ABSTRACT
Ral is a small GTPase of the Ras superfamily that is important for a number of cellular functions. Recently, we found that expression of Ral is regulated by store-operated calcium entry (SOCE) in Drosophila neurons. In this study, through genetic and behavioural experiments, we show that Ral function is required in differentiated muscles for flight. Reducing Ral function in muscles, specifically reduced duration of flight bouts but not other motor functions, like climbing. Interestingly, unlike in the nervous system, Ral expression in the muscle is not regulated by SOCE. Moreover, either knockdown or genetic inhibition of SOCE in muscles does not affect flight. These findings demonstrate that a multiplicity of signalling mechanisms very likely regulate Ral expression in different tissues.
KEYWORDS:
Introduction
Ral GTPases, members of the Ras superfamily regulate a variety of cellular processes including vesicle trafficking, cell polarity, and even oncogenesis.Citation1,2 In mammals, two Ral GTPase genes are present — RalA and RalBCitation3 and they interact with the exocyst complex.Citation4 Ral function in various cell types has been studied, neuronal RalA and RalB regulate neurotransmitter release and neurite branching.Citation5,6 RalA is critical for insulin secretion from pancreatic cells.Citation7 Besides secretion, Ral proteins are also important in membrane targeting of proteins, like the Glut transporter in adipocytes. Citation8
In the genetic model system Drosophila, there exists a single homolog for Ral.Citation9 Like the mammalian Ral proteins, Drosophila Ral also mediates its functions through the exocyst complex.Citation10 It has several functions in development Citation11,12 , including membrane transportCitation13 and receptor trafficking.Citation14 In a recent study, we identified Ral, as a regulator of Drosophila flight. Reduction of either Ral levels or function, across all neurons led to significant flight defects. However, Ral mutant flies were almost flightless Citation15, suggesting that Ral function might be required in tissues other than the nervous system, to regulate flight. In Drosophila, like most insects, the duration of flight bouts is regulated primarily by neuronal activity and muscle function.Citation16-Citation18 Ral is expressed in and functions in multiple tissues besides the nervous system.Citation9,13,19 Thus, in this study we have investigated whether Ral function in muscles is required for flight.
Store-operated Calcium Entry (SOCE) is a mode of calcium entry in the cell that is triggered by emptying of endoplasmic reticular calcium stores by extracellular stimuli Citation20, and is involved in a variety of cellular processes like regulating gene transcription Citation15,21 , muscle contraction Citation22 and cell migration.Citation23 SOCE is mediated primarily by the endoplasmic reticular calcium sensor STIM Citation24 and the plasma membrane calcium channel Orai.Citation25 Knockdown of either of these components in Drosophila neurons results in flight defects.Citation26 In the previous study, we identified Ral expression to be regulated by SOCE Citation15 in neurons and thus we have tested for similar regulation in the muscle in this paper.
Results
A dominant negative form of Ral, which harbours a single point mutation RalS25N in the GTP binding domain of Ral (henceforth referred to as RalDN ) reduces Ral function.Citation13,19 Flies in which UAS-RalDN expression was driven using a muscle-specific Mef2-GAL4 Citation27 were tested for their ability to fly using the single flight assay.Citation15 Flies with reduced Ral function in muscles had significantly lower flight durations than the corresponding controls (). Reduction in Ral levels in muscles using an RNAi against Ral (RalIR ) also significantly reduced the flight duration as compared with controls (). This reduction in flight was not due to altered wing morphology because wings of flies, with Ral perturbations in muscles, appeared normal (). A muscle requirement for Ral in the context of flight was further tested with another muscle driver, Mhc-GAL4, which expresses exclusively in differentiated muscles Citation28 as compared with Mef2-GAL4 which also expresses in myoblasts. Mhc-GAL4 control flies themselves had slightly impaired flight bout durations as compared to the Mef2-GAL4 control ( and ) indicating an effect of the genetic background on flight. Still, flies with expression of RalDN using Mhc-GAL4 had significantly reduced flight bout durations as compared to controls with no effect on wing morphology (). Taken together, these data demonstrate that the small GTPase Ral is also required in differentiated muscles for regulating the duration of Drosophila flight.
