Abstract
Immune escape is consequential for cancer development. Identifying abnormalities of the immune microenvironment during early carcinogenesis can provide insight into disease pathogenesis and unravel new preventive or therapeutic targets. We recently conducted a comprehensive immune gene expression analysis in endometriosis and endometriosis-associated ovarian cancer and explored new mechanistic roles for the complement pathway.
Introduction
Despite concerted efforts in the last decade, the rate of ovarian cancer mortality has only improved marginally. The majority of patients continue to present with late-stage disease, typically associated with low 5-year survival. The lack of effective screening procedures for early detection is partly due to the highly diverse pathology of ovarian epithelial tumors. For several types of cancer (cervical, colon, pancreas, breast, and prostate), the premalignant lesions are well defined (human papillomavirus-associated cervicitis, colon polyposis, pancreatitis, breast intraductal neoplasia, and prostate intraepithelial neoplasia, respectively) and prevention and/or early detection of these conditions can be designed and implemented.Citation1 Consequently, it is estimated that the screening and treatment of the above precursors prevent a significant number of cancers each year, although some approaches (e.g., in cervical cancer) are more effective, whereas others (such as in prostate cancer) still struggle to prove efficacy. Notably absent from the list is a precursor for ovarian cancer, the most lethal gynecologic malignancy.
Several studies in the past decade suggest that endometriosis, a commonly diagnosed, chronic benign inflammatory disease, may predispose to endometrioid, clear-cell, and low-grade serous ovarian cancers, also termed endometriosis-associated ovarian cancer (EAOC).Citation2 Endometriosis consists of endometrial-like tissue (glandular epithelia and stromal cells) ectopically implanted outside the uterine cavity and is usually treated through a combination of hormone therapy and surgical excision of lesions. Endometriosis implants develop in a highly inflammatory milieu and are often recurrent, resistant to treatment, and debilitating. Furthermore, these lesions have attributes that resemble ovarian cancer, including multifocality, genetic instability, increased proliferation, and capacity to invade surrounding tissues. In advanced stages, epithelial cells in endometriotic lesions display signs of “atypia,” defined by histological criteria such as cellular crowding and large nuclei with moderate to marked pleomorphism. Often found adjacent to a tumor, atypical endometriosis (AE) may represent the transitioning entity from benign lesions to malignant variants.Citation3
In response to the invasive and sometimes recurrent endometriotic lesions, the host's immune system often makes ineffective attempts to “repair and renew” that are believed to contribute to the transition from endometriosis to EAOC. Thus, a better understanding of the immune microenvironment in precursor lesions may provide mechanistic insight to EAOC pathogenesis. To date, cellular and molecular characterization of the endometriosis-associated inflammatory milieu has mostly focused on circulating chemokines and peritoneal immune cells (macrophages, natural killer [NK] cells).Citation4 We recently reported results from the first comprehensive immune gene expression analysis of pelvic inflammation in benign endometriosis, AE, and EAOC.Citation5 Our 120-case cohort revealed gene expression changes associated with each disease state. Benign endometriosis cases displayed a mixed immune gene profile, with one third of the patients showing immune responses more similar to cancer. However, when analyzing the AE cases, we observed that the majority (85%) of patients had a cancer-like immune environment further supporting AE as a tumor precursor lesion (Fig. 1).
Figure 1. Approach to profiling immune dysregulation in endometriosis and ovarian cancer. Endometrioid, clear cell and low grade serous ovarian tumors may develop from endometriosis, a chronic benign inflammatory condition consisting of endometrial-like epithelial glands (ovals) surrounded by immune stroma (circles). Atypical endometriosis (defined by morphological criteria) is considered to be the immediate cancer precursor. We recently profiled the immune microenvironment in normal endometrium, benign and atypical endometriosis and endometriosis-associated ovarian cancer (EAOC). Our results show that (1) while some of the atypical endometriosis cases have signs of benign inflammation (blue arrow), (2) 85% of cases have a cancerlike immune gene signature (red arrow). (3) Pathway analyses revealed complement activation and humoral immunity in endometriosis and EAOC. In parallel, mechanistic studies in ovarian cells from genetically engineered mice show that complement upregulation in epithelial cells does not trigger antibody-induced and complement-mediated cytotoxicity. Moreover, downregulation of complement inhibits tumor cell proliferation.
