Abstract
The progression of genital human papillomavirus (HPV) infections into preneoplastic lesions suggests that infected/malignant cells are not adequately recognized by the immune system. In this study, we demonstrated that cervical/vulvar cancer cells secrete factor(s) that affect both the maturation and function of dendritic cells (DC) leading to a tolerogenic profile. Indeed, DC cocultured with cancer cell lines display both a partially mature phenotype after lipopolysaccharide (LPS) maturation and an altered secretory profile (IL-10high and IL-12p70low). In addition, tumor-converted DC acquire the ability to alter T-cell proliferation and to induce FoxP3+ suppressive T cells from naive CD4+ T cells. Among the immunosuppressive factors implicated in DC alterations in genital (pre)neoplastic microenvironment, we identified receptor activator of nuclear factor kappa-B ligand (RANKL), a TNF family member, as a potential candidate. For the first time, we showed that RANKL expression strongly increases during cervical progression. We also confirmed that RANKL is directly secreted by cancer cells and this expression is not related to HPV viral oncoprotein induction. Interestingly, the addition of osteoprotegerin (OPG) in coculture experiments reduces significantly the inhibition of DC maturation, the release of a tolerogenic cytokine profile (IL-12low IL-10high) and the induction of regulatory T (Treg) cells. Our findings suggest that the use of inhibitory molecules directed against RANKL in cervical/vulvar (pre)neoplastic lesions might prevent alterations of DC functionality and represent an attractive strategy to overcome immune tolerance in such cancers.
Abbreviations:
- APC, antigen presenting cells; DC, dendritic cells
- GILZ, glucocorticoid-induced leucine zipper; HPV, human papillomavirus
- HSIL, high grade intraepithelial lesions
- IHC, immunohistochemistry
- ILT3, Immunoglobulin-like transcript 3
- KN, normal keratinocytes
- LC, Langerhans cells; LPS, lipopolysaccharide
- LSIL, low grade intraepithelial lesion
- MFI, mean fluorescence intensity
- OPG, osteoprotegerin
- PBMC, peripheral blood mononuclear cells; pDC, plasmacytoid dendritic cells
- RANKL, Receptor activator of nuclear factor kappa-B ligand
- SCC, squamous cell carcinoma
- SIL, squamous intraepithelial neoplasia
- Treg cells, regulatory T cells
- VIN, vulvar intraepithelial neoplasia
Introduction
HPV infection has been found to be associated with the majority of cervical and anal cancers. Half of vulvar and penile neoplasms are also infected by one or several HPV genotypes. Development of HPV-related cancers corresponds to a multistep process preceded by well-defined changes in the epithelium known as squamous intraepithelial lesions (SIL). Although HPV oncoproteins play a key role in carcinogenesis, HPV alone is not sufficient for cancer development. Host immune response appears to be critical in determining the outcome of infection as shown by the high prevalence, persistence and recurrence of HPV infections in immunodeficient women.Citation1
In the last decade, accumulating data demonstrated that the progression of HPV infections into (pre)neoplastic lesions is associated with altered local immune responses and immunotolerance. Indeed, (pre)neoplastic cells create a tolerant microenvironment by activating a plethora of immunosuppressive mechanisms, including the modulation of antigen-presenting cells (APC), upregulation of immunosuppressive factors and accumulation of Treg cells.Citation2 Furthermore, SIL exhibit a high expression of type II cytokines, a downregulation of IFNγ and a reduced density and function of Langerhans cells (LC).Citation3,4 The activation and maturation of these latter cells depend on the local microenvironment and may be altered or polarized by different factors. In the cancer setting, tumor cells have been reported to produce factors such as IL-10, VEGF, TGF-β, IL-6, prostanoids (PGE2) and M-CSF which inhibit or suppress both the differentiation and activation of DC.Citation5 Cancers developing in the anogenital tract are also known to secrete immunosuppressive factors, but little is known about their effect on DC function.
RANKL is a cytokine belonging to the TNF family that is expressed on the surface of numerous cell types.Citation6 At least three forms of RANKL exist and their expression is controlled by numerous cytokines and hormones, commonly known as regulators of the immune system and calcium homeostasis. All variants can remain on the cell surface or can be proteolytically cleaved into biologically active soluble forms. RANKL has been shown to regulate many physiological processes such as bone remodeling. In the cancer microenvironment, the importance of RANKL and its decoy receptor OPG has been highlighted in many reports demonstrating its role in regulating tumor cell metastasis to bone.Citation7 Indeed, an aberrant production of RANKL by tumor cells was reported to induce unregulated osteoclast-mediated bone destruction and an increased tumor burden.Citation8 By inducing proliferative changes, recent studies demonstrated a crucial function of RANKL in breast cancer.Citation9
The present study was designed to assess the effects of molecules secreted by cervical cancer cells on DC maturation and function. Our data showed that supernatants from tumor epithelial cells significantly inhibit LPS-induced DC maturation. An upregulation of IL-10 associated with a decreased IL-12p70 secretion in response to LPS was also demonstrated. Furthermore, DC exposed to genital cancer microenvironment had a limited capacity to induce an allogeneic T-cell response while promoting T-cell regulatory activity. Interestingly, RANKL was overexpressed in SIL and squamous cell carcinoma (SCC) and led to the tolerogenic function of DC. Indeed, significant amounts of RANKL were detected in the supernatants of tumor cells and OPG suppressed its tolerogenic effect. Moreover, the tolerogenic function of DC was shown to be mediated by ILT3 expression. Taken together, our data suggest that neutralization of RANKL might be an attractive approach to restore tumor-associated DC functions in cancer.
Results
DC differentiated in the presence of genital SCC cell lines display a semi-mature phenotype and a defective functional activity
DC were cultured either alone (control DC), with normal keratinocytes (KN) or genital SCC cell lines for 6 d and then stimulated for maturation by LPS during 24 h. We found that DC cultured in the presence of SCC cell lines presented a semi-mature phenotype determined by monitoring the expression of surface markers CD80, CD83, CD86, HLA-DR, and CCR7. Indeed, in terms of percentages of cells expressing these markers () and mean fluorescence intensity (MFI) (Fig. S1), we observed that all DC maturation markers studied (CD80, CD83, CD86 and HLA-DR) were significantly reduced after exposure to SCC cell lines when compared to control DC. Interestingly, expression of CCR7, the lymph node homing receptor, was significantly increased on DC when they were cocultured with genital SCC cell lines. However, these latter results were only observed when MFI was calculated. Except for the costimulatory marker CD80, the percentages of DC expressing the maturation markers CD83, CD86, HLA-DR and CCR7 were not statistically different when DC were cocultured with KN or alone (control DC), showing that KN were not able to affect DC phenotype. For this reason, DC cultured alone were used as a control (corresponding to 100% of maturation marker expression) for the experiments with DC cocultured with genital SCC cell lines.
Figure 1. DC cultured in a cervical/vulvar tumor microenvironment display a semi-mature phenotype and functional defects. (A) DC were either cultured alone (control DC) or in the presence of normal (KN) or malignant cells (CaSki, SiHa, C4II and A431) in a Transwell Chamber assay. At day 6, epithelial cells were removed and DC were stimulated with LPS for 24 h. DC phenotype was then assessed by flow cytometry (maturation markers: CD80, CD83, CD86, HLA-DR, and CCR7). Data were normalized to control DC (= 100%). Data are from 21 different experiments and mean values are shown as percentages of positive cells ± standard deviation. Statistical differences were determined by performing the one-way ANOVA test between control DC or KN and the other cell culture conditions (CaSki, SiHa, C4II, A431) (*P < 0.05; **P < 0.01; ***P < 0.001, ns: not significant). (B) DC differentiated in the presence of SCC cell lines produce high levels of IL-10 and low amount of IL-12. Data are from 20 different experiments and mean values are shown as cytokine concentration (pg/mL) ± standard deviation (**P < 0.01; ***P < 0.001). (C) DC cocultured with SCC cell lines inhibit T-cell proliferation and induce suppressive T cells in MLR assays. MLR of DC cocultured with genital CaSki cell line, KN or alone (Control) and then cultured with allogeneic T lymphocytes for 7 d. Responder cells: lymphocytes T; effector cells: DC. Data represent mean ± standard deviation of 3H-Tdr incorporation (**P < 0.01; ***P < 0.001). (D) Suppressor activity of T cells originally primed by DC cocultured with CaSki, SiHa, C4II, KN or alone (control DC). Allogeneic CD4+ T cells were purified and cocultured during 7 d with irradiated control DC or DC cocultured with CaSki, SiHa, C4II or KN. Cell mixture was than mixed with freshly isolated T cells and with other allogeneic DC. 3H-Tdr incorporation was measured after 5 d of culture. Data represent mean ± standard deviation of 3H-Tdr incorporation from six experiments (*P < 0.05; **P < 0.01; ***P < 0.001).