Figure 1. Ral function is required in the muscle for flight but not climbing. (A and B) Representative images of flies from the indicated genotypes. Wing posture is normal for all genotypes tested (top). A box plot of flight durations of flies measured by single flight assay of the indicated genotypes is shown below. In the box plots, centre lines show the medians; crosses indicate the means; box limits indicate the 25th and 75th percentiles; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles, individual data points are represented as open circles and the numbers below represent “n” for each box. Flies from two crosses were used in these experiments. (C) Number of flies, out of ten that could climb 8cm in 12 seconds of the indicated genotypes are shown as bar graphs of mean values and standard errors of mean. Alphabets over the box plots/ bar graphs represent statistically indistinguishable groups (one-way ANOVA with a post hoc Tukey's test p < 0.05). Pairwise comparisons were performed by unpaired, two-tailed Student's t-test and the exact p-values are indicated. (D) Confocal images of thoracic indirect flight muscles of the indicated genotypes. The upper and lower panels show representative images of dorsal ventral muscles (DVMs) and dorsal longitudinal muscles (DLMs) respectively. Muscle membranes are marked by mRFP allowing visualization of the fibres. BF indicates brightfield image. Orientation of the thorax is indicated by A (anterior) and P (posterior). Scale bar indicates 10µm.
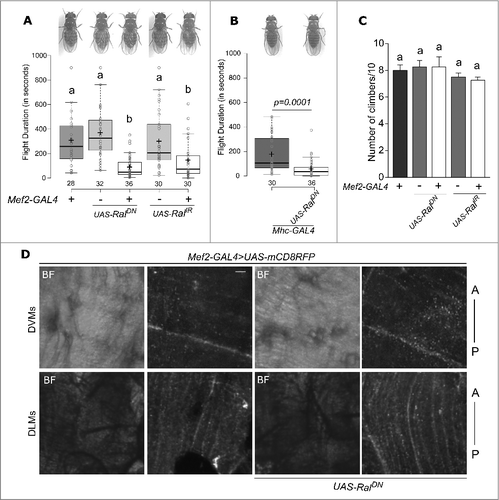
Both the muscle GAL4s used, also express in muscles other than flight muscles. Therefore, next we tested if Ral function is specific to flight muscles or required more generally. For this, we tested the ability to climb 8 cm in 12 seconds. Flies with Mef2-GAL4 driven expression of either RalDN or RalIR in muscles could climb at a rate that was statistically indistinguishable from controls () suggesting that Ral function is very likely specific to flight muscles. If Ral function is important in the development of the indirect flight muscles, this could reflect in their morphology in adults.Citation29 Thus, we looked at the morphology of the indirect flight muscles in the thorax which consist of dorsal longitudinal muscles (DLMs) and dorsal ventral muscles (DVMs).Citation30 Mutations in genes required for formation of these muscle fibres can lead to malformed muscle fibres.Citation31 However, expression of RalDN with Mef2-GAL4 in all muscles through development and in adults resulted in muscle fibres that look identical to control ().
