ABSTRACT
A tight relationship between apico-basal polarity and trafficking is essential for epithelial physiology and tissue homeostasis. Recent studies have described how some Rab GTPases, key components of the intracellular traffic machinery, contribute to the establishment of cell polarity in vertebrates. We have demonstrated a novel connection between cell polarity and trafficking: in Drosophila epithelia, the apical determinant aPKC is recycled via Rab11-Nuf-recycling endosomes to maintain cell polarity. Furthermore, the phosphorylation of Nuf by aPKC allows aPKC to control the sub-cellular localization of Nuf and its own membrane accumulation. Here we review these data and show the different contribution of the 2 Drosophila Rab11 adaptor proteins, Nuf and Rip11, to the maintenance of Drosophila embryonic ectoderm polarity.
Cell polarity is a basic characteristic required for their proper physiological function of most cells. A number of cell polarity determinants are required to establish and maintain the different functional domains in the cell (reviewed in ref. Citation1). Among these polarity determinants stand out those forming the apical (Crumbs-PATJ-PALS1 and Par3-Par6-aPKC) and the basolateral (DLG-LGL-Scribble) complexes. Proper trafficking toward, and recycling from, the membrane is required for establishment and maintenance of cell polarity. Once established, cell polarity defines the cell domains that organize the direction for cargo delivery. Thus, an interconnection between cell polarity and trafficking is essential to maintain each other and for proper cell physiology.
Rab proteins (the largest family of small Ras-like GTPases) are involved in regulating all the steps of intracellular trafficking by their capability to interact with specific membranes and with other proteins such as SNARES (involved in vesicle fusion), adaptor proteins that link Rabs to the cytoskeletal motors or exocyst complex members.Citation2
Several recent reports have shown the involvement of Rab proteins in the establishment of apico-basal polarity and lumen formation in MDCK 3D cell cultures.Citation3,4 For example, Rab11 and Rab8a direct the traffic of podocalyxin (PDX) to the apical membrane initiation site where PDX is essential to initiate the formation of a new lumen. Before PDX deposition Par3 is already located at the edge of cell-cell contact where it is required for PDX delivery. In accordance, Par3 knockdown impairs PDX membrane delivery and promotes its cytoplasmic accumulation.Citation3 Thus, Par 3 seems to mark the position of the pre-apical patch and where apical lumen formation begins by Rab-vesicle delivery.
Rab35, another Rab GTPase required for cytokinesis in MDCK cells, is also necessary for the proper delivery of PDX containing vesicles to the apical membrane initiation site. Rab35 tethers these vesicles to the membrane by its direct interaction with PDX. These vesicles also contain the apical determinants aPKC and Crumbs3, coupling in this way cytokinesis with apical polarity formation.Citation4
The functional communication between cell polarity and trafficking is not limited to the initiation of cell polarization but it is also required to maintain the different cell domains. The internalization and the delivery of vesicles from different regions in the membrane are defined by the polarity of the cell and also, the polarity determinants must be recycled to give certain plasticity to the cell during epithelia remodeling. E-Cadherin (E-Cad) trafficking is a well-studied example: both in vertebrates and in invertebrates it has been shown the trafficking of E-Cad to the membrane through Rab11-recycling endosomes (RE) (reviewed in ref. Citation5). Also Rab11-REs have been involved in the maintenance of the Crumbs (Crb) apical determinant membrane levelsCitation6 although Crb membrane levels are also regulated by the retromer.Citation7,8
