ABSTRACT
Adipose tissue contains various types of immunocompetent cells, and these cells of innate and adaptive immunity control adipose tissue inflammation that blunts insulin sensitivity. Recent studies have shown that adipocytes express CD1d and present lipid antigen(s) to activate natural killer T (NKT) cells. The function of adipocytes is in turn modulated by cytokines that NKT cells produce to alter the expression of anti-inflammatory adipokine(s) and the production of inflammatory and chemoattractant cytokines. These in vitro studies imply that the interaction between adipocytes and NKT cells might affect the development of not only obesity but also obesity-related diseases. To test the importance of the interaction between NKT cells and adipocytes, we examined whether an adipocyte-specific CD1d deletion affected the development of obesity, which had been demonstrated with B6.CD1d−/− (CD1d KO). We found that the interaction is indeed important to induce adipose tissue inflammation and insulin resistance in response to lipid excess. In this commentary, the advances and controversies on NKT cells and obesity are discussed based on our recent report that NKT cells play a pivotal role in the regulation of adipose tissue by communicating with adipocytes via CD1d.
Introduction
Adipose tissue (AT) harbors various types of immunocompetent cells and thus it can be viewed as an ancestral immune organ.Citation1 Interactions between adipocytes and cells of the stromal vascular fraction, including immune cells, maintain tissue integrity and physiological function. Adipocytes are now recognized as multi-functional cells which store excess energy as fat and secrete adipokines, such as TNF-α, IL-6, leptin, adiponectin, and many other biological response modifiers.Citation2,3 The level of adiposity alters the expression of these adipokines, and either aggravates or ameliorates the development of obesity. Obesity, especially visceral-type, also affects the development of various diseases in a direct or indirect way. These include not merely the lifestyle-related diseases explosively increasing worldwide in recent years, such as type 2 diabetes, hypertension, and atherosclerosis, but also airway diseases, infectious diseases and cancers.Citation4-8 Many studies, which focused on discovering the mechanisms of obesity-associated aggravation, have revealed that chronic low-grade inflammation contributes to the derangement of cellular and molecular homeostasis in the various organs.Citation9, 10 The low-grade but generalized inflammation in the visceral AT, abundantly distributed as mesenteric, paranephric or perigonadal AT in the abdominal cavity, along with the altered production of adipokines induces insulin resistance and results in glucose intolerance at the whole-body level.Citation11-13 To date, various immunocompetent cells have been found to be involved as either enhancers or suppressors of AT inflammation. For example, M1-macrophages, CD8+ T cells, and NK cells can all induce insulin resistance by producing Th1-based inflammatory cytokines,Citation14-16 whereas regulatory cells, such as M2-macrophages, Treg cells, and ILC2s, suppress AT inflammation and obesity.Citation17,18 These findings contribute to the idea that an immune-based intervention may control the development of obesity and obesity-associated diseases.
Natural killer T (NKT) cells and obesity
NKT cells are a unique subset of T cells that recognize lipid antigens in the context of CD1d, which is mainly expressed by professional antigen-presenting cells (APCs), such as macrophages, dendritic cells, and B cells. NKT cells are promptly activated upon TCR stimulation and produce large amounts of various cytokines that modulate immune balance.Citation19,20 α-galactosylceramide is a prototype ligand recognized by invariant NKT (iNKT) cells bearing an invariant TCR α-chain, Vα14-Jα18 in mice and Vα24-Jα18 in humans.Citation21 Another type of NKT cell, variant NKT (vNKT cells) which expresses diverse TCRs, is supposed to recognize a variety of lipid antigens, such as sulfatide.Citation22 Since NKT cells are one of the AT-resident cells,Citation1 they have been assumed to be either enhancers or suppressors of the immune balance in AT inflammation and to play some roles in obesity and other diseases related to lipid metabolism.
