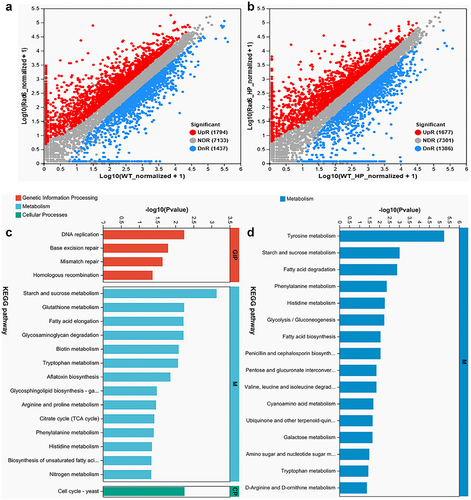
To explore the effect of Rad6 on the oxidant stress response, we analysed the transcriptome of WT and Δrad6 in CDA and CDA-H2O2 plates. In the differentially expressed genes (DEGs) analysis, 1794 up-regulated and 1437 down-regulated DEGs were shown between WT and Δrad6 incubated on the CDA medium. On the other hand, 1677 up-regulated and 1386 down-regulated DEGs were found between the WT and Δrad6 incubated on the CDA- H2O2 medium (). In the transcriptomic validation experiment, all 12 genes that exhibited downregulation in the CDA and CDA-derived media transcriptomics data were consistently and significantly downregulated in qRT-PCR. Similarly, all 12 genes that showed upregulation in the transcriptomics data displayed significant upregulation in the qRT-PCR results (Supplemental Figure S2). In the normal cultured transcriptome, the top 20 KEGG pathways are categorized into three groups: Genetic Information Processing (GIP), Metabolism (M), and Cellular Process (CP). The GIP group includes DNA replication, base excision repair, mismatch repair, and homologous recombination KEGG pathways. The M group comprises starch and sucrose metabolism, glutathione metabolism, fatty acid elongation, glycosaminoglycan degradation, biotin metabolism, tryptophan metabolism, aflatoxin biosynthesis, glycosphingolipid biosynthesis-ganglio series, arginine and proline metabolism, citrate cycle (TCA cycle), phenylalanine metabolism, histidine metabolism, biosynthesis of unsaturated fatty acids, and nitrogen metabolism KEGG pathways. The CP group solely contains the cell cycle KEGG pathway (). Interestingly, all of the top 20 KEGG pathways in the stressed transcriptome fall into the M group. In descending order of significance, they are tyrosine metabolism, starch and sucrose metabolism, fatty acid degradation, phenylalanine metabolism, histidine metabolism, glycolysis metabolism, fatty acid biosynthesis, penicillin and cephalosporin biosynthesis, pentose and glucuronate interconversions, valine, leucine and isoleucine degradation, cyanoamino acid, ubiquinone and other terpenoid-quinone biosynthesis, galactose metabolism, amino sugar and nucleotide sugar metabolism, tryptophan metabolism, and D-arginine and D-ornithine metabolism KEGG pathways ().
Figure 5. Transcriptomic insight into the regulatory role of Rad6 in B. bassiana. (a,b) Volcano plots under normal and oxidative stress incubation conditions, respectively; (c,d) KEGG analysis of cultures from normal (left) and oxidative stress (right) strains. (Note: The ordinate represents the KEGG pathway, and the abscissa ordinate represents the significance level of enrichment. The smaller the FDR, the greater the -log10 (FDR) value, indicating a more significant enrichment of the KEGG pathway.).
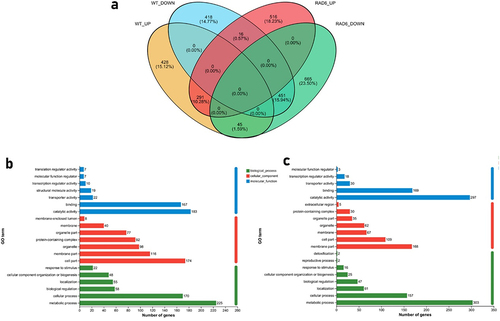
A comprehensive analysis of the two groups (normal vs H2O2 treated) yielded interesting results. Venn diagram showed that among the DEGs in the two groups, only 26% of DEGs in H2O2-Rad6 vs CDA-Rad6 showed the same trends as the DEGs in H2O2-WT vs CDA-WT (), indicating that the absence of Rad6 affects the cellular response to the oxidant stress at the gene transcription level. GO analysis revealed that the up- and down-regulated DEGs (which exhibited reversed regulation trends or remained unchanged in the WT) were primarily associated with catalytic activity and metabolic processes ().