Despite the requirement for both processes to be synchronized, only a few examples of interplay between them have been described.Citation3,9-14 Recently, our laboratory has shown a new interacting point between cell polarity and trafficking at the level of the polarity determinant aPKC and the Rab11-adaptor protein Nuclear fallout (NufCitation15). aPKC is a Ser/Thr kinase of the Par complex essential in most of the polarity processes. Depending on the process aPKC interacts with and phosphorylates different substrates modifying their sub-cellular localization. Nuf belongs to the Rab11-family interacting proteins (Rab11-FIPCitation16) and is the Drosophila homolog of FIP3, which functions as Rab11 adaptor to the microtubule motor protein kinesin and the dynein complex.Citation15,17 We have shown that aPKC directly interacts with and phosphorylates Nuf. Remarkably aPKC phosphorylation of Nuf modifies Nuf sub-cellular localization: a non-phosphorylatable version of Nuf accumulates at the apico-lateral cortex, while a phospho-mimetic protein, although also apically accumulated, avoids this lateral domain. While aPKC-dependent phosphorylation state of Nuf does not modify Nuf binding to Rab11 or to cytoskeleton motor proteins, distribution of the Rab11-Nuf-RE is affected by loss of aPKC. However the levels or distribution of known Rab11-RE cargoes such as DE-CadCitation18 or DeltaCitation19 are unaffected in cells overexpressing any of the 2 phosphorylation variants of Nuf. The AJ marker Bazooka (ortologue of Par-3 in Drosophila) or the apical-determinant Crb are also unaffected in these conditions but, interestingly, aPKC levels are increased in cells expressing the non-phosphorylatable Nuf. These results point to aPKC as a cargo of the Rab11-RE in Drosophila. We further demonstrate Rab11-dependent traffic of aPKC by depletion of Rab11 or Nuf, which causes a reduction of aPKC at the apical cortex. In accordance, interfering with exocytosis (in cells mutant for the exocyst components sec5 and sec6) result in the accumulation of aPKC at the apical cytoplasmic region. Furthermore, our results indicate that aPKC seems to be regulating its own recycling by phosphorylation and displacement of Nuf from the apico-lateral cortex. Remarkably, this Rab11 dependent trafficking of aPKC is in an already formed epithelia, that of the imaginal discs, indicating that aPKC recycling occurs when the polarity is established as it is the case of E-Cadherin.Citation20
We next studied whether Rab11-dependent trafficking of aPKC also takes place during polarity establishment. Similar to vertebrate cell culture, Drosophila embryogenesis is a very adequate system for studying cell polarity establishment. In fact, several studies performed in the Drosophila embryo have helped to clarify how cell polarity builds up (reviewed in ref. Citation21). The Drosophila zygote experiences 13 rapid nuclear divisions without cytokinesis forming a syncytial embryo. At mitotic cycle 14, cytokinesis first occurs when what formed the zygotes's membrane begins to grow inwards surrounding the nuclei and forming, at the membrane front, the furrow canals (analogous to the cytokinetic contractile ring).Citation22 The polarization of this plasma membrane begins toward the end of cellularization (stage 5) when the accumulation of Par3 at the apico-lateral membrane and the redistribution from basal to apical AJs of the E-Cad cause the first signs of apico-basal polarity in the cell,Citation23 both of which are dependent on microtubule trafficking.Citation24
During cellularization, Rab11 and the microtubule cytoskeleton are required to transport vesicles to the growing membrane. Nuf was first described by the phenotype of nuf mutant embryos, showing disrupted cellularization, incomplete membrane furrow formation and presence of nuclei inside the embryo.Citation25 Nuf protein locates to the centrosomes co-localizing with Rab11 from nuclear cycle 14 to the end of cellularization.Citation25 Interfering with Rab11 function disrupts membrane recruitment to the furrow, a phenotype also observed in nuf mutants.Citation26,27
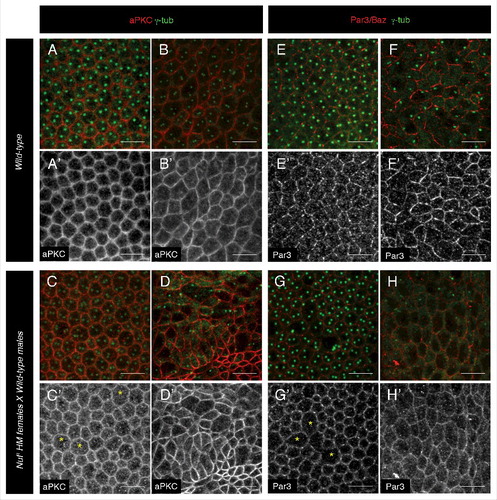
Because cellularization precedes polarity formation and Nuf is required in the imaginal discs to maintain aPKC levels, Nuf could also be required for recruiting aPKC to the membrane in the establishment of cell polarity during embryogenesis. However this is not the case as in nuf embryos aPKC can be normally detected in the apical membrane ( compare with 1A, A′ stage 5 and stage 6 (gastrulation) compare with 1B, B′). Previous data showed that the apical recruitment of aPKC is Baz dependent.Citation28 We observed that the distribution of Baz was not affected in nuf mutant background at these early stages ( stage 5 compare with 1E, E′ and stage 6 compare with 1F, F′). These results indicate that Nuf-Rab11 REs, although required for membrane growth and microtubule network formation in early cellularization, do not play a role in the establishment of apico-basal polarity.