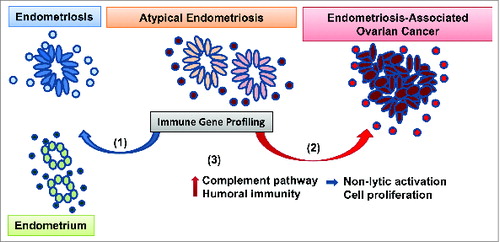
Using differentially expressed genes in all disease categories, we identified complement as the most significantly dysregulated immune pathway in endometriosis and EAOC. Notably, the complement was not among the top pathways altered in serous ovarian cancer, suggesting that complement involvement may be specific to endometriosis and EAOC. We also identified activation of humoral immunity, suggesting antibody-dependent, complement-mediated immunity as a potential mechanism of epithelium–immune stoma interaction. To further explore this mechanism, we employed triple transgenic mice that progress to EAOC closely mirroring the human disease.Citation6,7 Using this preclinical model, we demonstrated that complement upregulation in epithelial cells occurs early in carcinogenesis. However, exposure of complement-expressing ovarian tumor epithelial cells to in vivo-generated antibodies that can recognize and bind to the cell-surface mucin 1 (MUC1) tumor antigen does not trigger antibody-induced, complement-mediated cell death, demonstrating that, at least in this system, complement engagement is a rather ineffective tumor lytic mechanism. Furthermore, inhibition of 1 of the effector complement genes (C7) triggered suppression rather than stimulation of tumor cell proliferation, generating the hypothesis that complement activation, despite antibody presence, may bolster tumor growth (Fig. 1). This hypothesis has been supported by a series of recent studies that demonstrate a tumor-supporting role for complement, via several mechanisms that coexist in the tumor environment: directly, by stimulating tumor cell proliferation, or indirectly, by stimulating immune suppression and neovascularization.Citation8,9
Currently, there are no standardized screening methods for endometriosis or early ovarian carcinogenesis. Serum CA125 continues to be the mostly widely used protein biomarker, despite its limited specificity and sensitivity. Large studies on transvaginal sonography have failed to provide support for this approach. As a concerted effort to identify molecular abnormalities during the transition from endometriosis to EAOC, we have profiled microRNA (miRNA) expression in tissue (manuscript in preparation) and in patients’ plasma,Citation10 and determined that plasma miRNA expression signatures could be used as biomarkers for endometriosis-to-EAOC progression.Citation10 The immune gene expression profileCitation5 further demonstrates that cancer-like signatures may develop early in patients with precursor lesions. Given that immune effectors can distinctly sense early molecular changes of cells undergoing transformation, it is conceivable that profiling the immune response in tissue may provide molecular clues for patients with EAOC risk and new targets for preventive or therapeutic vaccines. We hope our hypothesis will stimulate further studies, in larger cohorts, that can validate (or disprove) the clinical applicability of immune profiles as biomarkers and of the complement pathway as a potential therapeutic target.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were declared.
References
- Sankaranarayanan R. Screening for cancer in low- and middle-income countries. Ann Glob Health 2014; 80:412–7; PMID:25512156; http://dx.doi.org/10.1016/j.aogh.2014.09.014
- Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund KG, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol 2012; 13:385–94; PMID:22361336; http://dx.doi.org/10.1016/S1470-2045(11)70404-1
- Fukunaga M, Nomura K, Ishikawa E, Ushigome S. Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology 1997; 30:249–55; PMID:9088954; http://dx.doi.org/10.1046/j.1365-2559.1997.d01-592.x
- Funamizu A, Fukui A, Kamoi M, Fuchinoue K, Yokota M, Fukuhara R, Mizunuma H. Expression of natural cytotoxicity receptors on peritoneal fluid natural killer cell and cytokine production by peritoneal fluid natural killer cell in women with endometriosis. Am J Reprod Immunol 2014; 71:359–67; PMID:24495049; http://dx.doi.org/10.1111/aji.12206
- Suryawanshi S, Huang X, Elishaev E, Budiu RA, Zhang L, Kim S, Donnellan N, Mantia-Smaldone G, Ma T, Tseng G, et al. Complement pathway is frequently altered in endometriosis and endometriosis-associated ovarian cancer. Clin Cancer Res 2014; 20:6163–74; PMID:25294912; http://dx.doi.org/10.1158/1078-0432.CCR-14-1338
- Budiu RA, Elishaev E, Brozick J, Lee M, Edwards RP, Kalinski P, Vlad AM. Immunobiology of human mucin 1 in a preclinical ovarian tumor model. Oncogene 2013; 32:3664–75; PMID:22964632; http://dx.doi.org/10.1038/onc.2012.397
- Budiu RA, Diaconu I, Chrissluis R, Dricu A, Edwards RP, Vlad AM. A conditional mouse model for human MUC1-positive endometriosis shows the presence of anti-MUC1 antibodies and Foxp3+ regulatory T cells. Dis Model Mech 2009; 2:593–603; PMID:19841240; http://dx.doi.org/10.1242/dmm.002535
- Pio R, Ajona D, Lambris JD. Complement inhibition in cancer therapy. Semin Immunol 2013; 25:54–64; PMID:23706991; http://dx.doi.org/10.1016/j.smim.2013.04.001
- Rutkowski MJ, Sughrue ME, Kane AJ, Ahn BJ, Fang S, Parsa AT. The complement cascade as a mediator of tissue growth and regeneration. Inflamm Res 2010; 59:897–905; PMID:20517706; http://dx.doi.org/10.1007/s00011-010-0220-6
- Suryawanshi S, Vlad AM, Lin HM, Mantia-Smaldone G, Laskey R, Lee M, Lin Y, Donnellan N, Klein-Patel M, Lee T, et al. Plasma microRNAs as novel biomarkers for endometriosis and endometriosis-associated ovarian cancer. Clin Cancer Res 2013; 19:1213–24; PMID:23362326; http://dx.doi.org/10.1158/1078-0432.CCR-12-2726