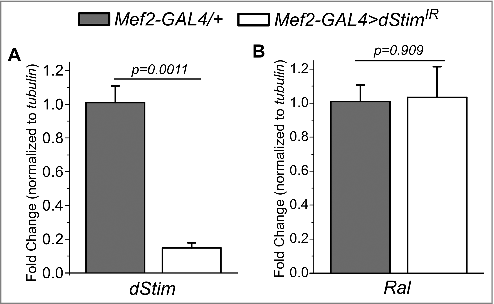
SOCE in neurons regulates flight Citation32 and expression of Ral.Citation15 Calcium dynamics in the muscle, mediated by Sarco-Endoplasmic Reticulum Calcium ATPase (SERCA) are important for muscle function.Citation33 SERCA interacts with SOCE components in Drosophila neurons.Citation32 Having identified a role for Ral in flight muscles, we therefore tested whether SOCE-regulates Ral expression in muscles. We knocked down levels of dStim, a calcium sensor on the endoplasmic reticulum that initiates SOCE upon store-depletion Citation24 in muscles with an RNAi against dStim (dStimIR ) and Mef2-GAL4. Flies with dStim knockdown in muscles had normal wings and flight durations, no different from controls () despite significant reduction in dStim levels (). Next, we tested the role of another molecule that mediates SOCE, the calcium release-activated calcium (CRAC) channel, Orai.Citation25 Expression of the dominant-negative form of dOrai, dOraiE180A results in abrogation of SOCE.Citation34 Flies with UAS-dOraiE180A without any GAL4 by itself had lower flight durations than other controls ( compared to and ). Expression of dOraiE180A in the muscle using the Mef2-GAL4 however, resulted in no difference in flight times compared to the corresponding control (). These data indicate that flight muscles do not require SOCE through the STIM/Orai pathway, and suggest that Ral expression in flight muscles is independent of SOCE. This idea was tested next by measuring Ral mRNA levels by qRT-PCR from dissected thoraces, in which the predominant tissues are flight muscles. Ral expression was the same between RNA from thoraces with knockdown of dStim and controls ( and ) confirming that Ral levels in muscles are not regulated by SOCE.
Figure 2. SOCE function in muscle is not required for flight. Representative images of flies from the indicated genotypes showing normal wing posture (top) and a box plot of flight durations of flies measured by single flight assay (bottom) from flies with dStim knockdown in the muscle (A) or dOrai dominant negative expression in the muscle (B). Box plot symbols are as described for . All flies tested were from the same cross. Pairwise comparisons were performed by unpaired, two-tailed Student's t-test and the exact p-values are indicated.
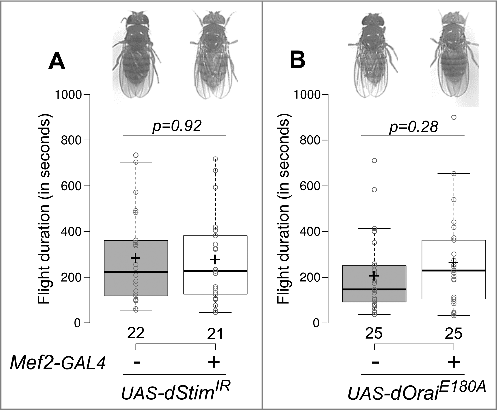
Figure 3. SOCE does not regulate Ral expression in the muscle. Change in the levels of dStim (A) and Ral (B) in the indicated genotypes, normalized to tubulin as measured by qRT-PCR. Flies used were from the same cross as that for . Bars represent means and error bars, standard errors of mean of the fold change. Pairwise comparisons were performed by unpaired, two-tailed Student's t-test and the exact p-values are indicated.
Discussion
In this study, we have identified a role for Ral in flight muscles of Drosophila flight. We also find that in muscles, unlike the nervous system, Ral expression is not regulated by store-operated calcium entry (SOCE).
Perturbation of Ral function with both Mef2-GAL4 and Mhc-GAL4 resulted in flight defects. However, Mef2-GAL4 is expressed predominantly in developing muscles Citation27,35 whereas Mhc-GAL4 is expressed mostly in differentiated muscles.Citation28 Therefore, Ral function maybe required in developing as well as differentiated flight muscles. Based on the similar flight deficits observed in Mef2>RalDN and Mhc>RalDN ( and ) it is more likely that Ral function is primarily in differentiated flight muscles. Moreover, although Mef2-GAL4 expresses in myoblasts, the gross anatomy of the adult thoracic flight muscles was not affected upon expression of RalDN supporting the idea that Ral function is not required for development of the flight muscles. However, our data does not rule out the possibility that loss of Ral function might affect the ultrastructure of these muscles. The requirement of Ral in differentiated muscles supports a role for Ral in muscle physiology. Further experiments are required to identify the temporal requirement of Ral in flight muscles which might help understand its function in this cell type better. Ral function in regulating the addition of post-synaptic membranes in differentiated muscles has been demonstrated earlier Citation13, and may be the underlying cause for the observed flight deficits. This previous study had also demonstrated a role for Ca2+ in activation of Ral but because perturbing SOCE in muscles did not lead to observed flight deficits, calcium through SOCE is unlikely to activate Ral.