Figure 1. Cell polarity formation is not affected in nuf mutant embryos. Drosophila embryos derived from wild-type (A–B', E -F') or nufCitation1 females (C–D', G–H') were stained with the centrosome marker γ-tubulin (green, A–H) and aPKC (red, A–D and white, A′-D′) or Baz (red, E–H and white, E′-H′) both at cellular blastoderm (stage 5, A, C, E and G) or at gastrulation (stage 6, B, D, F and H). At these stages of development, no differences in the localization or levels of these apical determinants can be detected between nufCitation1 and wild-type female embryos. Yellow asterisk in C′ and G′ mark cells with more than one nucleus due to a cellularization failure typical of nuf mutants. Scale bars 10 µm.
Rab11 is able to interact with other FIPs in addition to Nuf. In vertebrates, there are 5 Rab11-FIPs members classified in 2 groups depending on their interacting domains.Citation16 In Drosophila there are 2 FIPs: Nuf belonging to Class I and homologous to FIP3, is able to interact with kinesin and dynein; and Rip11, a Class II homologous to FIP2, which interacts with Myosin V. Rip11 and Rab11 are involved in the transport of Rhodopsin during photoreceptor development.Citation29 However no embryonic phenotypes have been described for Rip11 save those related with the recycling of DE-Cad in the trachea formation.Citation18 We wondered whether Rip11 was expressed during cellularization in the embryo and was required during early stages of embryogenesis. As shown in Rip11 is expressed from early stages () colocalizing with Nuf (). Despite such co-localization, Rip11 appears not to be required for cellularization process since in embryos lacking the Rip11 maternal component (Rip11 GLC) no delay in the membrane growth during cellularization or presence of nuclei inside the embryo can be detected.
Figure 2. Rip11 is expressed in the embryo but it is not required for cell polarity establishment. Drosophila wild-type embryos stained for Rip11 before (red in A and white in A′) and during cellularization and (red in B and white in B′). DAPI marks DNA in blue in A–B. At both stages Rip11 (red in C and D and white in C″ and D″) co-localizes with Nuf (green, C and D and white in C′ and D′) surrounding the centrosomes (marked by γ-tubulin in blue, C, D and white in C″′ and D″′). (E–H) In embryos derived from Rip11KG02485 germ line clones, aPKC (red, E and F and white in E′ and F′) or Par3/Baz (red, G and H and white in G′ and H′) membrane localization is not affected at cellularization (E and G) or blastoderm stages (F and H). γ-tubulin is marking centrosomes in green in E–H. Scale bars 10 µm.
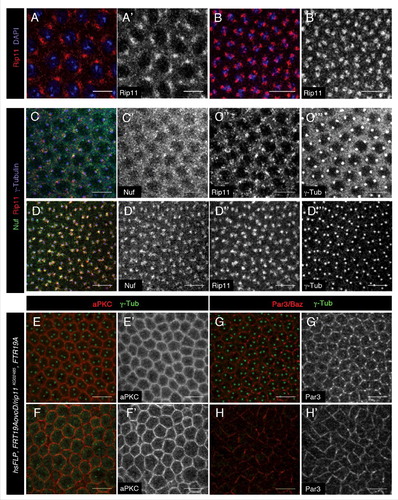
Rip11 is neither required for the establishment of cell polarity, as both aPKC (, stage 5 and F, F′, stage 6) and Baz (, stage 5 and H, H′, stage 6) are properly located in the membrane of the Rip11 embryos at cellular blastoderm stage and during early gastrulation.