Figure 6. Regulation of Rad6 on the transcriptome of B. bassiana under normal and oxidative stress. (a) Venn diagram of differentially expressed genes between WT and Rad6 under CDA and H2O2 cultures; (b) GO analysis of differential gene sets up-regulated in Δrad6 but unchanged or down-regulated in WT between CDA and H2O2 groups; (c) GO analysis of differential gene sets down-regulated in Δrad6 but unchanged or up-regulated in WT between CDA and H2O2 groups.
Discussion
Our findings elucidate the pivotal roles of Rad6 in maintaining conidial properties, stress tolerance, and hyphal resistance to various oxidative, cell wall-disturbing, and DNA-damaging agents in B. bassiana. Furthermore, the reduced virulence and enzyme activity underscore the critical contribution of Rad6 to the biological control potential. Additionally, our investigation reveals the indispensable role of rad6 in modulating gene transcription patterns in B. bassiana under normal and oxidative culture conditions, shedding light on the noteworthy role of Rad6 in adapting to environmental stresses, as discussed below.
Conidia are the primary active ingredient of the fungicide [Citation40], making their properties and stress tolerance crucial for successful field applications. The biocontrol process of filamentous fungi typically initiates with adhesion to the pest surface [Citation41], where the subsequent germination speed determines conidial survival under stressful conditions. The delayed GT50 in the mutant strain aligns with previous studies indicating that disruption of MoRad6 severely impairs conidial germination in Magnaporthe oryzae and B. bassiana [Citation36,Citation37]. As global warming transitions from speculation to reality [Citation42], the thermal tolerance of conidia and hyphae holds significant implications for fungicide effectiveness. A previous study highlighted the importance of Rad6/Bre1 in H3K4 methylation, which is essential for the thermal tolerance of Cryptococcus neoformans [Citation43]. The decreased LT50 of conidia and heightened sensitivity of fungal hyphae to prolonged thermal stress suggest that Rad6 plays a similar role in B. bassiana, critical for adaptation to a warmer Earth. Beyond thermal stress, UV-B irradiation poses another indiscriminate harmful factor [Citation44]. The pronounced drop in UV-B resistance in the Δrad6 strain is consistent with findings in yeast [Citation45]. Hydroxyurea (HU), methyl methanesulfonate (MMS), and camptothecin (CPT) are three types of DNA-damaging agents, functioning as DNA synthesis inhibitors, DNA-damaging chemicals, and topoisomerase I inhibitors, respectively [Citation46]. The diminished tolerance of the rad6 disruption strain to HU and MMS confirms Rad6’s role in DNA repair, a well-established function in Saccharomyces cerevisiae [Citation47,Citation48].
Aside from its essential role in regulating conidial properties and stress tolerance, Rad6 is vital for the virulence of B. bassiana. In our studies, the survival curve of the WT strain is much smoother than that of the rad6 disruption mutant in two infection modes, which is consistent with its role in Magnaporthe oryzae [Citation36]. The successful infection requires conidia to penetrate the surface layer of the body of an insect, which consists of proteins, chitin, and fats [Citation49]. Pr1 proteases have been identified as vital virulence factors in B. bassiana [Citation50]. The transcriptome analysis showed that the expression levels of pr1A1, pr1A2, pr1B1, pr1C, and pr1F1 in Δrad6 were significantly increased, while pr1B2 and pr1G were significantly decreased. This result suggested a fluctuation in Pr1 activity in Δrad6. The reduced Pr1 activity is consistent with the transcriptome results. These data suggest that Rad6 may play a role in regulating the transcriptional expression of Pr1-associated genes, which is essential for the complete virulence of B. bassiana. On the other hand, extracellular (cuticle-degrading) enzymes (ECEs) are another crucial factor affecting fungal virulence [Citation51]. Our experimental data showed a dramatic loss in ECEs activities in Δrad6. The changes in Pr1 and ECEs could explain, at least in part, the delayed LT50 of G. mellonella infected by Δrad6.