Ral expression in neurons is down-regulated by knockdown of dSTIM.Citation15 However, in muscles, Ral levels were unaffected upon dStim knockdown. Concurrently, perturbations of SOCE in muscles did not alter flight durations. SOCE mediated by STIM and Orai has been documented in mammalian muscles Citation36 but so far has not been shown in Drosophila muscles. Expression of dStim in Drosophila thoracic muscles (), indicates the presence of SOCE. Thus, Ral expression is differentially regulated in the two tissues — neurons and muscles. The functional significance of this differential regulation needs further investigation.
Materials and methods
Fly rearing and stocks
Flies were reared on media containing cornmeal supplemented with yeast extract. Fly crosses were set up and allowed to lay eggs at 25°C, vials containing larvae were moved to 29°C and were maintained at the elevated temperature until testing. Fly strains used are as follows: Mef2-GAL4 (BL27390), Mhc-GAL4 (BL55133), UAS-RalDN (BL32094), UAS-RalIR (BL29580) and UAS-mcd8RFP (BL32219) from Bloomington Drosophila Stock Centre; dStimIR (v47073) from Vienna Drosophila Resource Centre and UAS-dOraiE180A .Citation34
Fly images
Fly images were acquired using a Pro-Series camera attached to a stereo-microscope and images were obtained at the same zoom and image acquisition settings for all flies.
Single flight assay
In order to measure the ability of the flies to initiate and sustain flight, single flight assay was performed as described in.Citation15 Briefly, 2—5 days old flies of either sex were anaesthetized on ice for about a minute and then tethered on to a thin metal wire between the head and thorax. After allowing a short duration of recovery, a gentle, mouth blow air-puff was given as a stimulus to initiate flight, and the duration of time from initiation to cessation of flight was noted at flight duration. Flight duration observation was capped at fifteen minutes.
Climbing assay
Flies were tested for their climbing ability using an assay described in.Citation34 Briefly, a batch of ten 2—5 day old flies of either sex were dropped in a graduated glass cylinder, and tapped to collect them at the bottom. The number of flies that crossed the 8cm mark after this, in 12 seconds were noted as climbers. At least 3 independent batches of flies were used for this assay per genotype.
Preparation of thoracic flight muscles and microscopy
DVMs were dissected from adults of corresponding genotypes in ice cold 1x phosphate buffered saline (PBS) and fixed with 4% Paraformaldehyde (PFA) for an hour on ice. For visualizing DLMs, the thoraces were fixed with 4% PFA on ice for an hour and then cut longitudinally using a sharp blade. Both were mounted in 60% glycerol. The samples were imaged using a Leica SP5 confocal microscope using a 20x objective under similar image acquisition settings. Complete z-projects were obtained using Fiji Citation37 and no post-acquisition processing was performed.
