Abstract
Adoptive transfer of lymphocytes expressing engineered T cell receptors (TCR) is a promising option for cancer treatment and could include hepatocellularcarcinoma (HCC), where therapeutic options are limited. We have recently investigated whether hepatitis B viral antigens can act as a HCC-specific antigen and thus be targeted by adoptively transferred HBV-specific TCR redirected T cells.
Keywords:
HCC is the third leading cause of cancer deaths worldwide and is often associated with HBV or HCV chronic infections. In Asia, where the incidence of HBV infection is high, HBV accounts for at least 80% of HCC. HBV predisposes to the development of HCC by causing chronic inflammation but also by integrating into the genome of hepatocytes. A high frequency of HBV integrations has been observed in HBV-related HCC tumors,Citation1 that might result in the expression of HBV antigens in tumor cells.
We thought that this particular feature of HBV related HCC could be exploited for immunotherapy since targeting viral non-self antigens with T cells can have advantages over the targeting of classical tumor-associated antigens.
T cells specific for classical tumor-associated antigens (like NY-Eso-1, glycan-3, α-feto protein, Mage-3) are present in HBV-related HCC patients.Citation2 However, the use of T cells engineered with TCR specific for classical tumor antigens can lead to severe side effects due to the targeting of normal tissueCitation3 or induce a state of tolerance in tumor-specific T cells.Citation4 Furthermore, TCR specific for tumor associated antigens are often of low affinity and require modifications in order to efficiently recognize tumor cells. In contrast, T cells recognizing HBV antigens present in patients that resolved acute HBV infection express high affinity TCR. Transfer of these HBV-specific TCRs can be used to produce therapeutic T cell that lyse not only HBV infected cells but also natural HCC line with HBV-DNA integration.Citation5 Furthermore, these TCR-redirected T cells are not inhibited by the HBV antigen present in patients' sera,Citation5 a problem that might instead theoretically affect HBV-specific CAR-T cells.Citation6
The use of HBV antigen as a target for immunotherapy has however one important drawback: in patients with chronic HBV infection who developed HCC, HBV antigens are expressed not only by HCC cells with HBV-DNA integration but also by HBV infected hepatocytes. The risk of inducing a severe liver damage due to the targeting of normal infected hepatocytes cannot be ignored.
Our reportCitation7 describes a unique clinical scenario with a much reduced risk of potential damage to normal liver tissue. This patient had a liver transplant and then presented with extrahepatic HCC metastasis with HBV-DNA integration producing only surface protein of HBV (HBsAg) while complete HBV-DNA, a sign of HBV replication was absent in blood and in the transplanted liver.
We first tested whether his extrahepatic HCC cells present HBV antigen in a conformation recognizable by HLA-class I restricted TCR. The presence of HLA-A0201/HBV peptide complexes on the surface of extrahepatic HCC metastasis was tested using a TCR-like antibody specific for the complex HLA-A0201/HBs183-91. The HCC cells were not only producing HBsAg, but were able to process and present HLA-A0201/HBV peptide complexes. Importantly, the expression of HBV-peptide complexes was homogeneously present in all the HCC cells derived from the metastasis.
A specific TCR targeting the complexes HLA-A0201/HBs183-91 was selected and used to engineer HBV-TCR specific T cells under good manufacturing practice for use in a compassionate, specials' license treatment. Autologous in vitro activated T cells were transduced using a retroviral vector and the patient received a single dose of ˜1.2 × 104 TCR-redirected T cells per kg, a low dose in comparison with the ones used in immunotherapy of other tumors but similar to doses used in antiviral cell therapies. By the time of the treatment the patient's condition was very compromised with disseminated HCC metastasis in lung, bones and neck. Despite this frail condition, the cell infusion was very well tolerated. There were no signs of acute toxicities and in the 30 d of observation period after infusion we detected only a minimal alteration of ALT levels. Important observations were derived (). First, despite the lack or pre-emptive lymphodepletion or and cytokine therapy, TCR-redirected T cells efficiently expanded in the patient, A high frequency of TCR-redirected T cells (˜2% total CD8+ T cells) was detected 30 d after infusion This is interesting since HBV-specific CD8+ T cell in patients with CHB infection or in HBV/HCC patients are not only present at very low frequency (˜0.01/0.03% CD8+ cells) but are functionally impaired.Citation2 In contrast, the HBV-TCR-T cells transferred in the patient not only proliferate but seem to function in vivo, since HBsAg levels dropped dramatically (3200IU before therapy to ˜300 IU at day 30) in concomitance with the HBV-TCR-T cells expansion. Therefore, in this HBV-related HCC patient, HBV antigen acts like a tumor antigen that triggers a robust T cell expansion. This observation contrasts with the deletion/tolerization of HBV-specific T cells observed in CHB patients and might be caused by the selective extrahepatic HBV antigen presentation.
Figure 1. Schematic representation of the clinical history of the treated patient and graphs representing the clinical (ALT), virological (HBsAg) and immunological (frequency of TCR-redirected T cells) parameters after adoptive transfer of TCR-redirected T cells.
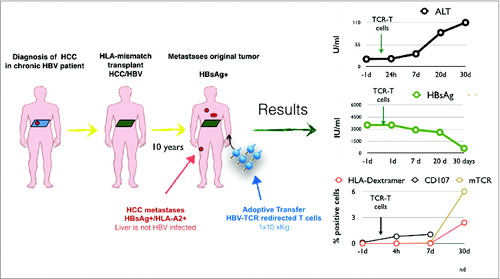
Regrettably, the robust HBsAg drop was not associated with any detectable reduction of the volume of the HCC metastasis. One month after T cell therapy the general conditions of the patient declined and at week 8 brain metastasis were detected that resulted in confusion, lethargy and death, 9 weeks after therapy.