Once the conidia successfully penetrate the haemolymph, the hyphal body needs to overcome the oxidant stress produced by the host [Citation52] and absorb nutrients to grow and reproduce. By analysing transcriptome data, we found that the deletion of rad6 affects the transcription levels of genes related to SOD and CAT. Under the H2O2 stress, the gene transcription levels of sod3, sod4, cat1, and cat6 in Δrad6 were significantly reduced, while the gene expression levels of sod5 and cat2 were significantly increased. The dysregulated transcription levels of SOD and CAT genes indicated a disordered SOD and CAT enzyme activity in the Δrad6. Our experimental results of the defects in oxidation tolerance to H2O2 and menadione in developing hyphal and the decreasing SOD and CAT enzymes activity reveal the second reason for the attenuated virulence of Δrad6 strain. Additionally, under H2O2 stress, Δrad6 showed a different transcription spectrum compared to WT, also suggesting that Rad6 is involved in the normal cellular response to the oxidant stress. Our findings revealed the potential of Rad6 involved in SOD and CAT enzyme activity by regulating the transcription model of their corresponding genes, and then further influencing the oxidative stress tolerance of B. bassiana.
The dimorphic switch is a vital index affecting the virulence of fungal pathogenicity [Citation53]. The significant decline in blastospore production and biomass in vitro indicates that Rad6 may play a role in regulating virulence by affecting the dimorphic switch. The in vivo haemolymph samples from the mutant strain confirmed the function of Rad6 in mediating dimorphic switch and explained the attenuated virulence of Δrad6 strain from a third aspect.
Moreover, the transcriptome analysis of normal and oxidative-stressed cultures indicates that rad6 has dual effects in B. bassiana. In Saccharomyces cerevisiae, Rad6 is the key for DNA-damage tolerance (DDT), which consists of two parallel pathways: error-free and error-prone TLS (translesion DNA synthesis) [Citation47]. Four significant KEGG pathways can categorize under the genetic information processing (GIP) subgroup in the standard cultured stains, consistent with the conventional understanding of Rad6 in other fungi [Citation47,Citation54,Citation55]. Intriguingly, no KEGG pathway belonged to GIP and cellular processes (CP) in the top 20 significantly enriched pathways when strains are cultured with an oxidant stress agent. Instead, all KEGG pathwaysfocus on metabolism, illustrating the role of Rad6 in adapting to a complex environment.
In summary, Rad6 governs conidial properties, stress tolerance and plays a crucial role in the virulence and associated cellular processes of B. bassiana. Furthermore, Rad6 exhibits diverse regulatory functions that aid the strain in adapting to stressful environments. Although these findings underscore the importance of Rad6 in fungal habitat adaptation and virulence, the mechanisms through which Rad6 controls these phenotypes via ubiquitination processes and the key upstream and downstream genes or proteins that cooperate with Rad6, remain unclear. This necessitates further exploration in future studies.
Materials and methods
Microbial strains and culture conditions
The wild-type (WT) strain B. bassiana ARSEF 2860 utilized in this study originated from the USDA-ARS Collection of Entomopathogenic Fungal Cultures in Ithaca, NY, USA. The rad6 gene knockout and complementary strains derived from the WT strains were produced at Zhejiang University and are currently stored at Fuzhou University. PCR verification and the transcriptional level of rad6 were assayed for the mutant confirmation (Figure S3a,b). The paired primer sequences (5′-3′) for PCR detection and qRT-PCR are CAAATGCTGCCATAATCCA/GGTACTGCTCCTCAAACTGC and GTGACTTCAAGCGAATGCAA/CGTTGGACTCCACCTGTTTT, respectively. To generate a sufficient conidial supply for the tolerance test, all strains underwent incubation in SDAY medium (4% glucose, 1% peptone, 1.5% agar, and 1% yeast extract) at the optimal conditions of 25°C with a light/dark cycle of 12:12 for eight days. Subsequently, each strain was cultivated on basic CDA agar plates (3% sucrose, 0.3% NaNO3, 0.1% K2HPO4, 0.05% KCl, 0.05% MgSO4, and 0.001% FeSO4, plus 1.5% agar) and CDA-derived media for stress tolerance analysis under a 25°C light/dark cycle of 0:24 for eight days.
