Abstract
We have developed a novel approach in cancer immunotherapy, the personalized peptide vaccination (PPV), in which human leukocyte antigen (HLA)-matched peptides are selected on the basis of preexisting host immunity before vaccination. Recently, we demonstrated the feasibility of PPV in previously treated patients with advanced colorectal cancer, thus warranting further clinical development of this approach.
Introduction
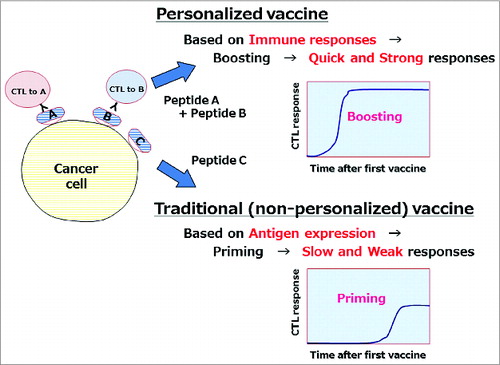
Recent advances in chemotherapy and/or targeted therapy have improved clinical outcomes in patients with advanced colorectal cancer (aCRC); however, the prognosis remains poor. The development of new therapeutic approaches, including immunotherapy, is thus urgently needed. However, limited numbers of clinical trials of immunotherapies have been reported for patients with aCRC. We have developed a novel approach of cancer immunotherapy, the personalized peptide vaccination (PPV), in which human leukocyte antigen (HLA)-matched peptides are individually selected from a panel of 31 cytotoxic T lymphocyte (CTL) epitope peptides derived from 15 tumor-associated antigens.Citation1,2 The most unique aspect of PPV is the personalized selection of ideal antigen peptides for individual patients on the basis of both HLA-class I types and preexisting immune responses to peptide vaccine candidates before vaccination.Citation1,2 In view of the heterogeneity of tumors and the complexity and diversity of immune responses, this approach might be more rational than the selection of non-personalized universal tumor antigens (). As tumor tissues are unavailable and difficult to precisely characterize in most of advanced patients, we selected and administered multiple (up to 4) antigens to increase the possibility that the antigens used for vaccination are expressed in tumor cells.
Figure 1. Advantage of personalized peptide vaccine. Personalized vaccine antigens selected on the basis of preexisting host immunity might be better than non-personalized antigens because they can induce quicker and stronger immune responses.
Early-phase clinical trials of PPV showed the feasibility of this new approach in patients with various types of cancers.Citation1-4 Recently, we conducted a phase 2 study to examine the feasibility of PPV in previously treated patients with aCRC who had failed at least 1 regimen of standard chemotherapies and/or targeted therapies.Citation5 Two to 4 HLA-matched peptides were individually selected from a pool of peptide vaccine candidates and administered subcutaneously without severe adverse events, as described previously.Citation1-4 The median overall survival (OS) time from the first vaccination was 498 d (95% confidence interval [CI], 233–654 days) with 1- and 2-year survival rates of 53% and 22%, respectively. Notably, patients, who had a treatment history of 2 or more regimens of standard chemotherapy and were refractory or intolerant to irinotecan, oxaliplatin, and fluoropyrimidines prior to enrollment showed median OS of 375 d (95% CI, 191–561 days) from the first vaccination, suggesting a potential survival benefit of PPV in previously treated patients with aCRC, even in the refractory stage. Boosting of CTL responses specific to the administered peptides was observed in 63% of patients who completed the first cycle of 6 vaccinations. Importantly, increased peptide-specific CTL responses after vaccination were significantly predictive of favorable OS independently of other factors, suggesting a causal relationship between the biological and clinical efficacies of PPV.
Several post-vaccination biomarkers, such as immune (CTL and/or immunoglobulin G [IgG]) responses to the vaccine antigens, delayed-type hypersensitivity, and autoimmunity, have been reported to be associated with clinical responses in cancer immunotherapies;Citation1,2,6,7 however, there are currently no validated pre-vaccination predictive biomarkers in widespread use. Not all patients showed clinical benefits from PPV; therefore, we tried to identify prognostic or predictive biomarkers in patients with aCRC who were treated with PPV.Citation5 By the Cox proportional hazards model, higher interleukin (IL)-6 and interferon gamma-inducible protein-10 (IP-10) and lower B-cell activating factor (BAFF) levels in pre-vaccination plasma were significantly associated with unfavorable OS (hazard ratio [HR] = 1.508, P = 0.043; HR = 1.579, P = 0.024; HR = 0.509, P = 0.002; respectively), although these factors might be not necessarily be predictive and unique to PPV. Notably, however, the pre-vaccination IP-10 level was predictive of the increase in CTL responses (odds ratio, 0.427; P = 0.039), which was associated with improved OS after vaccination, suggesting that IP-10 might potentially be useful for selecting patients with aCRC who would benefit from PPV. To more clearly assess the causal relation of IP-10, CTL responses, and OS as well as to elucidate prognostic versus the predictive relevance of such biomarkers, future randomized, controlled clinical trials with or without PPV would be required.