RNA isolation and qRT-PCR
RNA was isolated from thoraces of adult female flies. Four thoraces were used per sample to isolate RNA using TRIzol (Invitrogen), following manufacturer's protocol. Approximately 500ng of total RNA was treated with DNAseI (Invitrogen) and reverse transcribed to cDNA using M-MLV (Invitrogen) as described in.Citation34 For quantitative PCR, Kapa SYBR Fast qPCR kit (KAPA Biosystems) was used in a 10µl reaction on a ABI QuantStudio3 system. Three biological replicates from independently isolated RNA samples were used for each experiment. A melt curve was performed to ensure a single product. Fold changes were calculated using the ΔΔCt method.Citation38 β-Tubulin was used as an internal control. Primer sequences used are as follows (5′-3’):
β-Tub(F) — CCAAGGGTCATTACACAGAGG, β-Tub(R)—ATCAGCAGGGTTCCCATACCdStim(F) — GAAGCAATGGATGTGGTTCTG, dStim(R) — CCGAGTTCGATGAACTGAGAG
Ral(F) — GACTACGAGCCCACCAAG, Ral(R) — CGGCATAATCCTCCTGGC
Data representation and statistics
Flight data is represented as box plots generated using BoxPlotR.Citation39 Otherwise, data is represented as bar graphs representing means and error bars, standard errors of mean. For analyses with more than two test conditions, One-way Analysis of Variance (ANOVA), followed by pairwise Tukey's test was used. Statistical significance post ANOVA is denoted with small alphabets, where different alphabets indicate statistical significance at p < 0.05 whereas same alphabet indicates statistically indistinguishable groups. For analyses comparing two conditions, unpaired, two-tailed Student's t-test was used and the exact p-values are indicated within the figures.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Siddharth Jayakumar for performing confocal imaging. We thank CIFF, NCBS for access to the confocal microscope. Fly strains from Bloomington Drosophila Stock Center (National Institutes of Health P40OD018537) were used in this study.
Funding
This study was supported by core funds from the National Centre for Biological Sciences, Tata Institute of Fundamental Research, Bangalore.
References
- Feig LA. Ral-GTPases: Approaching their 15 minutes of fame. Trends in Cell Biology. 2003;13:419-425. https://doi.org/10.1016/S0962-8924(03)00152-1. PMID:12888294
- Shirakawa R, Horiuchi H. Ral GTPases: Crucial mediators of exocytosis and tumourigenesis. J. Biochem. 2015;157:285-299. https://doi.org/10.1093/jb/mvv029. PMID:25796063
- Chardin P, Tavitian A. Coding sequences of human ralA and ralB cDNAs. Nucleic Acids Res. 1989;17:4380. https://doi.org/10.1093/nar/17.11.4380. PMID:2662142
- Moskalenko S, Tong C, Rosse C, Mirey G., Formstecher E, Daviet L, Camonis J, White MA. Ral GTPases Regulate Exocyst Assembly through Dual Subunit Interactions. J. Biol. Chem. 2003;278:51743-51748. https://doi.org/10.1074/jbc.M308702200. PMID:14525976
- Li G, Han L, Chou T, Fujita Y, Arunachalam L, Xu A, Wong A, Chiew SK, Wan Q, Wang L, et al. RalA and RalB function as the critical GTP sensors for GTP-dependent exocytosis. J. Neurosci. 2007;27:190-202. https://doi.org/10.1523/JNEUROSCI.2537-06.2007. PMID:17202486
- Lalli G, Hall A. Ral GTPases regulate neurite branching through GAP-43 and the exocyst complex. J. Cell Biol. 2005;171:857-869. https://doi.org/10.1083/jcb.200507061. PMID:16330713
- Lopez JA, Kwan EP, Xie L, He Y, James D, Gaisano HY The RalA GTPase is a central regulator of insulin exocytosis from pancreatic islet beta cells. J. Biol. Chem. 2008;283:17939-17945. https://doi.org/10.1074/jbc.M800321200. PMID:18426794