Stress tolerance analysis for strains
For stress tolerance assays, the 8-day-old cultures of each strain were collected and subjected to ultrasonic oscillation in a 1.5 mL tube with 1 mL of 0.02% Tween 80. 1 µL of spore suspension at a concentration of 106/mL was applied to plates (9 cm in diameter) of CDA, or CDA amended with various stress agents: (i) 2 mM H2O2 or 0.03 mM menadione for oxidative stress; (ii) Congo red (3 μg/ml) or calcofluor white (5 μg/ml) for cell wall-disturbing stress; (iii) hydroxyurea (HU: 10 mM), methane sulphonate (MMS: 0.1%), or camptothecin (CPT: 0.1 μM) for DNA-damaging stress. To assess the hyphal thermal tolerance in the strains, the plates of each strain were exposed to a 42°C incubator for 0, 3, 6, and 9 hours after 2 days in the optimal environment, followed by returning the thermally stressed plates to the original chamber. Following an 8-day incubation, the diameter of each colony in both CDA and CDA-derived plates was measured to calculate the relative inhibition rate (RGI) of the chemical agents. The formula for RGI is as follows:
Here, SC represents the colony area in the CDA plates, and ST represents the colony area in CDA amended with different chemical agents.
Conidial stress tolerance analysis and properties analysis
The suspension of 107 conidia for each strain was evenly distributed on GM plates to measure the median germination time (GT50, h) under optimal conditions for 24 hours. Conidial germination rates were observed under a microscope at 6, 7, 8, 9, 10, 11, 12, 16, 20, and 24 hours post-incubation. The LD50 index, which represents UVB resistance, was determined following established protocols [Citation2,Citation45]. Briefly, 100 mL aliquots of 107 conidia were evenly spread on GM plates and air-dried for approximately 10 minutes. Subsequently, the uncovered plates were placed in the sample room of a Bio-Sun++ UV irradiation chamber (Vilber Lourmat, Marne-la-Vallée, France) and exposed to UVB (312 nm wavelength) doses ranging from 0.1 to 0.6 J/cm2 or left untreated for control. To assess the thermal tolerance of conidia, 2 mL aliquots of 107 conidia were incubated in a glass tube in a 45°C water bath. One hundred microlitres of conidial suspension were sampled every 15 minutes, plated onto GM plates, and then incubated at the optimal temperature of 25°C. The germination rate of each strain in control and experimental groups was examined and calculated after 24 hours. To assess the mean size and complexity (density), three stained samples containing 2 × 104 conidia per strain underwent flow cytometry analysis to measure the readings of the FSc and SSc detectors.
Bioassay for virulence
The virulence of B. bassiana conidia against Galleria mellonella was assessed using two infection methods. In the case of standard infection, 40 larvae were immersed in a 40 mL suspension with a concentration of 107/mL for 10 seconds. For cuticle-bypassing infection, 5 μL of a 105 conidial suspension was injected into each larva. Two batches of the bioassay experiments were carried out. At least 3 replicates were designed for each treatment. Subsequently, both immersed and injected G. mellonella larvae were incubated at 25°C and monitored at 12 or 24-hour intervals for survival/mortality. Mortality data were subjected to probit analysis to determine LT50 (d) as a virulence index. The deceased larvae from both infection modes were preserved under optimal conditions to observe fungal outgrowths on cadaver surfaces.
Hyphal body in vivo and dimorphic transition rates in vitro
Haemolymph samples were collected from surviving larvae either injected or immersed, 3 and 4 days or 5 and 6 days post-infection, respectively. Subsequently, these samples underwent microscopic examination using a haemocytometer to quantify the concentration of hyphal bodies in vivo. For in vitro assessment of dimorphic transition rates, 50 mL aliquots of a suspension at a concentration of 106/mL in TPB (3% trehalose, 0.5% peptone, 0.1% K2HPO4, 0.05% KCl, 0.05% MgSO4, and 0.001% FeSO4) were cultured on a shaking bed (150 rpm, 25°C) for a duration of 3 days. Blastospore production was determined under a microscope using a haemocytometer. After obtaining samples for blastospore concentration measurement, the remaining cultures were filtered through filter paper and dried to acquire hyphal biomass.