Therefore trafficking mediated by Rab11 and their effectors Nuf and Rip11 appears no to be involved in the establishment of the embryonic polarity, although later on Rab11-REs are required for recycling of polarity determinants such as DE-Cad or Crb.Citation6,18 In the tracheal tree, the correct intercalation of the tracheal cells depends on the Rab11-Rip11 recycling of DE-Cad.Citation18 However it has not been determined which FIP is associated with Rab11-REs dependent traffic of Crb or DE-Cad in the embryonic ectoderm. We examined the involvement of Nuf and Rip11 in the maintenance of embryonic epidermis polarity. Loss of the maternal Nuf component causes early embryonic lethality, probably due to the defects in cellularization. However, some nuf embryos manage to cellularize and begin gastrulation reaching later stages of development (). In these embryos, the sub-cellular distribution of aPKC is severely affected ( compare with A′) as well as Par3/Baz (not shown). DE-Cad localization is also disturbed; however to a lesser extent than aPKC since some DE-Cad staining can still be detected in places where aPKC is completely lost (, compare with 3A and A″). It can also be observed a decreased accumulation of Crb compared with the wild-type epidermis (, compare with B, B′) indicating a failure of Crumb membrane deposition. However, considering that Crb membrane stabilization depends on aPKCCitation30 and that its membrane localization in nuf embryos is less compromised than that of aPKC, it cannot be ruled out that the failure in Crumbs deposition could be a secondary effect of aPKC membrane loss.
Figure 3. Maintenance of apical polarity is compromised in nuf mutants. Embryos stained for aPKC (white in A′, C′ and E′ and green in A″′, C″′ and E″′) and DE-Cad (white in A, A,″ C, C,″ E and E″ and red in A″′, C″′ and E″′) or Crumbs (B, D, F) at stage 11 of development in wild-type (A, B), or embryos derived from nufCitation1 females (C, D) or Rip11KG02485 (E, F) lacking maternal contribution (the embryos proceeded from hsFLP, ovoD1-18, FRTG19/ rip11KG0248, FRT19A females, which were heat shocked on second instar larval to induce germ line clones and were mated to Oregon R males). aPKC is completely lost from the membrane of nuf embryos while DE-Cad can still be detected in a few spots and Crb is at lower levels in the membrane. In Rip11 mutant embryos polarity is affected to a lesser extent. (G–H) en-G4 driven overexpression of an aPKC′s non-phosphorylatable Nuf variant fused to Myc (blue in G) or a Nuf phospho-mimetic variant (H) stained against aPKC (green in G and H and white in G′ and H′) and DE-Cad (red in G and H and white in G″ and H″). Only the non-phosphorylatable version enhances the level of aPKC in the membrane (yellow arrow) but does not affect DE-Cad levels. (I–J) Imaginal discs containing clones of Rip11KG02485 marked by the absence of β-Gal (green). DE-Cad pattern (red in I) is partially disrupted in the mutant cells (arrows in I′ and asterisks in I″), while aPKC (red in J) is unaffected (J′–J″). I″ and J″ are close-ups of the yellow dashed line boxes in I′ and J′ respectively. Scale bars 10 µm.
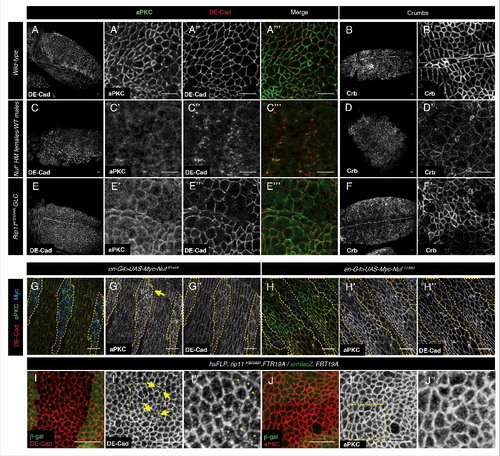
The defects in Rip11 mutants related with membrane polarity are in general less severe than those of nuf mutants: Rip11 embryos reach late stages of development without apparent morphological defects apart from those on trachea formation already described.Citation18 In some embryos patches of epidermis devoid of aPKC () or E-Cad () can be detected, but in this case similarly affected. Crumbs distribution is also affected but in a different manner than in nuf mutants, since instead of showing decreased levels in the membrane, it is absent in some cells ().
Thus the analysis of the nuf and Rip11 mutants corroborates the requirement of the Rab11-RE in the maintenance of apical cell polarityCitation6 and also suggests a different role of the 2 family of FIPs in the control of the cargos transported by the RE, as described in vertebrates.Citation31 aPKC maintenance in the membrane is heavily affected in nuf mutant embryos indicating that, as in the imaginal discs,Citation15 aPKC is recycled in the embryonic epidermis and this recycling is dependent on Nuf. In fact, the overexpression of the non-phosphorylatable version of Nuf (Nuf S155ACitation15) in the embryo enhances the levels of aPKC in the membrane without affecting other apical membrane markers such as DE-Cad () while the non-phosphorylatable version (Nuf S155D) has no effect (), mimicking the effect of the expression of these phosphorylation mutant versions of Nuf on aPKC distribution in the imaginal disc cells.Citation15
Our data and that of others, strongly suggest that Rip11 is involved mainly in the recycling of the AJ determinant E-Cad. In Rip11 mutants other polarity determinants such as Crb, Par3 or aPKC are also affected but to a lesser or similar extent than DE-Cad. Accordingly, in the epithelia of the imaginal discs, cells devoid of Rip11 show disruptions in the DE-Cad adherens junction belt (), while aPKC apical localization is unaffected ().