Accordingly, many studies have been done to date to examine whether NKT cells play beneficial or harmful roles and the results have been recapitulated by Wu and Van Kaer in Adipocyte,Citation23 and by Rakhshandehroo et al.Citation24 There are 3 potential outcomes for the involvement of NKT cells in the development of obesity; namely protective, aggravating, or neutral. Some groups have reported a protective role, because the development of obesity was aggravated when iNKT cells were deficient. They demonstrated that iNKT cells in adipose tissue produce anti-inflammatory cytokines, such as IL-4 and IL-10, in contrast to those in the spleen and liver.Citation25-27 These Th2 cytokines induced M2 macrophages and suppressed obesity-associated inflammation.Citation28 The AT iNKT (atNKT) cell constitutes a specialized subset of NKT cells expressing the transcription factor E4BP4 that develops independently from PLZF, which controls the development of an iNKT cell in other tissues. However, other groups, including ours, have reported their aggravating role because the development of obesity was ameliorated in the absence of NKT cells. In their reports, iNKT cells produced pro-inflammatory cytokines, such as IFN-γ, in response to lipid excess in the body,Citation29,30 and type II NKT cells also exacerbated diet-induced obesity in the absence of iNKT cells.Citation31 The rest reported a neutral role where NKT cells have no active role for skewing the environment toward either a Th1- or Th2-bias during the development of obesity.Citation32
The three types of outcome in body mass by NKT cells corresponded to the alterations of metabolic phenotypes in respective models. ‘Aggravating group’ demonstrated that the insulin level in blood was significantly higher in wild-type (WT) mice fed on HFD than that of CD1d KO mice where NKT cells were deficient, suggesting that insulin resistance was induced in the background where NKT cells were present and exposed to lipid excess.Citation29,31 It was further confirmed that the hypoglycemia after insulin administration was blunted with insulin tolerance test (ITT) in WT mice on HFD when compared with that in CD1d KO mice on HFD. More precisely, insulin resistance was demonstrated with hyperinsulinemic–euglycemic clamp where decreased activity of insulin was implied with the decreased rate of glucose infusion necessary for maintaining euglycemia in WT mice than that of CD1d KO mice.Citation31 One of the mechanisms in insulin resistance appeared to be primarily mediated through pro-inflammatory cytokines, such as TNF-α from NKT cells and inflammatory Mφ on target cells, although various factors affect glucose homeostasis in vivo.Citation33 On the other hand, by ‘protecting group’, insulin resistance was revealed to be induced in the absence of either iNKT cells in Jα18 KO mice or all CD1d-resitricted NKT cells in CD1d KO mice as lesser hypoglycemic response with ITT.Citation25 The activation of iNKT cells with α-GalCer in WT mice demonstrated lesser increase of blood sugar after IPGTT or better hypoglycemic response with ITT, both of which were abrogated with administration of anti-IL-10 and anti-IL-4 antibodies.Citation25 Of note, a protective group demonstrated that insulin resistance implied by ITT or IPGTT was better notified in Jα18 KO or CD1d KO than WT on low fat diet (LFD).Citation26 Interestingly, a beneficial role of CD1d, but not via iNKT cells, was reported by ‘another neutral group’, which could be explained by vNKT cells or really by the function of CD1d independent of NKT cells.Citation34
At any rate, there may be several reasons for these divergent (more than 3) outcomes, such as differences in the mouse strains, the fat components in their diet, and their gut microbiomes, which still remain elusive. Since NKT cells are likely to respond in either pro- or anti-inflammatory manners to the above differences, we need to find the critical condition for the particular switch.