Oxidation and virulence-related enzyme activities
Samples from each strain (WT, Δrad6, and Δrad6::rad6) were obtained from SDAY cultures initiated with a 100 μL suspension of 107/mL spores, maintained under optimal conditions at 25°C with a 12/12 light/dark cycle for five days. To extract intracellular components, 0.1 g of ground mycelium was pulverized into a powder and combined with 1 mL of extraction solution. The mixture was centrifuged at 8000 g, 4°C for 10 minutes, and the resulting supernatant was promptly placed on ice for subsequent testing. Superoxide dismutase (SOD) and catalase (CAT) activities were determined using the SOD activity detection kit and CAT activity detection kit (Solarbio, Beijing, China), respectively.
The overall enzyme activity of extracellular enzymes (ECEs) and Pr1 protease, relevant to virulence, was assessed using our prior methodology [Citation45]. In summary, a 100 mL culture of 104 spores/mL CDA, supplemented with 0.3% bovine serum albumin (BSA) as the sole nitrogen source, was incubated for five days at 25°C with continuous shaking (150 r/min). Subsequently, 2 mL of the liquid culture underwent centrifugation at 13,200 g for 1 minute at 4°C, yielding a crude extract containing extracellular enzymes and Pr1 protease. The activities of total ECE and Pr1 in the supernatant (crude extract) were gauged by measuring optical densities at 442 nm (OD442) and 410 nm (OD410), respectively. One unit of enzyme activity was defined as the amount of enzyme required for a 0.01 increase in each OD value after a 1-hour reaction of each extract compared to a control. The total activity was then quantified as U ml−1 in the supernatant.
Transcriptome analysis and verification
Five-day-old cultures of the ∆rad6 and wild-type (WT) strains, cultivated on cellophane-overlaid CDA and CDA plates supplemented with 2 mM H2O2, were sent to Meiji BioTech Company (Hangzhou, China) for RNA extraction and subsequent transcriptomic analysis using an Illumina HiSeq™ platform. All strains were incubated at the optimal conditions of 25 °C and a 12:12 light-dark cycle. Raw reads obtained from cDNA sequencing underwent filtering to produce clean tags, followed by alignment to the B. bassiana genome [Citation51]. Differentially expressed genes (DEGs) were identified based on log2 R(Δrad6/WT) values ≤ −1 or ≥ 1, with statistical significance set at p value < 0.05. For KEGG analysis, all DEGs were enriched in various KEGG pathways, and the significance level was maintained at p value < 0.05, as performed on the Meiji BioTech Company website (https://vip.majorbio.com/). For transcriptome data verification, we isolated RNA from WT and disruption strains that were incubated under the same conditions as the transcriptome samples. Six downregulated and upregulated genes from the transcriptome data were selected as targets to verify data reliability through real-time quantitative (qPCR) analysis and the primers are listed in Supplementary Table S1.
Statistic analysis
All experiments in this study have three replicates. The one-way ANOVA was used to measure biochemical and phenotypic assays. The Tukey’s honest significance test (Tukey’s-HSD) was selected to determine the significance level of each assay.
Author contributions
YG and LBZ designed the experiments, analysed the data and prepared the manuscript. HMH and YHG performed the experiments. LBZ and HMH finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download MS Word (540.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analysed during this study are deposited in figshare, DOI: https://doi.org/10.6084/m9.figshare.25699368. The transcriptome sequencing data were deposited in NCBI (https://www.ncbi.nlm.nih.gov/bioproject) database with the accession number PRJNA1037315.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21505594.2024.2362748