We have demonstrated that IL-6 might be useful for predicting OS in PPV-treated patients with various types of cancers including aCRC.Citation5,8,9 As IL-6 has recently been reported to induce suppressive immune cell subsets, such as myeloid-derived suppressor cells and Th17, high levels of IL-6 may inhibit immune responses to cancer vaccines by inducing these suppressive cells. Based on these findings, an early-phase clinical trial is underway to examine whether inhibition of IL–6-mediated inflammatory signaling with a humanized anti–IL-6 receptor monoclonal antibody, tocilizumab, would be beneficial for enhancing the immune and/or clinical responses after PPV in patients with aCRC who show higher plasma IL-6 levels. Interestingly, we demonstrated that the IL-6R 48892A>C polymorphism might have a significant effect on OS in patients with aCRC after PPV: the patients bearing the IL-6R 48892C/C or 48892A/C genotypes tended to have a better prognosis than those carrying the IL-6R 48892A/A genotype.Citation5 As the IL-6R 48892A>C polymorphism has been reported to show no effects on prognosis in patients with some cancers, such as esophageal squamous cell carcinoma and neuroblastoma, without cancer vaccines, the prognostic significance of this polymorphism might be unique to vaccinated patients.
In summary, our recently conducted phase 2 trial demonstrated that PPV induced substantial immune responses to vaccine antigens without severe adverse events and showed a potential clinical benefit in previously treated patients with aCRC even in the refractory stage.Citation5 Nevertheless, because it was a small study with a limited number of patients, some of whom received combined chemotherapies and/or targeted therapies during the vaccination period, the clinical efficacy of PPV, as well as the clinical utility of the identified biomarkers, in patients with aCRC remain to be confirmed in future, larger scale, randomized trials of PPV without combined chemotherapies or targeted therapies.
Disclosure of Potential Conflicts of Interest
Kyogo Itoh owns stock in the Green Peptide Co., Ltd., and has received research funding from Taiho Pharmaceutical Co., Ltd. No potential conflicts of interests were declared by the other authors.
Funding
This study was supported by funding to a research program of the Project for Development of Innovative Research on Cancer Therapeutics (P-Direct) of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Noguchi M, Sasada T, Itoh K. Personalized peptide vaccination: a new approach for advanced cancer as therapeutic cancer vaccine. Cancer Immunol Immunother 2013; 62:919-29; PMID:23197273; http://dx.doi.org/10.1007/s00262-012-1379-1
- Sasada T, Yamada A, Noguchi M, Itoh K. Personalized peptide vaccine for treatment of advanced cancer. Curr Med Chem 2014; 21:2332-45; PMID:24524766; http://dx.doi.org/10.2174/0929867321666140205132936
- Terasaki M, Shibui S, Narita Y, Fujimaki T, Aoki T, Kajiwara K, Sawamura Y, Kurisu K, Mineta T, Yamada A, et al. Phase I trial of a personalized peptide vaccine for patients positive for human leukocyte antigen–A24 with recurrent or progressive glioblastoma multiforme. J Clin Oncol 2011; 29:337-44; PMID:21149665; http://dx.doi.org/10.1200/JCO.2010.29.7499
- Noguchi M, Kakuma T, Uemura H, Nasu Y, Kumon H, Hirao Y, Moriya F, Suekane S, Matsuoka K, Komatsu N, et al. A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol Immunother 2010; 59:1001-9; PMID:20146063; http://dx.doi.org/10.1007/s00262-010-0822-4
- Kibe S, Yutani S, Motoyama S, Nomura T, Tanaka N, Kawahara A, Yamaguchi T, Matsueda S, Komatsu N, Miura M, et al. Phase II study of personalized peptide vaccination for previously treated advanced colorectal cancer. Cancer Immunol Res 2014; 2:1154-62; PMID:25351849; http://dx.doi.org/10.1158/2326-6066.CIR-14-0035
- Disis ML. Immunologic biomarkers as correlates of clinical response to cancer immunotherapy. Cancer Immunol Immunother 2011; 60:433-42; PMID:21221967; http://dx.doi.org/10.1007/s00262-010-0960-8
- Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, Humphrey R, Blumenstein B, Old L, Wolchok J. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 2010; 102:1388-97; PMID:20826737; http://dx.doi.org/10.1093/jnci/djq310
- Komatsu N, Matsueda S, Tashiro K, Ioji T, Shichijo S, Noguchi M, Yamada A, Doi A, Suekane S, Moriya F, et al. Gene expression profiles in peripheral blood as a biomarker in cancer patients receiving peptide vaccination. Cancer 2012; 118:3208-21; PMID:22071976; http://dx.doi.org/10.1002/cncr.26636
- Yoshitomi M, Yutani S, Matsueda S, Ioji T, Komatsu N, Shichijo S, Yamada A, Itoh K, Sasada T, Kinoshita H. Personalized peptide vaccination for advanced biliary tract cancer: IL-6, nutritional status and pre-existing antigen-specific immunity as possible biomarkers for patient prognosis. Exp Ther Med 2012; 3:463-9; PMID:22969912