- Chen XW, Leto D, Chiang SH, Wang Q, Saltiel AR. Activation of RalA Is Required for Insulin-Stimulated Glut4 Trafficking to the Plasma Membrane via the Exocyst and the Motor Protein Myo1c. Dev. Cell. 2007;13:391-404. https://doi.org/10.1016/j.devcel.2007.07.007. PMID:17765682
- Sawamoto K, Winge P, Koyama S, Hirota Y, Yamada C, Miyao S, Yoshikawa S, Jin MH, Kikuchi A, Okano H. The Drosophila Ral GTPase regulates developmental cell shape changes through the Jun NH(2)-terminal kinase pathway. J. Cell Biol. 1999;146:361-72. https://doi.org/10.1083/jcb.146.2.361. PMID:10427090
- Moskalenko S, et al. The exocyst is a Ral effector complex. Nat. Cell Biol. 2002;4:66-72. https://doi.org/10.1038/ncb728. PMID:11740492
- Holly RM, Mavor LM, Zuo Z, Blankenship JT. A rapid, membrane-dependent pathway directs furrow formation through RalA in the early Drosophila embryo. Development. 2015;142:2316-2328. https://doi.org/10.1242/dev.120998. PMID:26092850
- Cho B, Fischer JA. Ral GTPase promotes asymmetric Notch activation in the Drosophila eye in response to Frizzled/PCP signaling by repressing ligand-independent receptor activation. Development. 2011;138:1349-1359. https://doi.org/10.1242/dev.056002. PMID:21350007
- Teodoro RO, Pekkurnaz G, Nasser A, Higashi-Kovtun ME, Balakireva M, McLachlan IG, Camonis J, Schwarz TL. Ral mediates activity-dependent growth of postsynaptic membranes via recruitment of the exocyst. EMBO J. 2013;32:2039-55. https://doi.org/10.1038/emboj.2013.147. PMID:23812009
- Klose M, Duvall L, Li W, Liang X, Ren C, Steinbach JH, Taghert PH. Functional PDF Signaling in the Drosophila Circadian Neural Circuit Is Gated by Ral A-Dependent Modulation. Neuron. 2016;90:1-14. https://doi.org/10.1016/j.neuron.2016.04.002. PMID:27054611
- Richhariya S, Jayakumar S, Abruzzi K, Rosbash M, Hasan G. A pupal transcriptomic screen identifies Ral as a target of store-operated calcium entry in Drosophila neurons. Sci. Rep. 2017;7:42586. https://doi.org/10.1038/srep42586. PMID:28195208
- Dickinson MH, Tu MS. The function of dipteran flight muscle. Comparative Biochemistry and Physiology — A Physiology. 1997;116:223-238. https://doi.org/10.1016/S0300-9629(96)00162-4
- Fry SN, Sayaman R, Dickinson MH. The aerodynamics of free-flight maneuvers in Drosophila. Science. 2003;300:495-8. https://doi.org/10.1126/science.1081944. PMID:12702878
- Sadaf S, Reddy OV, Sane SP, Hasan G. Neural control of wing coordination in flies. Curr. Biol. 2015;25:80-86. https://doi.org/10.1016/j.cub.2014.10.069. PMID:25496964
- Ghiglione C, Devergne O, Cerezo D, Noselli S. Drosophila RalA is essential for the maintenance of Jak/Stat signalling in ovarian follicles. EMBO Rep. 2008;9:676-82. https://doi.org/10.1038/embor.2008.79. PMID:18552769
- Prakriya M, Lewis R S. Store-Operated Calcium Channels. Physiol. Rev. 2015;95:1383-1436. https://doi.org/10.1152/physrev.00020.2014. PMID:26400989
- Feske S Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 2007;7:690-702. https://doi.org/10.1038/nri2152. PMID:17703229
- Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, et al. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat. Cell Biol. 2008;10:688-697. https://doi.org/10.1038/ncb1731. PMID:18488020
- Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 Are Critical for Breast Tumor Cell Migration and Metastasis. Cancer Cell. 2009;15:124-134. https://doi.org/10.1016/j.ccr.2008.12.019. PMID:19185847