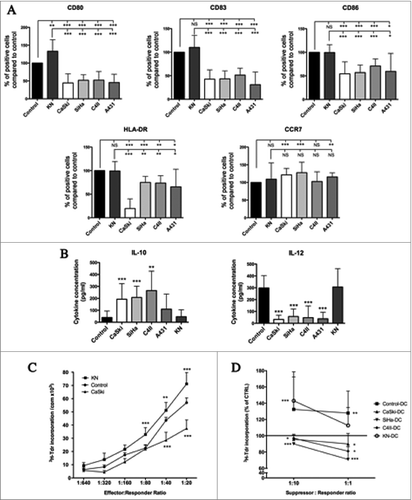
It is well known that IL-12 plays a significant role in the activation of the Th1 immune response and, at the same time, that IL-10 action results in a negative regulation of this process. Here, we showed that DC cocultured with the CaSki, SiHa and C4II cell lines secrete significantly more IL-10 than control DC (). No significant increase was observed with A431 cells. In contrast to control DC, DC cocultured with the genital cell lines CaSki, SiHa, C4II and A431 presented a reduced ability to produce IL-12p70 in response to LPS (). Therefore, the IL-10/IL-12 ratio was significantly higher in DC cocultured with genital SCC cell lines compared with control DC.
In order to determine if the downregulation of costimulatory molecules observed at the surface of DC exposed to genital SCC cell lines is able to influence DC stimulation of T-cell response, a mixed lymphocyte reaction (MLR) assay was performed. We showed that, compared to control DC, DC cocultured with genital SCC cell lines, present a decreased ability to stimulate the proliferation of allogeneic T lymphocytes, especially when the effector-to-responder ratio was the highest (1:40, 1:20) ( and Fig. S2). This result suggests that DC exposed to genital cancer microenvironment do not have the capacity to provide accessory signals for an efficient proliferation of allogeneic T lymphocytes.
As DC showing an IL-10high IL-12low phenotype are well-known to promote Treg cells differentiation, we performed a T-cell suppressor assay to determine if DC exposed to the genital cancer microenvironment in coculture assays have a similar property. Allogeneic T cells were cultured with control DC or with DC previously cocultured with keratinocytes (SiHa, CasKi, C4II or KN) for 7 d (= MLR1) and then, in order to determine whether some of these T cells exhibit a regulatory function, they were cocultured with allogeneic DC and fresh T cells for 5 d (= MLR2). As expected, T cells stimulated by control DC or KN-DC did not present a suppressive function, as they did not inhibit T-cell proliferation in the MLR2. In contrast, DC exposed to genital SCC cell lines (CasKi, SiHa and C4II) induced suppressive T cells. Indeed, we observed, in the MRL2 assay, a significantly reduced proliferation of T cells when compared to the internal control corresponding to T cells only stimulated by DC but not exposed to the MLR1 cell mixture and arbitrary determined as 100% of proliferation ().
Genital carcinoma cells express RANKL in vitro and in situ
We then investigated the molecular mechanisms potentially involved in the acquisition of the DC immunosuppressive functionality observed after exposure to genital SCC cell lines. By performing immunohistochemistry (IHC), we observed that RANKL is expressed in the uterine exocervical epithelium but also in the metaplastic squamous epithelium lining the transformation zone and in (pre)neoplastic lesions. Normal epithelial tissues (both exocervical and metaplastic) displayed a weak RANKL immunoreactivity (). In contrast, preneoplastic lesions (low-grade squamous intraepithelial lesions (LSIL) and high-grade intraepithelial lesions (HSIL)) and SCC were found to be strongly positive for this cytokine with a diffuse cytoplasmic staining ().
Figure 2. Squamous carcinoma cells express RANKL in vitro and in situ. (A) RANKL expression in cervical biopsy specimens. (a) Normal exocervix, (b) squamous epithelial metaplasia, (c) low-grade squamous intraepithelial lesions, (d) high-grade squamous intraepithelial lesions, (e) cervical SCC. Original magnification: X100. The RANKL immunoreactivity is observed in the epithelial compartment. (f) Semi-quantitative evaluation of RANKL expression in normal exocervix (n = 22), epithelial metaplasia (n = 9), LSIL (n = 18), HSIL (n = 27) and SCC (n = 12). Asterisks indicate statistically significant differences (*P < 0.05; ***P < 0.001). (B) RANKL secretion by SCC cell lines (SiHa, CaSki, A431, C4II) and normal keratinocytes (KN) was determined by an ELISA assay. Data represent mean ± standard deviation of RANKL concentration (pg/mL/106 cells) from five independent experiments (*P < 0.05; **P < 0.01). (C) RANKL mRNA expression by SCC cell lines (CaSki, SiHa, C4II, A431) and KN was determined by classical PCR. Densitometric analysis (ratio) shows that RANKL mRNA level is higher in SCC cell lines compared to KN.
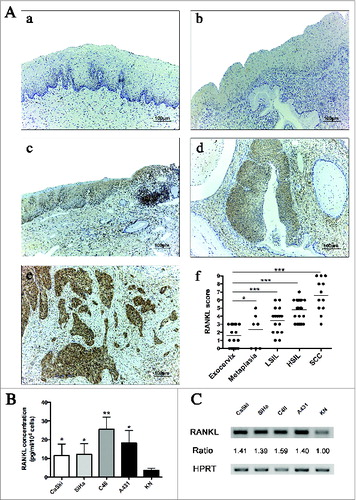
Compared to normal cervical tissues, a semi-quantitative evaluation of RANKL intraepithelial expression indicated that preneoplastic lesions and SCC are significantly associated with an upregulation of this cytokine. Moreover, RANKL expression increased progressively during cervical carcinogenesis, suggesting that it might be involved in the progression of SCC ().
Furthermore, RANKL secretion was also found in supernatants of the genital cell lines used in the coculture assays. By performing an ELISA assay, we showed that SiHa, CaSki, A431 and C4II cell lines secrete significantly higher levels of RANKL compared to both KN () and other non-epithelial cell lines (Fig. S3). This difference in RANKL secretion could be related to the transcription rate observed in each cell. Indeed, an increased level of RANKL transcripts was observed in cancer cells compared to KN. ().
RANKL is able to modify the phenotype and the cytokine secretion profile of DC, an effect reversed by OPG treatment
To determine whether RANKL is able to inhibit DC maturation and modify their functionality, DC were incubated in the presence of human recombinant RANKL (0.1 or 0.5 μg/mL). Similar to DC cocultured with the genital SCC cell lines, DC incubated in the presence of RANKL (0.1 and 0.5 μg/mL) also showed a semi-mature phenotype. Indeed, we observed that the percentage of DC positive for the different costimulatory molecules studied (CD80, CD83, CD86 and HLA-DR) was significantly reduced when these cells were cultured with 0.1 or 0.5 μg/mL of RANKL compared to control DC. The percentage of DC expressing CCR7 was also significantly increased after DC incubation with RANKL (). Furthermore, DC incubated with recombinant RANKL exhibited a reduced ability to produce IL-12p70 and showed an increased expression of IL-10 in response to LPS ().