Additional information
Funding
References
- Zhang LB, Feng MG. Antioxidant enzymes and their contributions to biological control potential of fungal insect pathogens. Appl Microbiol Biot. 2018;102(12):4995–13. doi: 10.1007/s00253-018-9033-2
- Wang DY, Fu B, Tong SM, et al. Two photolyases repair distinct DNA lesions and reactivate UVB-inactivated conidia of an insect mycopathogen under visible light. Appl Environ Microb. 2019;85(4):e02459–18. doi: 10.1128/AEM.02459-18
- Engelberg D, Klein C, Martinetto H, et al. The UV response involving the ras signaling pathway and AP-1 transcription factors is conserved between yeast and mammals. Cell. 1994;77(3):381–390. doi: 10.1016/0092-8674(94)90153-8
- Griffiths HR, Mistry P, Herbert KE, et al. Molecular and cellular effects of ultraviolet light-induced genotoxicity. Crit Rev Cl Lab Sci. 1998;35(3):189–237. doi: 10.1080/10408369891234192
- Cox MM, Goodman MF, Kreuzer KN, et al. The importance of repairing stalled replication forks. Nature. 2000;404(6773):37–41. doi: 10.1038/35003501
- Osborn AJ, Elledge SJ, Zou L. Checking on the fork: the DNA-replication stress-response pathway. Trends Cell Biol. 2002;12(11):509–516. doi: 10.1016/S0962-8924(02)02380-2
- Friedberg EC. Suffering in silence: the tolerance of DNA damage. Nat Rev Mol Cell Bio. 2005;6(12):943–953. doi: 10.1038/nrm1781
- Zhang HS, Lawrence CW. The error-free component of the RAD6/RAD18 DNA damage tolerance pathway of budding yeast employs sister-strand recombination. P Natl Acad Sci USA. 2005;102(44):15954–15959. doi: 10.1073/pnas.0504586102
- Xu X, Blackwell S, Lin A, et al. Error-free DNA-damage tolerance in Saccharomyces cerevisiae. Mutat Res-Rev Mutat. 2015;764:43–50. doi: 10.1016/j.mrrev.2015.02.001
- Daigaku Y, Davies AA, Ulrich HD. Ubiquitin-dependent DNA damage bypass is separable from genome replication. Nature. 2010;465(7300):951–955. doi: 10.1038/nature09097
- Karras GI, Jentsch S. The RAD6 DNA damage tolerance pathway operates uncoupled from the replication fork and is functional beyond S phase. Cell. 2010;141(2):255–267. doi: 10.1016/j.cell.2010.02.028
- Parker JL, Ulrich HD. Mechanistic analysis of PCNA poly-ubiquitylation by the ubiquitin protein ligases Rad18 and Rad5. Embo J. 2009;28(23):3657–3666. doi: 10.1038/emboj.2009.303
- Das-Bradoo S, Nguyen HD, Wood JL, et al. Defects in DNA ligase I trigger PCNA ubiquitylation at Lys 107. Nat Cell Biol. 2010;12(1):74–9; sup pp 1–20. doi: 10.1038/ncb2007
- Silva GM, Finley D, Vogel C. K63 polyubiquitination is a new modulator of the oxidative stress response. Nat Struct Mol Biol. 2015;22(2):116–123. doi: 10.1038/nsmb.2955
- Piro AS, Mayekar MK, Warner MH, et al. Small region of Rtf1 protein can substitute for complete Paf1 complex in facilitating global histone H2B ubiquitylation in yeast. P Natl Acad Sci USA. 2012;109(27):10837–10842. doi: 10.1073/pnas.1116994109
- Kim J, Roeder RG. Direct Bre1-Paf1 complex interactions and RING finger-independent Bre1-Rad6 interactions mediate histone H2B ubiquitylation in yeast. J Biol Chem. 2009;284(31):20582–20592. doi: 10.1074/jbc.M109.017442
- Van Oss SB, Shirra MK, Bataille AR, et al. The histone modification domain of Paf1 complex subunit Rtf1 directly stimulates H2B ubiquitylation through an interaction with Rad6. Mol Cell. 2016;64(4):815–825. doi: 10.1016/j.molcel.2016.10.008
- Wu Z, Liu J, Zhang QD, et al. Rad6-Bre1-mediated H2B ubiquitination regulates telomere replication by promoting telomere-end resection. Nucleic Acids Res. 2017;45(6):3308–3322. doi: 10.1093/nar/gkx101
- Fu Y, Zhu Y, Zhang K, et al. Rad6-Rad18 mediates a eukaryotic SOS response by ubiquitinating the 9-1-1 checkpoint clamp. Cell. 2008;133(4):601–611. doi: 10.1016/j.cell.2008.02.050