Interestingly, both Nuf and Rip11mutant phenotypes are milder in the epithelia of imaginal discs than in the embryonic epidermis. This can be explained because the active morphogenetic movements during embryogenesis require continuous recycling of polarity proteins. On the other hand, in the imaginal disc the epithelium is more stable and cell polarity is fully established making this tissue less sensitive to changes in recycling efficiency of polarity proteins.
A tight communication between the cell polarity and cell trafficking machineries is necessary for the maintenance of tissue homeostasis. Although different studies are revealing new points of interaction between them, many questions remain unanswered. For example, are the role of the different Rabs in the establishment or maintenance of apical basal polarity evolutionarily conserved? In vertebrates Rab11 and Rab8 are involved in the establishment of apico-basal cell polarity in MDCK cyst formation.Citation3 In Drosophila, although Rab11 is required for the maintenance of this polarity Citation6 it has not been addressed whether Rab11-dependent trafficking plays a role in early events of polarity formation. Recently, Rab8 has been related to the growth of the cell membrane during the cellularization of the Drosophila embryo.Citation32 It would be interesting to investigate whether, as in MDCKs system, Rab8 is also required later in Drosophila embryonic ectodermal cells for the initiation of the localization of Baz and DE-Cad in the apico-lateral cell membrane. Rab35 has been involved in the attachment of vesicles containing PDX, aPKC, Crb and Cdc42 to the apical membrane initiation site region during lumen formation.Citation4 In Drosophila Rab35 is maternally provided (BDGP expression data) and thus, is present in the embryo from the first stages of development. It is attractive to think about a similar function for Rab35 in the early establishment of embryonic polarity as in vertebrate cells.
Material and methods
Fly strains
We used the following stocks: Oregon R (wild-type), nuf,Citation1, 25 UAS-Myc-NufS155A and UAS-Myc-NufS155D.Citation15 Null embryos for rip11 were generated by inducing germ line clones (GLC) in hsFLP::FRTG19:: ovo1-18 / rip11KG02485::FRT19ACitation29 females, which were heat shocked on second larval instar for 1 hour at 37°C and mated to Oregon R males. nuf embryos were obtained from nufCitation1 homozygous females mated to Oregon R males. Clones in imaginal disc were generated at 48–72 h after egg laying in the HSFlip, rip11KG0248, FRT19A/ armLacZ, FRT19A genotype and dissected and stained at 96–120 hours after egg laying.
We used en-Gal4 as driver line.
Immunohistochemistry
Embryos were fixed in 1:1 formaldehyde 4% in PBS:n-heptane for 20 minutes at room temperature and stained according to standard protocols. Imaginal discs were fixed for 20 minutes in paraformaldehyde 20 % followed by a second fixation of 20 minutes in paraformaldehyde 20 %–0.1 % TritonX-100.
The following primary antibodies were used: anti-Nuf and anti-Baz (1:500Citation15), anti-γ-Tubulin (Sigma, 1:350), mouse and rabbit anti-aPKC (1:100 and 1:500 respectively) from Santa Cruz Biotechnology; mouse anti-Myc (Cell Signaling 1:500); anti-Crb (1:50) and anti-DECad (1:50) from Developmental Studies HybridomaBank; anti-Rip11 (a gift from Dr. D. Ready, 1:200), anti-ßgal (Promega, 1:10.000) Secondary antibodies were coupled to Alexa488, Alexa555 or Alexa647 (Molecular Probes, 1:500). DNA was stained with DAPI (Molecular Probes, 1:500).
Images were taken on a SPE Leica confocal microscope and processed using FIJI and Adobe Photoshop programs. Panels are projections of 10 confocal images of 0.30 µm thickness of embryonic epidermis using the average intensity algorithm (ImageJ).