Figure 3. RANKL induces a semi-mature phenotype in DC and modifies their IL-10/IL-12 secretion. (A) DC were cultured in the presence of human recombinant RANKL (0.1 or 0.5 μg/mL). At day 6, DC were stimulated by LPS for 24 h. DC phenotype was then assessed by flow cytometry (maturation markers: CD80, CD83, CD86, HLA-DR, and CCR7). Data were normalized to control DC (= 100%). Data are from six different experiments and mean values are shown as percentages of positive cells ± standard deviation (*P < 0.05; **P < 0.01; ***P < 0.001). (B) IL-10 and IL-12 production by both control DC and DC cultured with RANKL. Data are from 11 different experiments and mean values are shown as cytokine concentration (pg/mL) ± standard deviation (***P < 0.001).
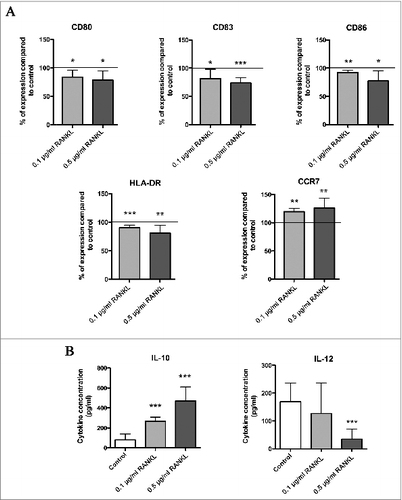
These findings indicate that the inhibition of DC maturation and the release of a tolerogenic cytokine profile (IL-12low IL-10high) induced by genital SCC cell lines may be mediated by RANKL.
Next we tested whether the inhibition of RANKL by OPG could restore DC phenotype and IL-10/IL-12 secretion after LPS stimulation. We compared the percentage of DC expressing the maturation markers (CD80, CD83, CD86, HLA-DR and CCR7) after their exposure to 0.5 μg/mL RANKL alone or in combination with OPG, its inhibiting receptor. We showed that treatment of DC with RANKL in the presence of OPG no longer affected DC maturation. OPG statistically inhibited RANKL effect on DC maturation, leading to a percentage of cells positive for the different maturation markers similar to that observed in the absence of RANKL. We also confirmed that OPG treatment had no effect on DC maturation status (). Furthermore, RANKL inhibition by OPG restored IL-10 and IL-12 secretion ().
Figure 4. OPG inhibits RANKL effect on DC. (A) OPG restores the phenotype of DC cultured in the presence of RANKL. DC were cultured either alone or in the presence of OPG alone, human recombinant RANKL (0.5 μg/mL) or RANKL and OPG. At day 6, DC were stimulated by LPS for 24 h. The expression of DC maturation markers (CD80, CD83, CD86, HLA-DR, and CCR7) was assessed by flow cytometry. Data were normalized to control DC (= 100%). Data are from five different experiments and mean values are shown as percentages of positive cells ± standard deviation (*P < 0.05). (B) IL-10 and IL-12 production by control DC and DC cultured with RANKL (0.5 μg/mL) or with RANKL and OPG. Data are from eight different experiments and mean values are shown as cytokine concentration (pg/mL) ± standard deviation (*P < 0.05). ns: not significant; OPG: osteoprotegerin.
Blockade of the RANK/RANKL signaling pathway partially abolishes the alterations of DC induced by genital SCC cell lines and restores their functionality
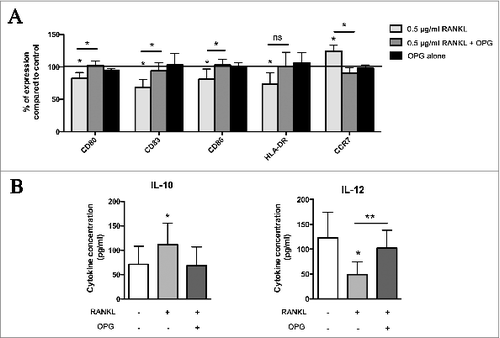
To further assess the contribution of RANKL to DC functional impairment in the presence of genital SCC cell lines, cocultures were treated with the RANKL antagonist, OPG. illustrates that the neutralizing receptor for RANKL reverses the effects of genital SCC cell lines on costimulatory molecules and CCR7 expression. Indeed, inhibition of RANKL increased significantly the percentage of DC positive for CD80, CD83, CD86 and HLA-DR compared to cocultures in the absence of OPG. The expression of the different analyzed molecules was similar to that observed in control DC (). Moreover, cytokine secretions were modified in the presence of RANKL inhibitor. Indeed, addition of OPG in the coculture experiments resulted in significantly higher levels of IL-12 p70 production by DC compared with DC exposed to cell lines alone. In contrast, IL-10 levels showed the opposite pattern. DC cultured in the presence of OPG and genital SCC cell lines produced significantly lower levels of RANKL than DC cultured in the exclusive presence of SCC cell lines. Interestingly, OPG had no significant effect on the cytokine production by control DC ().
Figure 5. RANKL inhibition is sufficient to reverse the tolerogenic profile of DC. (A) Analysis of DC phenotype after coculture in the presence of SCC cell lines treated or not with human OPG. After 6 d of coculture and 24 h of incubation with LPS, the expression of CD80, CD83, CD86, HLA-DR and CCR7 was assessed by flow cytometry. Data are from seven independent experiments and were normalized to control DC (= 100%). Mean values are shown as percentages of positive cells ± standard deviation (*P < 0.05, **P < 0.01). (B) Secretion levels of IL-10 and IL-12p70 in supernatant of DC cocultured with cancer cell lines in the presence or not of OPG. The secretion levels were measured by ELISA after 6 d of coculture and 24 h of incubation with LPS. DC cultured alone or with OPG were used as controls. Data are presented as means ± standard deviation of six independent experiments (*P < 0.05; **P < 0.01). (C) FACS analysis of ILT3 expression on DC cocultured with SCC cell lines in the presence or not of OPG. Data were normalized to control DC (= 100%). Data are from five different experiments and mean values are shown as percentages of positive cells ± standard deviation (*P <0.05; **P < 0.01). (D) DC cocultured in the presence of OPG induced a lower Treg cell differentiation in vitro. Real-time RT-PCR analysis of FoxP3 expression in allogeneic CD4+ T cells cultured with DC isolated from previously described coculture experiments. DC cultured alone were used as control. Data represent mean ± standard deviation of four independent experiments (*P < 0.05; **P < 0.01).
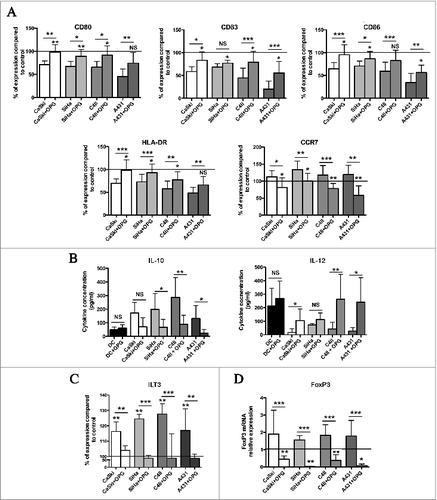
In order to determine the mechanism by which genital SCC cell lines induce tolerogenic DC, we analyzed expression of inhibitory receptors known to be implicated in the suppressive function of DC. Interestingly, whereas we observed no increase in the expression of ICOSL, ILT4, CD273 (PD-L2) or CD274 (PD-L1) on DC cocultured with genital SCC cell lines, FACS analysis indicated a significant increase of ILT3, compared to control DC. Moreover, incubation with OPG during the coculture time led to a significant decrease in the expression of ILT3, restoring the production levels observed in the absence of cell lines ().
By using a Treg cell induction assay, we confirmed that DC cocultured with genital SCC cell lines stimulates the differentiation of naive CD4+ T cells into Treg cells as shown by the expression of Foxp3 transcripts in CD4+ T cells. Similar results were obtained at the protein level (Fig. S8). More interestingly, the decreased expression of ILT3, by the addition of OPG to the cocultures, prevented the induction of Treg cells from CD4+ T cells ().