- Song Y-H, Ahn SH. A Bre1-associated protein, large 1 (Lge1), promotes H2B ubiquitylation during the early stages of transcription elongation. J Biol Chem. 2010;285(4):2361–2367. doi: 10.1074/jbc.M109.039255
- Nakanishi S, Lee JS, Gardner KE, et al. Histone H2BK123 monoubiquitination is the critical determinant for H3K4 and H3K79 trimethylation by COMPASS and Dot1. J Cell Bio. 2009;186(3):371–377. doi: 10.1083/jcb.200906005
- Robzyk K, Recht L, Osley MA. Rad6-dependent ubiquitination of histone H2B in yeast. Science. 2000;287(5452):501–504. doi: 10.1126/science.287.5452.501
- Schulze JM, Jackson J, Nakanishi S, et al. Linking cell cycle to histone modifications: SBF and H2B monoubiquitination machinery and cell-cycle regulation of H3K79 dimethylation. Mol Cell. 2009;35(5):626–641. doi: 10.1016/j.molcel.2009.07.017
- Rhie B-H, Song Y-H, Ryu H-Y, et al. Cellular aging is associated with increased ubiquitylation of histone H2B in yeast telomeric heterochromatin. Biochem Biophys Res Commun. 2013;439(4):570–575. doi: 10.1016/j.bbrc.2013.09.017
- Latham JA, Chosed RJ, Wang S, et al. Chromatin signaling to kinetochores: transregulation of Dam1 methylation by histone H2B ubiquitination. Cell. 2011;146(5):709–719. doi: 10.1016/j.cell.2011.07.025
- Huang H, Kahana A, Gottschling DE, et al. The ubiquitin-conjugating enzyme Rad6 (Ubc2) is required for silencing in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17(11):6693–6699. doi: 10.1128/MCB.17.11.6693
- Albrecht D, Hurlimann HC, Ceschin J, et al. Multiple chemo-genetic interactions between a toxic metabolite and the ubiquitin pathway in yeast. Curr Genet. 2018;64(6):1275–1286. doi: 10.1007/s00294-018-0843-7
- Storchova Z, Gil APR, Janderova B, et al. The involvement of the RAD6 gene in starvation-induced reverse mutation in Saccharomyces cerevisiae. Mol Gen Genet. 1998;258(5):546–552. doi: 10.1007/s004380050766
- de Faria MR, Wraight SP. Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control. 2007;43(3):237–256. doi: 10.1016/j.biocontrol.2007.08.001
- Ying SH, Feng MG. Insight into vital role of autophagy in sustaining biological control potential of fungal pathogens against pest insects and nematodes. Virulence. 2018;9(1):1–4. doi: 10.1080/21505594.2017.1320012
- Tong SM, Feng MG. Insights into regulatory roles of MAPK-cascaded pathways in multiple stress responses and life cycles of insect and nematode mycopathogens. Appl Microbiol Biot. 2019;103(2):577–587. doi: 10.1007/s00253-018-9516-1
- Amobonye A, Bhagwat P, Singh S, et al. Enhanced xylanase and endoglucanase production from Beauveria bassiana SAN01, an entomopathogenic fungal endophyte. Fungal Biol. 2021;125(1):39–48. doi: 10.1016/j.funbio.2020.10.003
- Mishra S, Malik A. Nutritional optimization of a native beauveria bassiana isolate (HQ917687) pathogenic to housefly, Musca domestica L. J Parasit Dis. 2013;37(2):199–207. doi: 10.1007/s12639-012-0165-5
- Aynalem B, Muleta D, Venegas J, et al. Molecular phylogeny and pathogenicity of indigenous beauveria bassiana against the tomato leafminer, tuta absoluta meyrick 1917 (lepidoptera: gelechiidae), in Ethiopia. J Genet Eng Biotechnol. 2021;19(1):127. doi: 10.1186/s43141-021-00227-x
- Gencer D. Isolation and characterization of a high-efficacy Beauveria bassiana strain from the European tent caterpillar, Malacosoma neustria Linnaeus (Lepidoptera: Lasiocampidae). Linnaeus (Lepidoptera: Lasiocampidae). Folia Microbiol (Praha). 2023;68(4):579–586. doi: 10.1007/s12223-023-01037-z
- Shi H-B, Chen G-Q, Chen Y-P, et al. MoRad6-mediated ubiquitination pathways are essential for development and pathogenicity in magnaporthe oryzae. Environ Microbiol. 2016;18(11):4170–4187. doi: 10.1111/1462-2920.13515
- Luo XC, Yu L, Xu SY, et al. Rad6, a ubiquitin conjugator required for insect-pathogenic lifestyle, UV damage repair, and genomic expression of Beauveria bassiana. Microbiol Res. 2024;281:127622. doi: 10.1016/j.micres.2024.127622