Adipocytes as an APC for NKT cells
We now know that NKT cells affect the development of obesity in vivo, even though the outcomes are divergent. However, we have not identified the major cell-type that expresses CD1d to interact or activate NKT cells in adipose tissue. Recent studies, including ours, focus on the interaction of NKT cells and adipocytes, because adipocytes express CD1d, suggesting that the adipocytes may function as the APC. In vitro studies revealed that adipocytes could stimulate NKT cells through up-regulating CD1d by activating Pparg, a member of the peroxisome proliferator-activated receptor family, during adipocyte maturation.Citation35 Furthermore, CCAAT-enhancer-binding protein (C/EBP) β and δ isoforms appeared to be critical regulators of CD1d expression, and the Ag presentation through CD1d was also controlled by MTP (microsomal triglyceride transfer protein) in adipocytes.Citation36 To clarify the role of the interaction between NKT cells and adipocytes in vivo, we employed an adipocyte-specific deletion of CD1d by crossbreeding CD1d1-floxed miceCitation37 and adipoq-cre miceCitation38 and directly testing whether the development of obesity could be affected in CD1df/f-adipoq-cre mice fed on a high fat diet (HFD).Citation39 The CD1df/f-adipoq-cre mice gained less body weight compared with the control mice fed with a HFD, whereas no significant differences were observed when fed on a standard fat diet (SFD). Glucose intolerance in CD1df/f-adipoq-cre mice was suppressed and the level of serum insulin was lower than that of control mice, suggesting that insulin resistance was ameliorated when CD1d was deleted only in adipocytes. NKT cells in adipose tissue were activated and produced more IFN-γ in the control mice than in the CD1df/f-adipoq-cre mice. Meanwhile, IFN-γ modulated functions of adipose tissue by increasing expression of CD1d1, Ccl2 and Cxcl16, which drove AT inflammation by recruiting more macrophages and NKT cells, whereas decreasing that of Adipoq-induced anti-inflammatory function. Indeed, macrophage infiltration into adipose tissue was significantly reduced in CD1df/f-adipoq-cre mice compared with the control mice. These results suggest that AT inflammation and the harmful outcomes are significantly suppressed by the inhibition of the interaction between NKT cells and adipocytes without deletion of CD1d in other cells and tissues.Citation39 It is worthy of note that the deletion of CD1d that is limited to adipocytes is almost as effective as the deletion of CD1d in the whole body as seen in B6.CD1d1−/−.Citation31
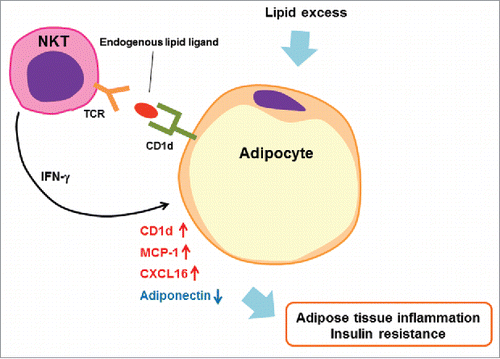
Collectively, our report indicates that adipocytes function as APCs for NKT cells by presenting putative Ag(s) via CD1d, and that NKT cells have a vital role in response to lipid excess in adipocytes that induce adipose tissue inflammation and thus significant influence in systemic glucose metabolism ().
Figure 1. A causative interaction for adipose tissue inflammation between NKT cells and adipocytes. Mature adipocytes express CD1d and act as antigen-presenting cells for NKT cells. NKT cells are activated by endogenous lipid ligand(s) presented via CD1d, which may be synthesized in adipocytes. NKT cells produce IFN-γ due to this interaction which modulates the function of adipocytes to induce adipose tissue inflammation by further increasing the expression of CD1d, MCP-1, and CXCL16 and by decreasing anti-inflammatory adiponectin. NKT cells can foster an inflammatory milieu in adipose tissue, which contributes to the development of insulin resistance, obesity, and more.
Future perspectives
It has been revealed that the crosstalk between NKT cells and adipocytes is necessary to induce adipose tissue inflammation and insulin resistance. However, the endogenous ligand(s) needed to activate NKT cells in AT remains to be determined. It is probable that NKT cells alter their properties, such as cytokine production, depending on the chemical species of lipid ligands of endogenous or exogenous origins. Therefore, opposing results have been obtained in several studies to date on diet-induced obesity in respective laboratories.
The identification of the lipid ligands and the biosynthetic pathway for endogenous ligands may lead to the regulated generation of the ligands and could thus be applicable as a preventive or therapeutic measure for obesity itself and obesity-associated diseases. Those may include the developments of inhibitors for the lipid Ag synthesis, TCR-antagonism, and blockade of antigen presentation by adipocytes. To this end, employment of mice of Nurr77-EGFP reporterCitation40 from NKT cell-side and the metabolomics approach from adipocyte-side may help elucidate the interactions of these cells.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by Grant-in-Aid for Young Scientists (B) (#25870708) by Japan Society for the Promotion of Science, Grant-in-Aid for Project Research by Kitasato University Graduate School of Medical Sciences, Parent's Association (Keyaki Kai) Grant of Kitasato University School of Medicine, and BioLegend/Tomy Digital Biology Research Grant.