These results confirm that the genital SCC cell lines alter the DC functionality via the RANKL pathway. Moreover, DC tolerogenic activity is associated to their expression of ILT3 when these cells are exposed to the genital cancer microenvironment.
OPG expression is increased in (pre)neoplastic cervical lesions but remains stable during cervical cancer progression
Recently, expression of OPG has been reported in some cancers.Citation10,11 Analysis of OPG expression by IHC was performed in order to determine if this molecule could be expressed during cervical carcinogenesis to counteract RANKL effects in vivo. We showed that both exocervical and metaplastic squamous epithelia are often associated with a weak OPG immunoreactivity in the epithelium (). A stronger immunostaining was observed in (pre)neoplastic lesions when compared to the normal exocervix but the staining had a similar intensity regardless of the grade of the lesion observed (). Semi-quantitative evaluation of OPG epithelial expression indicated that the immunoreactivity of this molecule is significantly increased in LSIL when compared to the exocervix or metaplasia. Moreover, there was no significant difference in OPG expression between LSIL, HSIL and SCC ().
Figure 6. OPG expression is increased in (pre)neoplastic cervical lesions compared to normal tissues but, remains stable during cervical cancer progression. Representative images of OPG expression in exocervical epithelium (A), metaplasia (B), LSIL (C), HSIL (D) and SCC (E). The OPG immunoreactivity is observed in the epithelial compartment. (F) Semi-quantitative analysis shows a significant increased OPG expression in metaplasia (n = 9), LSIL (n = 8), HSIL (n = 11) and SCC (n = 10) compared to the exocervix (n = 5). Asterisks indicate statistically significant differences (**P < 0.01; ***P < 0.001). Original magnification: X100.
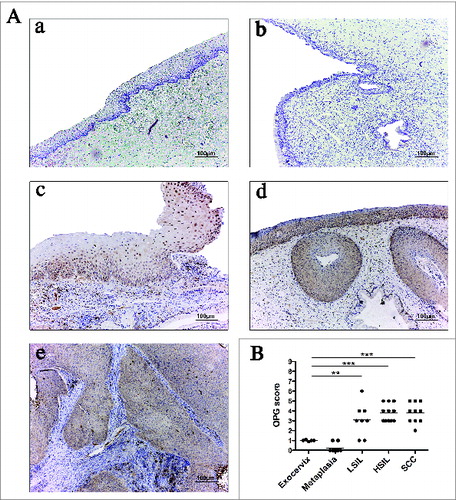
These results demonstrate that OPG expression is increased in preneoplastic lesions when compared to the normal exocervix but, in contrast to RANKL expression, OPG expression remains stable during cervical cancer progression, suggesting that OPG expression in cervical cancer is probably not sufficient to counteract RANKL effects on DC.
Discussion
Infection of the anogenital mucosa by HPV is a very frequently encountered event. It has been proposed that epithelial and/or inflammatory cells could create an immunosuppressive environment and promote malignant transformation that mainly occurs in the squamocolumnar junction by altering antitumor immunity.Citation12 The progression of genital HPV infections into preneoplastic lesions suggests that antigens are not adequately recognized by the innate immunity or presented to the adaptive immune system. Previous studies showed that the tumor microenvironment could commit DC to a tolerogenic function.Citation13 In the present study, we focused our attention on these latter cells and their potential role in the genital carcinogenesis.
We decided to analyze the effect of the tumor microenvironment on DC and not on LC because LC seem to present a certain tolerogenicity by nature. Indeed, they maintain tolerogenic function under a range of conditions that are commonly believed to induce immunogenicity in all DC subsets.Citation14 In addition, the paradigm stating that LC are the exclusive cells that can present skin-acquired antigens to T cells is obsolete. It has been shown that T-cell activation could be mediated by non-epidermal DC subsets.Citation15,16 Moreover, recent data obtained from mouse models suggest that the main epidermal subtype of DC able to cross-present antigens is the langerin+ CD103+ DC,Citation17 a DC subset presenting a dermal origin. Therefore, dermal DC and macrophages recruited to infected epithelium may be key players in the recognition of epithelial antigens and the induction of effector responses.Citation18
In this study, we demonstrated that the microenvironment created by genital cancer cells produce factor(s) that affect the maturation and function of DC leading to APC with a tolerogenic profile. DC cocultured with genital tumor cell lines retain a less mature phenotype after LPS maturation compared with control DC or DC cultured in the presence of normal keratinocytes. Indeed, they express low levels of accessory molecules (CD80, CD83, CD86 and HLA-DR) but retain, in some extent, the ability to upregulate the CCR7 molecule, suggesting that DC exposed to genital cancer microenvironment present an increased susceptibility to migrate to lymph nodes but are not able to stimulate a proper antitumor immune response because of their semi-mature phenotype. However, CCR7 modulation is not a mandatory tolerogenic indicator because DC can be active in the tumor itself.Citation19,20
We also demonstrated that DC cocultured with tumor cell lines show a tolerogenic cytokine profile (IL-10highIL-12p70low). This effect was not observed with normal keratinocytes. It is well-known that IL-12 plays a significant role in the activation of the Th1 immune response and, at the same time, that IL-10 action results in a negative regulation of this process. This shift might facilitate the tumor progression by subverting cellular immune surveillance mechanisms. Moreover, DC displaying this secretion profile have been reported to promote Treg cells.Citation21 In our study, tumor-converted DC acquired the ability to alter T-cell proliferation and to induce FoxP3+ suppressive T cells from naive CD4+ T cells. In agreement with these results, recent data showed that genital (pre)neoplastic lesions are infiltrated by an increased number of FoxP3+ Treg cells compared to surrounding healthy tissue.Citation2,22
Tumor cells can produce several factors that may negatively regulate the maturation and function of DC. Among the immunosuppressive factors potentially responsible for DC alterations in genital (pre)neoplastic microenvironment, RANKL represents an interesting candidate. Several studies showed that the RANK–RANKL interaction has a crucial role in the regulation of the immune system. For example, RANKL has been observed in keratinocytes of inflamed skin and its overexpression has been linked to functional alterations of epidermal DC and a systemic increase of Treg cells.Citation23 For the first time, we showed that RANKL expression increases during the cervical carcinogenesis and seems to play a role in vivo since the production of OPG is a constant feature in the cervical (pre)neoplastic lesions. Therefore, OPG could not prevent the activation of the RANK receptor by the overabundance of RANKL. Interestingly, this increased RANKL-OPG ratio has been also detected in several tumors related to bone or bone metastasis such as breast cancerCitation24 or osteosarcoma,Citation8 causing osteoclast activation and bone destruction. However, in these latter studies, the relationship with the immune system was not analyzed.
In this study, we confirmed that RANKL is directly expressed by tested genital cell lines. However, the origin of the elevated RANKL level in genital (pre)neoplastic lesions is not clear and several hypotheses are plausible. Among the factors that are known to induce overexpression of RANKL, PGE2 could explain our results. The amount of PGE2 increases RANKL mRNA in bone marrow cellsCitation25 and previous results from our laboratory showed that the expression of PGE2 enzymatic pathways is increased in SIL and SCC compared to the normal exocervix.Citation26 Moreover, RANKL expression is induced not only by cytokines (IL-1, IL-6, TNF-α, TGF-β), prostaglandins or UV-irradiationCitation27 but also by sex hormones.Citation28,29 Interestingly, the cervical transformation zone and the squamocolumnar junction may be at increased risk of developing (pre)neoplastic lesions because of a high sensitivity to sex hormone regulation.Citation30 The expression of sex hormone receptors in human cervical biopsies has also been shown to increase with the grade of SIL, strengthening the hormone-dependent establishment of cervical (pre)neoplastic lesions.Citation31 It should further be kept in mind that HPV should not be involved in the increased production of RANKL. Indeed, A431 vulvar HPV−cell line secreted high levels of this molecule and HPV positive or negative VIN display similar RANKL immunoreactivity (Fig. S9).