Abbreviations
| aPKC | = | atypical Protein Kinase C |
| AJ | = | Adherens junction |
| Baz | = | Bazooka |
| Crb | = | Crumbs |
| 3D culture | = | 3 Dimensional culture |
| DE-Cad | = | Drosophila E-Cadherin |
| DLG | = | discs large |
| GLC | = | Germ Line Clone |
| LGL | = | lethal giant larvae |
| MDCK | = | Madin-Darby canine kidney |
| Nuf | = | Nuclear fallout |
| PALS1 | = | Protein Associated with Lin Seven 1 |
| PAR | = | partitioning-defective |
| PATJ | = | Pals1 Associated Tight Junction |
| Rab11-FIP | = | Rab11-family interacting proteins |
| RE | = | Recycling endosomes |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank A. Satoh, D. Ready and M. Llimargas for reagents; J. Culí, J. C-G Hombría and S. Campuzando for suggestions to the manuscript.
Funding
This work was supported by grants of the MICINN/FEDER to S.S and J. C-G Hombría (BFU2010-15851 and BFU2013- 45866), and Junta de Andalucía (P11-CVI7256).
References
- Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol 2014; 15:225-42; PMID:24651541; https://doi.org/10.1038/nrm3775
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513-25; PMID:19603039; https://doi.org/10.1038/nrm2728
- Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peranen J, Martin-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 2010; 12:1035-45; PMID:20890297; https://doi.org/10.1038/ncb2106
- Klinkert K, Rocancourt M, Houdusse A, Echard A. Rab35 GTPase couples cell division with initiation of epithelial apico-basal polarity and lumen opening. Nat Commun 2016; 7:11166; PMID:27040773; https://doi.org/10.1038/ncomms11166
- Kowalczyk AP, Nanes BA. Adherens junction turnover: regulating adhesion through cadherin endocytosis, degradation, and recycling. Subcell Biochem 2012; 60:197-222; PMID:22674073; https://doi.org/10.1007/978-94-007-4186-7_9
- Roeth JF, Sawyer JK, Wilner DA, Peifer M. Rab11 helps maintain apical crumbs and adherens junctions in the Drosophila embryonic ectoderm. PLoS One 2009; 4:e7634; PMID:19862327; https://doi.org/10.1371/journal.pone.0007634
- Pocha SM, Wassmer T, Niehage C, Hoflack B, Knust E. Retromer controls epithelial cell polarity by trafficking the apical determinant Crumbs. Curr Biol 2011; 21:1111-7; PMID:21700461; https://doi.org/10.1016/j.cub.2011.05.007
- Zhou B, Wu Y, Lin X. Retromer regulates apical-basal polarity through recycling Crumbs. Dev Biol 2011; 360:87-95; PMID:21958744; https://doi.org/10.1016/j.ydbio.2011.09.009
- Georgiou M, Marinari E, Burden J, Baum B. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr Biol 2008; 18:1631-8; PMID:18976918; https://doi.org/10.1016/j.cub.2008.09.029
- Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol 2008; 18:1639-48; PMID:18976911; https://doi.org/10.1016/j.cub.2008.09.063
- Harris KP, Tepass U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J Cell Biol 2008; 183:1129-43; PMID:19064670; https://doi.org/10.1083/jcb.200807020
- Yoshihama Y, Sasaki K, Horikoshi Y, Suzuki A, Ohtsuka T, Hakuno F, Takahashi S, Ohno S, Chida K. KIBRA suppresses apical exocytosis through inhibition of aPKC kinase activity in epithelial cells. Curr Biol 2011; 21:705-11; PMID:21497093; https://doi.org/10.1016/j.cub.2011.03.029
- de Vreede G, Schoenfeld JD, Windler SL, Morrison H, Lu H, Bilder D. The Scribble module regulates retromer-dependent endocytic trafficking during epithelial polarization. Development 2014; 141:2796-802; PMID:25005475; https://doi.org/10.1242/dev.105403
- Balklava Z, Pant S, Fares H, Grant BD. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol 2007; 9:1066-73; PMID:17704769; https://doi.org/10.1038/ncb1627
- Calero-Cuenca FJ, Espinosa-Vazquez JM, Reina-Campos M, Diaz-Meco MT, Moscat J, Sotillos S. Nuclear fallout provides a new link between aPKC and polarized cell trafficking. BMC Biol 2016; 14:32; PMID:27089924; https://doi.org/10.1186/s12915-016-0253-6