References
- Caspar-Bauguil S, Cousin B, Galinier A, Segafredo C, Nibbelink M, André M, Casteilla L, Pénicaud L. Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett 2005; 579:3487-92; PMID:15953605; http://dx.doi.org/10.1016/j.febslet.2005.05.031
- Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol 2014; 220:T47-59; PMID:24403378; http://dx.doi.org/10.1530/JOE-13-0339
- Waki H and Tontonoz P. Endocrine functions of adipose tissue. Annu Rev Pathol 2007; 2:31-56; PMID:18039092; http://dx.doi.org/10.1146/annurev.pathol.2.010506.091859
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell 2001; 104:531-43; PMID:11239410; http://dx.doi.org/10.1016/S0092-8674(01)00240-9
- Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011; 121:2111-7; PMID:21633179; http://dx.doi.org/10.1172/JCI57132
- Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444:860-7; PMID:17167474; http://dx.doi.org/10.1038/nature05485
- Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, Yan QW, Miyoshi H, Mashek DG. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest 2011; 121:2102-10; PMID:21633178; http://dx.doi.org/10.1172/JCI46069
- Hegde V, Dhurandhar NV. Microbes and obesity–interrelationship between infection, adipose tissue and the immune system. Clin Microbiol Infect 2013; 19:314-20; PMID:23506525; http://dx.doi.org/10.1111/1469-0691.12157
- Knights AJ, Funnell AP, Pearson RC, Crossley M, Bell-Anderson KS. Adipokines and insulin action: A sensitive issue. Adipocyte 2014; 3:88-96; PMID:24719781; http://dx.doi.org/10.4161/adip.27552
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006; 444:847-53; PMID:17167472; http://dx.doi.org/10.1038/nature05483
- Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell 2015; 161:146-60; PMID:25815992; http://dx.doi.org/10.1016/j.cell.2015.02.022
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006; 116:1793-801; PMID:16823477; http://dx.doi.org/10.1172/JCI29069
- Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol 2014; 222:R113-27; PMID:25006217; http://dx.doi.org/10.1530/JOE-14-0283
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112:1796-808; PMID:25006217; http://dx.doi.org/10.1172/JCI200319246
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009; 15:914-20; PMID:19633658; http://dx.doi.org/10.1038/nm.1964
- Wensveen FM, Jelenčić V, Valentić S, Šestan M, Wensveen TT, Theurich S, Glasner A, Mendrila D, Štimac D, Wunderlich FT, et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol 2015; 16:376-85; PMID:25729921; http://dx.doi.org/10.1038/ni.3120
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009; 15:930-9; PMID:19633656; http://dx.doi.org/10.1038/nm.2002
- Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 2015; 160:74-87; PMID:25543153; http://dx.doi.org/10.1016/j.cell.2014.12.011
- Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol 2007; 19:354-64; PMID:17428648; http://dx.doi.org/10.1016/j.coi.2007.03.001
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol 2007; 25:297-336; PMID:17150027; http://dx.doi.org/10.1146/annurev.immunol.25.022106.141711
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. CD1d-restricted and TCR-mediated activation of Va14 NKT cells by glycosylceramides. Science 1997; 278:1626-9; PMID:9374463; http://dx.doi.org/10.1126/science.278.5343.1626
- Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest 2007; 117:2302-12; PMID:17641782; http://dx.doi.org/10.1172/JCI31602
- Wu L, Van Kaer L. Contribution of lipid-reactive natural killer T cells to obesity-associated inflammation and insulin resistance. Adipocyte 2013; 2:12-16; PMID:23700548; http://dx.doi.org/10.4161/adip.22296
- Rakhshandehroo M, Kalkhoven E, Boes M. Invariant natural killer T cells in adipose tissue: novel regulators of immune-mediated metabolic disease. Cell Mol Life Sci 2013; 70:4711-27; PMID:23835837; http://dx.doi.org/10.1007/s00018-013-1414-1
- Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O'Shea D, O'Farrelly C, Exley MA. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 2012; 37:574-87; PMID:22981538; http://dx.doi.org/10.1016/j.immuni.2012.06.016
- Schipper HS, Rakhshandehroo M, van de Graaf SF, Venken K, Koppen A, Stienstra R, Prop S, Meerding J, Hamers N, Besra G, et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest 2012; 122:3343-54; PMID:22863618; http://dx.doi.org/10.1172/JCI62739
- Sag D, Krause P, Hedrick CC, Kronenberg M, Wingender G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J Clin Invest 2014; 124:3725-40; PMID:25061873; http://dx.doi.org/10.1172/JCI72308
- Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C, Besra G, Vomhof-Dekrey EE, Tighe M, Koay HF, et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T(reg) cells and macrophages in adipose tissue. Nat Immunol 2015; 16:85-95; PMID:25436972; http://dx.doi.org/10.1038/ni.3047
- Wu L, Parekh VV, Gabriel CL, Bracy DP, Marks-Shulman PA, Tamboli RA, Kim S, Mendez-Fernandez YV, Besra GS, Lomenick JP, et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc Natl Acad Sci U S A 2012; 109:E1143-52; PMID:22493234; http://dx.doi.org/10.1073/pnas.1200498109
- Ohmura K, Ishimori N, Ohmura Y, Tokuhara S, Nozawa A, Horii S, Andoh Y, Fujii S, Iwabuchi K, Onoé K, et al. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler Thromb Vasc Biol 2010; 30:193-9; PMID:19910631; http://dx.doi.org/10.1161/ATVBAHA.109.198614
- Satoh M, Andoh Y, Clingan CS, Ogura H, Fujii S, Eshima K, Nakayama T, Taniguchi M, Hirata N, Ishimori N, et al. Type II NKT cells stimulate diet-induced obesity by mediating adipose tissue inflammation, steatohepatitis and insulin resistance. PLoS One 2012; 7:e30568; PMID:22383967
- Mantell BS, Stefanovic-Racic M, Yang X, Dedousis N, Sipula IJ, O'Doherty RM. Mice lacking NKT cells but with a complete complement of CD8+ T-cells are not protected against the metabolic abnormalities of diet-induced obesity. PLoS One 2011; 6:e19831; PMID:21674035
- Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell 2015; 160:816-27; PMID:25723161; http://dx.doi.org/10.1016/j.cell.2015.02.010
- Kotas ME, Lee H-Y, Gillum MP, Annicelli C, Guigni BA, Shulman GI, Medzhitov R. Impact of CD1d deficiency on metabolism. PLoS One 2011; 6:e25478; PMID:21980475
- Huh JY, Kim JI, Park YJ, Hwang IJ, Lee YS, Sohn JH, Lee SK, Alfadda AA, Kim SS, Choi SH, et al. A novel function of adipocytes in lipid antigen presentation to iNKT cells. Mol Cell Biol 2013; 33:328-39; PMID:23149942; http://dx.doi.org/10.1128/MCB.00552-12
- Rakhshandehroo M, Gijzel SM, Siersbæk R, Broekema MF, de Haar C, Schipper HS, Boes M, Mandrup S, Kalkhoven E. CD1d-mediated presentation of endogenous lipid antigens by adipocytes requires microsomal triglyceride transfer protein. J Biol Chem 2014; 289:22128-39; PMID:24966328; http://dx.doi.org/10.1074/jbc.M114.551242
- Bai L, Constantinides MG, Thomas SY, Reboulet R, Meng F, Koentgen F, Teyton L, Savage PB, Bendelac A. Distinct APCs explain the cytokine bias of α-galactosylceramide variants in vivo. J Immunol 2012; 188:3053-61; PMID:22393151; http://dx.doi.org/10.4049/jimmunol.1102414
- Wang ZV, Deng Y, Wang QA, Sun K, Scherer PE. Identification and characterization of a promoter cassette conferring adipocyte-specific gene expression. Endocrinology 2010; 151:2933-9; PMID:20363877; http://dx.doi.org/10.1210/en.2010-0136
- Satoh M, Hoshino M, Fujita K, Iizuka M, Fujii S, Clingan CS, Van Kaer L, Iwabuchi K. Adipocyte-specific CD1d-deficiency mitigates diet-induced obesity and insulin resistance in mice. Sci Rep 2016; 6:28473; PMID:27329323; http://dx.doi.org/10.1038/srep28473
- Holzapfel KL, Tyznik AJ, Kronenberg M, Hogquist KA. Antigen-dependent versus-independent activation of invariant NKT cells during infection. J Immunol 2014; 192:5490-8; PMID:24813205; http://dx.doi.org/10.4049/jimmunol.1400722