We then demonstrated that the in vitro treatment of DC with human recombinant RANKL is associated with a reduced expression of costimulatory molecules and the release of a tolerogenic cytokine profile (IL-12low IL-10high), which was reversed after OPG addition. Interestingly, in the same culture conditions, we also observed that RANKL does not affect plasmacytoid dendritic cells (pDC) phenotype, a subset of DC that often presents an immunosuppressive action in the tumor settingCitation32 (Fig. S10). These results suggest that the tolerogenic effect of RANKL could only be effective on DC. Our observations are however in disagreement with studies showing that treatment of DC with RANKL increases their activity by both inducing a partial maturationCitation33 and promoting the secretion of proinflammatory cytokines or IL-12.Citation34 However, the experimental conditions in these studies are radically different from those used in this work. Indeed, the observations were made in vivo in a mouse model using DC generated from bone marrow precursors and infused directly after incubation with RANKL. Moreover, other studies showed that murine DC isolated from mucosal tissues respond to RANKL by upregulating the expression of the suppressive cytokine IL-10, in contrast to DC isolated from peripheral lymphoid tissues or from spleen that respond to RANKL stimulation by increasing their secretion of IL-12.Citation35 This apparent divergence in function of RANKL might be explained by differences in the origin and precursor population of DC or microenvironment influences. Interestingly, DC generated in the latter study expressed CD207 and CD103 that could correspond to a recently identified cell population in the mouse, the dermal langerin+ DC.Citation36 These cells have the ability to capture antigens expressed in the skin and to transport them to the lymph nodes, suggesting a mucosal-like rather than peripheral blood DC function. However, as CD103 has not been validated as a reliable marker for these cells in the human skin,Citation37 we could not use it to state that DC used in our study correspond to these dermal DC.
In order to confirm that RANKL expression during genital carcinogenesis is potentially responsible for the acquisition of an altered tolerogenic phenotype by DC, we added OPG in our coculture experiments. The results indicated that the inhibition of DC maturation, the release of a tolerogenic cytokine profile (IL-12low IL-10high) and the induction of Treg cells by DC committed by genital SCC cell lines could be partially mediated by RANKL. Since the capacity of DC to deliver costimulatory and coinhibitory signals plays a significant role in determining whether responding T cells undergo activation or tolerance, we analyzed the expression of molecules implicated in the tolerogenic function of DC.Citation38 Involved in the negative regulation of adaptive responses,Citation39 PD-1/PD-L1 pathway was first studied. In contrast to a recent study which showed increased expression of PD-L1 to the membrane of DC, without any mechanistic explanation, in parallel with increasing SIL grade,Citation40 we did not detect overexpression of PD-L1 on the DC membrane following cocultures with genital SCC cell lines. However, the involvement of PD-L1 in genital carcinogenesis is a controversial issue. Indeed, another research demonstrated that PD-L1 is expressed in only a minority of cervical cancers and does not influence the survival of patients.Citation41 We neither revealed any modulation of ICOSL on DC in the presence of genital SCC cell lines, which was to our knowledge, not yet been studied.
Interestingly, DC displayed an increased ILT3 expression in the presence of genital SCC. This modulation was reversed by OPG addition, suggesting that RANKL secreted by the cell lines is implicated in this overexpression. The upregulation of ILT3 on DC is known to render them tolerogenic by conferring the ability to differentiate Treg cells.Citation42,43 RANKL, as an activator of NF-KappaB and MAPK, may have, rather than a direct effect on DC maturation and/or cytokine secretion, an indirect effect leading to the activation of immunosuppressive genes involved in the increased expression of ILT3 (e.g. GILZ).Citation44,45 Furthermore, RANKL could also increase cell surface ILT3 expression through STAT3 signaling pathway.Citation46,47 Due to the fact that many soluble factors are present in the tumor microenvironment, it is not surprising that the sole inhibition of RANKL was not sufficient to completely reverse the maturation and function of DC observed after coculture experiment with genital SCC cell lines. These findings suggest that additional secreted epithelial factors are involved in the induction of tolerogenic DC. Other factors such as PGE2, MUC1, TGF-β, VEGF or IL-10 have been shown to play a role in the inhibition of DC maturation.Citation26,48,49 Among these, MUC1 could be another potential candidate due to its high expression in HSIL (unpublished data).
Besides the negative effect of RANKL on the immune system in genital cancers, this cytokine is also secreted by other epithelial cancers such as prostate and breast tumors where it promotes an invasive phenotype by inhibiting the metastasis suppressor MaspinCitation50 or inducing an epithelial-to-mesenchymal transitionCitation51. Recent studies also reported a RANKL expression in “non-solid” tumors such as myelomaCitation52 as well as in gliomaCitation53 suggesting that the secretion of this soluble factor is not restricted to epithelial cancers. However, the secretion rates displayed by non-epithelial cancer cells could be considerably lower than those observed in genital cancer cell lines (Fig. S3).
Altogether these results suggest that RANKL may contribute to the poor prognosis of some cancers, epithelial or not, by modulating not only cancer cell properties but also by altering anti-tumor immune responses. Based on these findings, preclinical studies could be undertaken to assess the adequacy of a treatment targeting RANKL to counteract the immunosuppressive effects of genital cancers. To do so, a RANKL-blocking antibody (Denosumab), already approved for women with osteoporosis and for bone-related events in men with prostate cancer,Citation54 could be used.
In conclusion, we demonstrated a high expression of RANKL in cervical (pre)neoplastic lesions. RANKL is able to commit DC to a tolerogenic profile via ILT3 upregulation. These results could partially explain the functional alterations of APC during genital carcinogenesis. A better understanding of the microenvironmental alterations associated with genital (pre)neoplastic lesions and their potential consequences on DC functionalities may be crucial to elaborate new immunotherapies able to induce an effective antitumoral response.
Material and Methods
Dendritic cell generation
DC were generated from CD34+ haematopoietic precursors (HPC) obtained from cord blood samples and cultured for 7 d in the presence of human SCF (20 ng/mL, Peprotech), TPO (10 ng/mL, Peprotech), Flt3L (25 ng/mL, Peprotech), GM-CSF (200 U/mL, Amoytop Biotech) and IL-4 (100 U/mL, ImmunoTools), as previously described.Citation55 pDC were generated from CD34+ HPC as previously described.Citation56 In selected experiments, pDC were stimulated with type A CpG ODN at 12 μg/mL (5′-ggGGGACGATCGTCgggggg-3′; Eurogentec). All human samples were collected according to a protocol approved by the Ethics Committee of the University Hospital of Liège.
In selected experiments, DC were treated with human recombinant RANKL (0.1 or 0.5 μg/mL, R&D systems) for 6 d followed by a maturation with LPS (1 μg/mL, Sigma-Aldrich) for another day. OPG (Abcam), an inhibitor of RANKL, was also used at a concentration of 1 μg/mL, renewed once during the 7 d of culture.
Cocultures of DC in the presence of normal keratinocytes or cancer cell lines
Human exocervical epithelial cells were obtained from women who underwent total hysterectomy for non-cervical benign uterine disease. Cell cultures were established following a previously reported method.Citation57 The Ethics Committee of the University Hospital of Liège approved this study protocol.
Four genital SCC cell lines were obtained from the ATCC (American Type Culture Collection). SiHa, CaSki and C4II are cervical SCC cell lines infected by HPV16 (SiHa and CaSki) or HPV18 (C4II). A431 is a vulvar carcinoma cell line negative for HPV DNA. Melanoma (A2058), fibrosarcoma (HT1080), glioma (U87MG), lymphoma (U937) and leukemia (Jurkat) cell lines were also used. Cell cultures were maintained following ATCC-reported methods.
To evaluate the effects of the tumor environment on DC, cocultures were initiated by adding tumor cell lines (0.1 × 106 cells in 2 mL), normal keratinocytes (KN; 0.15 × 106 cells in 2 mL) or medium alone into 0.4-μm pore size membrane inserts (Nunc) placed in 6-well plates (Nunc) wherein DC (0.25 × 106 cells in 3 mL) were seeded. The medium used is constituted of complete RPMI 1640 supplemented with GM-CSF and IL-4. Inserts were extracted after 6 d of coculture and LPS (1 μg/mL) was added to the wells for 24 h in order to induce DC maturation. At day 7, DC were harvested for phenotypic and functional analysis. In selected experiments, human recombinant OPG (Abcam) was added to the culture, at a concentration of 1 μg/mL, and renewed once during the 7 d of culture. DC cultured alone or in the presence of OPG were used as controls.