- Horgan CP, McCaffrey MW. The dynamic Rab11-FIPs. Biochem Soc Trans 2009; 37:1032-6; PMID:19754446; https://doi.org/10.1042/BST0371032
- Riggs B, Fasulo B, Royou A, Mische S, Cao J, Hays TS, Sullivan W. The concentration of Nuf, a Rab11 effector, at the microtubule-organizing center is cell cycle regulated, dynein-dependent, and coincides with furrow formation. Mol Biol Cell 2007; 18:3313-22; PMID:17581858; https://doi.org/10.1091/mbc.E07-02-0146
- Shaye DD, Casanova J, Llimargas M. Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea. Nat Cell Biol 2008; 10:964-70; PMID:18641639; https://doi.org/10.1038/ncb1756
- Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, Gaitan MG, Knoblich JA. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell 2005; 122:763-73; PMID:16137758; https://doi.org/10.1016/j.cell.2005.08.017
- Langevin J, Morgan MJ, Sibarita JB, Aresta S, Murthy M, Schwarz T, Camonis J, Bellaiche Y. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev Cell 2005; 9:365-76; PMID:16224820; https://doi.org/10.1016/j.devcel.2005.07.013
- Laprise P, Tepass U. Novel insights into epithelial polarity proteins in Drosophila. Trends Cell Biol 2011; 21:401-8; PMID:21530265; https://doi.org/10.1016/j.tcb.2011.03.005
- Lecuit T. Junctions and vesicular trafficking during Drosophila cellularization. J Cell Sci 2004; 117:3427-33; PMID:15252125; https://doi.org/10.1242/jcs.01312
- McGill MA, McKinley RF, Harris TJ. Independent cadherin-catenin and Bazooka clusters interact to assemble adherens junctions. J Cell Biol 2009; 185:787-96; PMID:19468069; https://doi.org/10.1083/jcb.200812146
- Hain D, Langlands A, Sonnenberg HC, Bailey C, Bullock SL, Muller HA. The Drosophila MAST kinase Drop out is required to initiate membrane compartmentalisation during cellularisation and regulates dynein-based transport. Development 2014; 141:2119-30; PMID:24803657; https://doi.org/10.1242/dev.104711
- Rothwell WF, Fogarty P, Field CM, Sullivan W. Nuclear-fallout, a Drosophila protein that cycles from the cytoplasm to the centrosomes, regulates cortical microfilament organization. Development 1998; 125:1295-303; PMID:9477328
- Riggs B, Rothwell W, Mische S, Hickson GR, Matheson J, Hays TS, Gould GW, Sullivan W. Actin cytoskeleton remodeling during early Drosophila furrow formation requires recycling endosomal components Nuclear-fallout and Rab11. J Cell Biol 2003; 163:143-54; PMID:14530382; https://doi.org/10.1083/jcb.200305115
- Pelissier A, Chauvin JP, Lecuit T. Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis. Curr Biol 2003; 13:1848-57; PMID:14588240; https://doi.org/10.1016/j.cub.2003.10.023
- Harris TJ, Peifer M. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J Cell Biol 2005; 170:813-23; PMID:16129788; https://doi.org/10.1083/jcb.200505127
- Li BX, Satoh AK, Ready DF. Myosin V, Rab11, and dRip11 direct apical secretion and cellular morphogenesis in developing Drosophila photoreceptors. J Cell Biol 2007; 177:659-69; PMID:17517962; https://doi.org/10.1083/jcb.200610157
- Sotillos S, Diaz-Meco MT, Caminero E, Moscat J, Campuzano S. DaPKC-dependent phosphorylation of Crumbs is required for epithelial cell polarity in Drosophila. J Cell Biol 2004; 166:549-57; PMID:15302858; https://doi.org/10.1083/jcb.200311031
- Baetz NW, Goldenring JR. Rab11-family interacting proteins define spatially and temporally distinct regions within the dynamic Rab11a-dependent recycling system. Mol Biol Cell 2013; 24:643-58; PMID:23283983; https://doi.org/10.1091/mbc.E12-09-0659
- Mavor LM, Miao H, Zuo Z, Holly RM, Xie Y, Loerke D, Blankenship JT. Rab8 directs furrow ingression and membrane addition during epithelial formation in Drosophila melanogaster. Development 2016; 143:892-903; PMID:26839362; https://doi.org/10.1242/dev.128876