- Game JC, Williamson MS, Spicakova T, et al. The RAD6/BRE1 histone modification pathway in Saccharomyces confers radiation resistance through a RAD51-dependent process that is independent of RAD18. Genetics. 2006;173(4):1951–1968. doi: 10.1534/genetics.106.057794
- Sancar G, Sancar C, Bruegger B, et al. A global circadian repressor controls antiphasic expression of metabolic genes in neurospora. Mol Cell. 2011;44(5):687–697. doi: 10.1016/j.molcel.2011.10.019
- Mascarin GM, Jaronski ST. The production and uses of Beauveria bassiana as a microbial insecticide. World J Microb Biot. 2016;32(11):177. doi: 10.1007/s11274-016-2131-3
- Wang H, Peng H, Li W, et al. The toxins of Beauveria bassiana and the strategies to improve their virulence to insects. Front Microbiol. 2021;12:705343. doi: 10.3389/fmicb.2021.705343
- Alali S, Mereghetti V, Faoro F, et al. Thermotolerant isolates of Beauveria bassiana as potential control agent of insect pest in subtropical climates. PLOS ONE. 2019;14(2):e0211457. doi: 10.1371/journal.pone.0211457
- Liu R, Chen X, Zhao F, et al. The COMPASS complex regulates fungal development and virulence through histone crosstalk in the fungal pathogen Cryptococcus neoformans. J Fungi. 2023;9(6):672. doi: 10.3390/jof9060672
- Willocquet L, Colombet D, Rougier M, et al. Effects of radiation, especially ultraviolet B, on conidial germination and mycelial growth of grape powdery mildew. Eur J Plant Pathol. 1996;102(5):441–449. doi: 10.1007/BF01877138
- Vlckova V, Zuffova Z, Brozmanova J. UV-induced mutability in repair-deficientrad6-1 strains of Saccharomyces cerevisiae is caused by a suppressor gene. Folia Microbiol. 1992;37(4):267–272. doi: 10.1007/BF02814561
- Cai Q, Wang JJ, Shao W, et al. Rtt109-dependent histone H3 K56 acetylation and gene activity are essential for the biological control potential of Beauveria bassiana. Pest Manag Sci. 2018;74(11):2626–2635. doi: 10.1002/ps.5054
- Fan L, Bi T, Wang L, et al. DNA-damage tolerance through PCNA ubiquitination and sumoylation. Biochem J. 2020;477(14):2655–2677. doi: 10.1042/BCJ20190579
- Freiberg G, Mesecar AD, Huang HH, et al. Characterization of novel rad6/ubc2 ubiquitin-conjugating enzyme mutants in yeast. Curr Genet. 2000;37(4):221–233. doi: 10.1007/s002940050523
- Litwin A, Nowak M, Rozalska S. Entomopathogenic fungi: unconventional applications. Rev Environ Sci Bio. 2020;19(1):23–42. doi: 10.1007/s11157-020-09525-1
- Gao BJ, Mou YN, Tong SM, et al. Subtilisin-like Pr1 proteases marking the evolution of pathogenicity in a wide-spectrum insect-pathogenic fungus. Virulence. 2020;11(1):365–380. doi: 10.1080/21505594.2020.1749487
- Wang J, Ying SH, Hu Y, et al. Mas5, a homologue of bacterial DnaJ, is indispensable for the host infection and environmental adaptation of a filamentous fungal insect pathogen. Environ Microbiol. 2016;18(3):1037–1047. doi: 10.1111/1462-2920.13197
- Heller J, Tudzynski P. Reactive oxygen species in phytopathogenic fungi: signaling, development, and disease. Annu Rev Phytopathol. 2011;49(1):369–390. doi: 10.1146/annurev-phyto-072910-095355
- Zhang AX, Mouhoumed AZ, Tong SM, et al. BrlA and AbaA govern virulence-required dimorphic switch, conidiation, and pathogenicity in a fungal insect pathogen. mSystems. 2019;4(4):e00140–19. doi: 10.1128/mSystems.00140-19
- Selvam K, Rahman SA, Forrester D, et al. Histone H4 LRS mutations can attenuate UV mutagenesis without affecting PCNA ubiquitination or sumoylation. DNA Repair. 2020;95:102959.
- Li F, Ball LG, Fan L, et al. Sgs1 helicase is required for efficient PCNA monoubiquitination and translesion DNA synthesis in Saccharomyces cerevisiae. Curr Genet. 2018;64(2):459–468. doi: 10.1007/s00294-017-0753-0