Flow cytometry analysis
Double-staining immunofluorescence was performed by using a previously published procedure.Citation55 The following antibodies were used: CD1a-FITC, CD123-FITC, CD80-PE, CD83-PE, CD86-PE, HLA-DR-PE, ILT3-PE, ILT4-PE, CD273-PE, CD274-PE and CCR7-PE (). Fluorescence intensity and positive cell percentages were measured on a FACSCanto II flow cytometer (Becton Dickinson) and data were analyzed using FACSDiva software V 6.1.2 (Becton Dickinson).
Table 1. List of antibodies used for FACS detection
IL-10, IL-12p70 and RANKL ELISA
Cytokine production by tumor cells, DC alone or DC cultured in the presence of tumor cell lines was quantified by ELISA with the following commercial kits: IL-10 ELISA assay (Invitrogen), IL-12p70 ELISA assay (R&D systems) and RANKL ELISA assay (Biomedica) following the manufacturer's recommendations.
PCR targeting RANKL expression
One μg of total RNA extracted from cell cultures (Nucleospin RNA II, Macherey-Nagel) and quantified with a ND-1000 spectrophotometer (NanoDrop) was reverse-transcribed using Superscript II reverse transcriptase (Invitrogen) according to the manufacturer's instructions. PCR reactions were performed using the following primer sequences: RANKL: forward: 5′-TCA-GAG-CGC-AGA-TGG-ATC-CTA-A-3′; reverse: 5′-TGA-CTC-TCC-AGA-GTT-GTG-TCT-3′; HPRT: forward: 5′-GTT-GGA-TAT-AAG-CCA-GAC-TTT-GTT-G-3′; reverse: 5′-CAG-ATG-TTT-CCA-AAC-TCA-ACT-TGA-A-3′. Samples were run on 1.8% agarose gels containing ethidium bromide and visualized with an UV transilluminator. The mRNA levels were determined by densitometric analysis (Quantity One Software; Bio-Rad, Hercules, CA). HPRT was used as an internal control and the mRNA levels were normalized to this housekeeping gene.
Mixed lymphocyte reaction assay (MLR)
Priming of allogeneic T cells was assessed by 3H-Tdr (tritiated thymidine) uptake, as previously described.Citation58 The effector population corresponded to DC cultivated alone or in the presence of SCC cell lines (SiHa, CaSki, A431 or C4II) or KN. The responder cells are allogeneic PBMC. Results are presented as counts per minute (cpm).
T-cell suppressor assay
A first MLR was performed using allogeneic CD4+ T cells purified with the CD4+ isolation kit (Miltenyi Biotec) and cocultured for 7 d with irradiated DC (cultured alone or in the presence of keratinocytes). The ratio between responder (T cells) and effector (DC) cells was 10:1. A second MLR was performed using other allogeneic DC and T cells at a responder/effector ratio of 10:1. Cell mixtures harvested from the first MLR and considered as “suppressor” cells were then added at a ratio of 1:1 or 1:10 corresponding to 1 “suppressor” cell for 1 or 10 responder cell(s). 3H-Tdr incorporation was measured after 5 d of culture.
Treg cell induction assay
The assay was performed by culturing DC (stimulator cells) isolated from coculture experiments with allogeneic CD4+ T cells (responder cells) sorted from PBMC using the MACS CD4+ T-Cell Isolation Kit (Miltenyi Biotec), according to the manufacturer's protocol. The stimulator-to-responder ratio was 1:10 and cells were placed in RPMI 5% human pooled AB serum (Invitrogen) in 6-well plates (Nunc) for 6 d. DC cultured alone were used as controls.
Total RNA (1 μg) was extracted from T cells of the Treg cell induction assay (RNeasy minikit; Qiagen) and reverse-transcribed using Superscript II reverse transcriptase (Invitrogen) according to manufacturer's instructions. Quantitative real-time PCR was then performed using Power SYBR-green Master Mix (Eurogentec) and the following primer sequences: FoxP3 F: 5′-CAG-CAC-ATT-CCC-AGA-GTT-CCT-C-3′; FoxP3 R: 5′-GCG-TGTGAA-CCA-GTG-GTA-GAT-C-3′; GAPDH F: 5′-ACC-AGG-TGG-TCT-CCT-CTG-AC-3′; and GAPDH R: 5′-TGC-TGT-AGC-CAA-ATT-CGT-TG-3′ (Eurogentec). All experiments were performed in duplicate using the ABI-Prism 7700 Sequence Detection System (Applied Biosystems) and negative controls (master mix without any cDNA) were added in each run.
Tissue specimens
Eighty-seven paraffin-embedded cervical biopsy specimens were retrieved from the Tissue Biobank of the University of Liège. These biopsies included 22 normal exocervical tissues, 16 squamous metaplasia, 41 SILs (14 low-grade and 27 high-grade) and 10 invasive SCC. The histological diagnosis was confirmed after hematoxylin eosin staining. Vulvar tissue samples included high-grade intraepithelial neoplasia (VIN II and VIN III). The Ethics Committee of the University Hospital of Liege approved the protocol.
RANKL and OPG immunochemistry and scoring
Briefly, paraffin sections were deparaffinized in xylene, rehydrated in graded alcohols and antigens were retrieved in citrate buffer. Endogen peroxidase was blocked using a peroxidase-blocking reagent (Dako) and non-specific binding sites were blocked by the Protein Block Serum Free solution (Dako). The slides were then incubated with the primary antibody “anti-human RANKL (70525, R&D systems) or anti-OPG (polyclonal, Abcam)”. Immunoperoxidase staining was performed using the EnVision system (Dako) according to the supplier's recommendations. Colorimetric detection was completed with diaminobenzidine (Dako) for 5 min. Slides were then counterstained with hematoxylin. RANKL and OPG expression was evaluated, as previously described, by a semi-quantitative score of the intensity and extent of the staining according to an arbitrary scale.Citation59
Statistical analysis
Statistical analyses were performed by using the Student t-test or one- or two-way ANOVA test using GraphPad Prism 5.0c software.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
1008334_Supplementary_Materials.zip
Download Zip (38.9 MB)Acknowledgments
We thank the University of Liege Biobank and the GIGA imaging and flow cytometry facilities.
Funding
This study was supported by the Belgian National Fund for Scientific Research (FNRS) and by the Fonds Léon Frédéricq.