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE JR, Meyer T. STIM Is a Ca2+ Sensor Essential for Ca2+-Store-Depletion-Triggered Ca2+ Influx. Curr. Biol. 2005;15:1235-1241. https://doi.org/10.1016/j.cub.2005.05.055. PMID:16005298
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179-185. https://doi.org/10.1038/nature04702. PMID:16582901
- Venkiteswaran G, Hasan G. Intracellular Ca2+ signaling and store-operated Ca2+ entry are required in Drosophila neurons for flight. Proc. Natl. Acad. Sci. 2009;106:10326-10331. https://doi.org/10.1073/pnas.0902982106
- Ranganayakulu G, Schulz RA, Olson EN. Wingless Signaling Induces nautilus Expression in the Ventral Mesoderm of the Drosophila Embryo. Dev. Biol. 1996;176:143-148. https://doi.org/10.1006/dbio.1996.9987. PMID:8654890
- Klein P, Müller-Rischart AK, Motori E, Schönbauer C, Schnorrer F, Winklhofer KF, Klein R. Ret rescues mitochondrial morphology and muscle degeneration of Drosophila Pink1 mutants. EMBO J. 2014;33:341-355. https://doi.org/10.1002/embj.201284290. PMID:24473149
- Bernard F, Lalouette A, Gullaud M, Jeantet AY, Cossard R, Zider A, Ferveur JF, Silber J. Control of apterous by vestigial drives indirect flight muscle development in Drosophila. Dev. Biol. 2003;260:391-403. https://doi.org/10.1016/S0012-1606(03)00255-0. PMID:12921740
- Swank DM. Mechanical analysis of Drosophila indirect flight and jump muscles. Methods. 2013;56:69-77. https://doi.org/10.1016/j.ymeth.2011.10.015
- Mukherjee P, Gildor B, Shilo B, Vijayraghavan K, Schejter ED. The actin nucleator WASp is required for myoblast fusion during adult Drosophila myogenesis. Development. 2011;2357:2347-2357.
- Venkiteswaran G, Hasan G. Intracellular Ca2+ signaling and store-operated Ca2+ entry are required in Drosophila neurons for flight. Proc. Natl. Acad. Sci. U. S. A. 2009;106:10326-10331. https://doi.org/10.1073/pnas.0902982106. PMID:19515818
- Sanyal S, Consoulas C, Kuromi H, Basole A, Mukai L, Kidokoro Y, Krishnan KS, Ramaswami M. Analysis of conditional paralytic mutants in Drosophila sarco-endoplasmic reticulum calcium ATPase reveals novel mechanisms for regulating membrane excitability. Genetics. 2005;169:737-750. https://doi.org/10.1534/genetics.104.031930. PMID:15520268
- Pathak T, Agrawal T, Richhariya S, Sadaf S, Hasan G. Store-Operated Calcium Entry through Orai Is Required for Transcriptional Maturation of the Flight Circuit in Drosophila. J. Neurosci. 2015;35:13784-13799. https://doi.org/10.1523/JNEUROSCI.1680-15.2015. PMID:26446229
- Bryantsev AL, Baker PW, Lovato TL, Jaramillo MS, Cripps RM. Differential requirements for Myocyte Enhancer Factor-2 during adult myogenesis in Drosophila. Dev. Biol. 2012;361:191-207. https://doi.org/10.1016/j.ydbio.2011.09.031. PMID:22008792
- Pan Z, Brotto M, Ma J. Store-operated Ca2+ entry in muscle physiology and diseases. BMB reports. 2014;47:69-79. https://doi.org/10.5483/BMBRep.2014.47.2.015. PMID:24411466
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open source platform for biological image analysis. Nat. Methods. 2012;9:676-682. https://doi.org/10.1038/nmeth.2019. PMID:22743772
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2^-ΔΔCT Method. Methods. 2001;25:402-408. https://doi.org/10.1006/meth.2001.1262. PMID:11846609
- Spitzer M, Wildenhain J, Rappsilber J, Tyers M. BoxPlotR: a web tool for generation of box plots. Nat. Methods. 2014;11:121-2. https://doi.org/10.1038/nmeth.2811. PMID:24481215