It is clear that more data needs to be gathered to fully understand whether HBV-specific TCR redirected T cells can confer clinical efficacy in HCC patients. HBV-specific TCR cells can eradicate HCC tumors in SCID mice,Citation8 but the multiple HCC locations present in this patient could represent a tumor burden that is too advanced to test the HBV-TCR T cells therapeutic efficacy.
One other question connected to the clinical efficacy of HBV-TCR T cells is whether their therapeutic use should be selectively applied to the cases of HCC relapses in liver-transplanted patients.
Since HCC relapses after liver transplant can be very high (about 50% – 5 years in patients with liver transplant after HCC resectionCitation9) and serum HBsAg recurrence is accociated with HCC recurrence (7/8 patients, 87.5%Citation10) one possibility is to use HBV-TCR T cells not only as a therapy but as a prophylactic treatment to prevent HCC seeding in liver-transplanted patients with HBsAg positive HCC. In animal models HCC seeding was blocked with the use of HBV-TCR-T cells expressing transiently TCR trough mRNA electroporation.Citation8 mRNA TCR-T cells are easy to produce and even though these cells will not be able to reconstitute in patients an effector/memory T cell response, the transient expression of TCR might allow to perform a dose-escalating trials in groups, like the ones with CHB and HCC, where the potential toxicity of the HBV-specific TCR needs to be carefully tested.
At the moment, expanding the treatment to CHB patients with advanced HCC is hindered by our very limited knowledge of the difference between normal and transformed hepatocytes in term of HBV infectivity, expression, processing and presentation of viral antigen derived from HBV-DNA integration or infection.
We hope that this demonstration of the feasibility of targeting HBV antigen in HCC cells will boost the research in this area and make possible to offer a new alternative treatment to the many HBV-related HCC patients in need of an effective treatment.
Disclosure of Potential Conflicts of Interest
WQ and HS, are engaged in collaborations and receive research funding contracts from Catapult Therapy TCR Ltd. WQ is also collaborating and receive research support from Miltenyi Biotec to develop engineered cell therapy and is engaged with Sycona Ltd to develop engineered T cell therapies. AB is a co-founder of Lion TCR Ltd.
References
- Sung W-K, Zheng H, Li S, Chen R, Liu X, Li Y, Lee NP, Lee WH, Ariyaratne PN, Tennakoon C et al. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat Gen 2012; 44:765-9; PMID: 22634754; http://dx.doi.org/10.1038/ng.2295
- Gehring AJ, Ho ZZ, Tan AT, Aung MO, Lee KH, Tan KC, Lim SG, Bertoletti A. Profile of tumor antigen-specific CD8 T cells in patients with hepatitis B virus-related hepatocellular carcinoma. Gastroenterology 2009; 137:682-90; PMID:19394336; http://dx.doi.org/10.1053/j.gastro.2009.04.045
- Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan D-AN, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM et al. T cells targeting carcinoembryonic antigenCan mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 2009; 19:620-6; PMID: 21157437; http://dx.doi.org/10.1038/mt.2010.272
- Ghorashian S, Veliça P, Chua I, McNicol AM, Ben Carpenter, Holler A, Nicholson E, Ahmadi M, Zech M, Xue S-A et al. CD8 T cell tolerance to a tumor-associated self-antigen is reversed by CD4 T cells engineered to express the same T cell receptor. J Immunol 2014:1401703; PMID: 25539815; http://dx.doi.org/10.4049/jimmunol.1401703.
- Gehring AJ, Xue S-A, Ho ZZ, Teoh D, Ruedl C, Chia A, Koh S, Lim SG, Maini MK, Stauss H et al. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J Hepatol 2011; 55:103-10; PMID:21145860; http://dx.doi.org/10.1016/j.jhep.2010.10.025
- Krebs K, Böttinger N, Huang LR, Chmielewski M, Arzberger S, Gasteiger G, Jäger C, Schmitt E, Bohne F, Aichler M et al. T cells expressing a chimeric antigen receptor that binds hepatitis B virus envelope proteins control virus replication in mice. Gastroenterology 2013; 145:456-65; PMID:23639914; http://dx.doi.org/10.1053/j.gastro.2013.04.047
- Qasim W, Brunetto M, Gehring AJ, Xue S-A, Schurich A, Khakpoor A, Zhan H, Ciccorossi P, Gilmour K, Cavallone D et al. Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J Hepatol 2015; 62:486-92; PMID:25308176; http://dx.doi.org/10.1016/j.jhep.2014.10.001
- Koh S, Shimasaki N, Suwanarusk R, Ho ZZ, Chia A, Banu N, Wu Howland S, Ong ASM, Gehring AJ, Stauss H et al. A practical approach to immunotherapy of hepatocellular carcinoma using T cells redirected against hepatitis B virus. Mol Ther Nucleic Acids 2013; 2:e114; PMID:23941866; http://dx.doi.org/10.1038/mtna.2013.43
- Lai Q, Avolio AW, Lerut J, Singh G, Chan SC, Berloco PB, Tisone G, Agnes S, Chok KS, Sharr W et al. Recurrence of hepatocellular cancer after liver transplantation: The role of primary resection and salvage transplantation in East and West. J Hepatol 2012; 57:974-9; PMID:22771712; http://dx.doi.org/10.1016/j.jhep.2012.06.033
- Faria LC, Gigou M, Roque Afonso AM, Sebagh M, Roche B, Fallot G, Ferrari TCA, Guettier C, Dussaix E, Castaing D et al. Hepatocellular carcinoma is associated with an increased risk of hepatitis B virus recurrence after liver transplantation. Gastroenterology 2008; 134:1890-9; PMID:18424269; http://dx.doi.org/10.1053/j.gastro.2008.02.064