References
- Ellerbrock TV, Chiasson MA, Bush TJ, Sun XW, Sawo D, Brudney K, Wright TC Jr. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA 2000; 283:1031-7; PMID:10697063; http://dx.doi.org/10.1001/jama.283.8.1031
- Nakamura T, Shima T, Saeki A, Hidaka T, Nakashima A, Takikawa O, Saito S. Expression of indoleamine 2, 3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Sci 2007; 98:874-81; PMID:17433037; http://dx.doi.org/10.1111/j.1349-7006.2007.00470.x
- Giannini SL, Hubert P, Doyen J, Boniver J, Delvenne P. Influence of the mucosal epithelium microenvironment on Langerhans cells: implications for the development of squamous intraepithelial lesions of the cervix. Int J Cancer 2002; 97:654-9; PMID:11807793; http://dx.doi.org/10.1002/ijc.10084
- Nakayama Y, Asagoe K, Yamauchi A, Yamamoto T, Shirafuji Y, Morizane S, Nakanishi G, Iwatsuki K. Dendritic cell subsets and immunological milieu in inflammatory human papilloma virus-related skin lesions. J Dermatol Sci 2011; 63:173-83; PMID:21715145; http://dx.doi.org/10.1016/j.jdermsci.2011.05.006
- Gottfried E, Kreutz M, Mackensen A. Tumor-induced modulation of dendritic cell function. Cytokine Growth Factor Rev 2008; 19:65-77; PMID:18061513; http://dx.doi.org/10.1016/j.cytogfr.2007.10.008
- Walsh NC, Alexander KA, Manning CA, Karmakar S, Wang JF, Weyand CM, Pettit AR, Gravallese EM. Activated human T cells express alternative mRNA transcripts encoding a secreted form of RANKL. Genes Immun 2013; 14:336-45; PMID:23698708; http://dx.doi.org/10.1038/gene.2013.29
- Armstrong AP, Miller RE, Jones JC, Zhang J, Keller ET, Dougall WC. RANKL acts directly on RANK-expressing prostate tumor cells and mediates migration and expression of tumor metastasis genes. Prostate 2008; 68:92-104; PMID:18008334; http://dx.doi.org/10.1002/pros.20678
- Beristain AG, Narala SR, Di Grappa MA, Khokha R. Homotypic RANK signaling differentially regulates proliferation, motility and cell survival in osteosarcoma and mammary epithelial cells. J Cell Sci 2012; 125:943-55; PMID:22421365
- Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D, Dougall WC. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature 2010; 468:103-7; PMID:20881963; http://dx.doi.org/10.1038/nature09495
- Levidou G, Thymara I, Saetta AA, Papanastasiou P, Pavlopoulos P, Sakellariou S, Fragkou P, Patsouris E, Korkolopoulou P. TRAIL and osteoprotegerin (OPG) expression in bladder urothelial carcinoma: correlation with clinicopathological parameters and prognosis. Pathology 2013; 45:138-44; PMID:23277172; http://dx.doi.org/10.1097/PAT.0b013e32835c9891
- Owen S, Ye L, Sanders AJ, Mason MD, Jiang WG. Expression profile of receptor activator of nuclear-kappaB (RANK), RANK ligand (RANKL) and osteoprotegerin (OPG) in breast cancer. Anticancer Res 2013; 33:199-206; PMID:23267146
- Herfs M, Hubert P, Delvenne P. Epithelial metaplasia: adult stem cell reprogramming and (pre)neoplastic transformation mediated by inflammation? Trends Mol Med 2009; 15:245-53; PMID:19457719; http://dx.doi.org/10.1016/j.molmed.2009.04.002
- Hurwitz AA, Watkins SK. Immune suppression in the tumor microenvironment: a role for dendritic cell-mediated tolerization of T cells. Cancer Immunol Immunother 2012; 61:289-93; PMID:22237887; http://dx.doi.org/10.1007/s00262-011-1181-5
- Shklovskaya E, O'Sullivan BJ, Ng LG, Roediger B, Thomas R, Weninger W, Fazekas de St Groth B. Langerhans cells are precommitted to immune tolerance induction. Proc Natl Acad Sci USA 2011; 108:18049-54; PMID:22006331; http://dx.doi.org/10.1073/pnas.1110076108
- Loser K, Beissert S. Dendritic cells and T cells in the regulation of cutaneous immunity. Adv Dermatol 2007; 23:307-33; PMID:18159907; http://dx.doi.org/10.1016/j.yadr.2007.07.014
- Bosnjak L, Miranda-Saksena M, Koelle DM, Boadle RA, Jones CA, Cunningham AL. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol 2005; 174:2220-7; PMID:15699155; http://dx.doi.org/10.4049/jimmunol.174.4.2220
- Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol 2009; 10:488-95; PMID:19349986; http://dx.doi.org/10.1038/ni.1724
- Engelhardt JJ, Boldajipour B, Beemiller P, Pandurangi P, Sorensen C, Werb Z, Egeblad M, Krummel MF. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell 2012; 21:402-17; PMID:22439936; http://dx.doi.org/10.1016/j.ccr.2012.01.008
- Harimoto H, Shimizu M, Nakagawa Y, Nakatsuka K, Wakabayashi A, Sakamoto C, Takahashi H. Inactivation of tumor-specific CD8(+) CTLs by tumor-infiltrating tolerogenic dendritic cells. Immunol Cell Biol 2013; 91:545-55; PMID:24018532; http://dx.doi.org/10.1038/icb.2013.38
- Ng RL, Scott NM, Strickland DH, Gorman S, Grimbaldeston MA, Norval M, Waithman J, Hart PH. Altered immunity and dendritic cell activity in the periphery of mice after long-term engraftment with bone marrow from ultraviolet-irradiated mice. J Immunol 2013; 190:5471-84; PMID:23636055; http://dx.doi.org/10.4049/jimmunol.1202786
- Adikari SB, Pettersson A, Soderstrom M, Huang YM, Link H. Interleukin-10-modulated immature dendritic cells control the proinflammatory environment in multiple sclerosis. Scand J Immunol 2004; 59:600-6; PMID:15182256; http://dx.doi.org/10.1111/j.1365-3083.2004.01453.x
- Adurthi S, Mukherjee G, Krishnamurthy H, Sudhir K, Bafna UD, Umadevi K, Jayshree RS. Functional tumor infiltrating TH1 and TH2 effectors in large early-stage cervical cancer are suppressed by regulatory T cells. Int J Gynecol Cancer 2012; 22:1130-7; PMID:22872166; http://dx.doi.org/10.1097/IGC.0b013e318262aa53
- Loser K, Mehling A, Loeser S, Apelt J, Kuhn A, Grabbe S, Schwarz T, Penninger JM, Beissert S. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med 2006; 12:1372-9; PMID:17143276; http://dx.doi.org/10.1038/nm1518
- Santini D, Vincenzi B, Adamo V, Addeo R, Fusco V, Russo A, Montemurro F, Roato I, Redana S, Lanzetta G et al. Cigarette smoking habit does not reduce the benefit from first line trastuzumab-based treatment in advanced breast cancer patients. Oncol Rep 2011; 25:1545-8; PMID:21455582; http://dx.doi.org/10.3892/or.2011.1235
- Kanematsu M, Sato T, Takai H, Watanabe K, Ikeda K, Yamada Y. Prostaglandin E2 induces expression of receptor activator of nuclear factor-kappa B ligand/osteoprotegrin ligand on pre-B cells: implications for accelerated osteoclastogenesis in estrogen deficiency. J Bone Miner Res 2000; 15:1321-9; PMID:10893680; http://dx.doi.org/10.1359/jbmr.2000.15.7.1321
- Herfs M, Herman L, Hubert P, Minner F, Arafa M, Roncarati P, Henrotin Y, Boniver J, Delvenne P. High expression of PGE2 enzymatic pathways in cervical (pre)neoplastic lesions and functional consequences for antigen-presenting cells. Cancer Immunol Immunother 2009; 58:603-14; PMID:18802697; http://dx.doi.org/10.1007/s00262-008-0584-4
- Kukita A, Kukita T. Multifunctional properties of RANKL/RANK in cell differentiation, proliferation and metastasis. Future Oncol 2013; 9:1609-22; PMID:24156322; http://dx.doi.org/10.2217/fon.13.115
- Tanos T, Sflomos G, Echeverria PC, Ayyanan A, Gutierrez M, Delaloye JF, Raffoul W, Fiche M, Dougall W, Schneider P et al. Progesterone/RANKL is a major regulatory axis in the human breast. Sci Transl Med 2013; 5:182ra55; PMID:23616122; http://dx.doi.org/10.1126/scitranslmed.3005654
- Schramek D, Sigl V, Penninger JM. RANKL and RANK in sex hormone-induced breast cancer and breast cancer metastasis. Trends Endocrinol Metab 2011; 22:188-94; PMID:21470874; http://dx.doi.org/10.1016/j.tem.2011.02.007
- Remoue F, Jacobs N, Miot V, Boniver J, Delvenne P. High intraepithelial expression of estrogen and progesterone receptors in the transformation zone of the uterine cervix. Am J Obstet Gynecol 2003 189:1660-5; PMID:14710094; http://dx.doi.org/10.1016/S0002-9378(03)00852-4
- Monsonego J, Magdelenat H, Catalan F, Coscas Y, Zerat L, Sastre X. Estrogen and progesterone receptors in cervical human papillomavirus related lesions. Int J cancer 1991; 48:533-9; PMID:1646176; http://dx.doi.org/10.1002/ijc.2910480410
- Demoulin S, Herfs M, Delvenne P, Hubert P. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J Leukoc Biol 2013; 93:343-52; PMID:23136258; http://dx.doi.org/10.1189/jlb.0812397
- Schiano de Colella JM, Barbarat B, Sweet R, Gastaut JA, Olive D, Costello RT. Rank ligand stimulation induces a partial but functional maturation of human monocyte-derived dendritic cells. Eur Cytokine Netw 2008; 19:81-8; PMID:18632421; http://dx.doi.org/10.1684/ecn.2008.0125
- Josien R, Wong BR, Li HL, Steinman RM, Choi Y. TRANCE, a TNF family member, is differentially expressed on T cell subsets and induces cytokine production in dendritic cells. J Immunol 1999; 162:2562-8; PMID:10072496
- Williamson E, Bilsborough JM, Viney JL. Regulation of mucosal dendritic cell function by receptor activator of NF-kappa B (RANK)/RANK ligand interactions: impact on tolerance induction. J Immunol 2002; 169:3606-12; PMID:12244151; http://dx.doi.org/10.4049/jimmunol.169.7.3606
- Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med 2007; 204:3119-31; PMID:18086861; http://dx.doi.org/10.1084/jem.20071724
- Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev 2010; 234:120-41; PMID:20193016; http://dx.doi.org/10.1111/j.0105-2896.2009.00886.x
- Ramos RN, de Moraes CJ, Zelante B, Barbuto JA. What are the molecules involved in regulatory T-cells induction by dendritic cells in cancer? Clin Dev Immunol 2013; 2013:806025; PMID:23762097; http://dx.doi.org/10.1155/2013/806025
- Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother 2005; 54:307-14; PMID:15599732; http://dx.doi.org/10.1007/s00262-004-0593-x
- Yang W, Song Y, Lu YL, Sun JZ, Wang HW. Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology 2013; 139:513-22; PMID:23521696; http://dx.doi.org/10.1111/imm.12101
- Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, Melief CJ, van der Burg SH. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res 2009; 15:6341-7; PMID:19825956; http://dx.doi.org/10.1158/1078-0432.CCR-09-1652
- Manavalan JS, Rossi PC, Vlad G, Piazza F, Yarilina A, Cortesini R, Mancini D, Suciu-Foca N. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transpl Immunol 2003; 11:245-58; PMID:12967778; http://dx.doi.org/10.1016/S0966-3274(03)00058-3
- Vlad G, Suciu-Foca N. Induction of antigen-specific human T suppressor cells by membrane and soluble ILT3. Exp Mol Pathol 2012; 93:294-301; PMID:23018130; http://dx.doi.org/10.1016/j.yexmp.2012.09.011
- Hamdi H, Godot V, Maillot MC, Prejean MV, Cohen N, Krzysiek R, Lemoine FM, Zou W, Emilie D. Induction of antigen-specific regulatory T lymphocytes by human dendritic cells expressing the glucocorticoid-induced leucine zipper. Blood 2007; 110:211-9; PMID:17356131; http://dx.doi.org/10.1182/blood-2006-10-052506
- Cohen N, Mouly E, Hamdi H, Maillot MC, Pallardy M, Godot V, Capel F, Balian A, Naveau S, Galanaud P et al. GILZ expression in human dendritic cells redirects their maturation and prevents antigen-specific T lymphocyte response. Blood 2006; 107:2037-44; PMID:16293609; http://dx.doi.org/10.1182/blood-2005-07-2760
- Li C, Zhao J, Sun L, Yao Z, Liu R, Huang J, Liu X. RANKL downregulates cell surface CXCR6 expression through JAK2/STAT3 signaling pathway during osteoclastogenesis. Biochem Biophys Res Commun 2012; 429:156-62; PMID:23142594; http://dx.doi.org/10.1016/j.bbrc.2012.10.122
- Gur-Wahnon D, Borovsky Z, Liebergall M, Rachmilewitz J. The induction of APC with a distinct tolerogenic phenotype via contact-dependent STAT3 activation. PloS One 2009; 4:e6846; PMID:19718269; http://dx.doi.org/10.1371/journal.pone.0006846
- Burkholder B, Huang RY, Burgess R, Luo S, Jones VS, Zhang W, Lv ZQ, Gao CY, Wang BL, Zhang YM et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim Biophys Acta 2014; 1845(2):182-201; PMID:24440852; http://dx.doi.org/10.1016/j.bbcan.2014.01.004
- Anandkumar A, Devaraj H. Tumour immunomodulation: mucins in resistance to initiation and maturation of immune response against tumours. Scand J Immunol 2013; 78:1-7; PMID:23298229; http://dx.doi.org/10.1111/sji.12019
- Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, Cheresh DA, Karin M. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature 2007; 446:690-4; PMID:17377533; http://dx.doi.org/10.1038/nature05656
- Odero-Marah VA, Wang R, Chu G, Zayzafoon M, Xu J, Shi C, Marshall FF, Zhau HE, Chung LW. Receptor activator of NF-kappaB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells. Cell Res 2008; 18:858-70; PMID:18645583; http://dx.doi.org/10.1038/cr.2008.84
- Kim JK, Jin X, Sohn YW, Jin X, Jeon HY, Kim EJ, Ham SW, Jeon HM, Chang SY, Oh SY et al. Tumoral RANKL activates astrocytes that promote glioma cell invasion through cytokine signaling. Cancer Lett 2014; 353:194-200; PMID:25079688; http://dx.doi.org/10.1016/j.canlet.2014.07.034
- Buckle CH, De Leenheer E, Lawson MA, Yong K, Rabin N, Perry M, Vanderkerken K, Croucher PI. Soluble rank ligand produced by myeloma cells causes generalised bone loss in multiple myeloma. PloS One 2012; 7:e41127; PMID:22952578; http://dx.doi.org/10.1371/journal.pone.0041127
- Castellano D, Sepulveda JM, Garcia-Escobar I, Rodriguez-Antolin A, Sundlov A, Cortes-Funes H. The role of RANK-ligand inhibition in cancer: the story of denosumab. Oncologist 2011; 16:136-45; PMID:21285392; http://dx.doi.org/10.1634/theoncologist.2010-0154
- Herman L, Hubert P, Herfs M, Kustermans G, Henrotin Y, Bousarghin L, Boniver J, Delvenne P. The L1 major capsid protein of HPV16 differentially modulates APC trafficking according to the vaccination or natural infection context. Eur J Immunol 2010; 40:3075-84; PMID:21061438; http://dx.doi.org/10.1002/eji.201040571
- Demoulin S, Roncarati P, Delvenne P, Hubert P. Production of large numbers of plasmacytoid dendritic cells with functional activities from CD34(+) hematopoietic progenitor cells: use of interleukin-3. Exp Hematol 2012; 40:268-78; PMID:22245566; http://dx.doi.org/10.1016/j.exphem.2012.01.002
- Hubert P, Van Den Brule F, Giannini SL, Franzen-Detrooz E, Boniver J, Delvenne P. Colonization of in vitro-formed cervical human papillomavirus-associated (pre)neoplastic lesions with dendritic cells. Role of granulocyte/macrophage colony-stimulating factor. Am J Pathol 1999; 154:775-84; PMID:10079255; http://dx.doi.org/10.1016/S0002-9440(10)65324-2
- Hubert P, Greimers R, Franzen-Detrooz E, Doyen J, Delanaye P, Boniver J, Delvenne P. In vitro propagated dendritic cells from patients with human-papilloma virus-associated preneoplastic lesions of the uterine cervix: use of Flt3 ligand. Cancer Immunol Immunother 1998; 47:81-9; PMID:9769116; http://dx.doi.org/10.1007/s002620050507
- Caberg JH, Hubert P, Herman L, Herfs M, Roncarati P, Boniver J, Delvenne P. Increased migration of Langerhans cells in response to HPV16 E6 and E7 oncogene silencing: role of CCL20. Cancer Immunol Immunother 2009; 58:39-47; PMID:18438663; http://dx.doi.org/10.1007/s00262-008-0522-5
