Abstract
Myeloid cells contribute to increased malignancy and poor prognosis in breast cancer. We demonstrate that anti-CSF-1R therapy depletes a cell population sharing characteristics of tumor-associated macrophages (TAMs) and dendritic cells (DCs). Intravital imaging combined with cellular characterization has refined our understanding of anti-CSF-1R therapy on the tumor microenvironment.
Tumor-infiltrating Myeloid Cells Promote Tumor Progression
Tumors function as complex organs that shape their microenvironment through the interaction with stromal cells, consisting of fibroblasts, adipocytes, blood and lymph vessels, and immune cells.Citation1 As tumors progress, tumor-derived growth factors lead to evolving phenotypes of tumor-associated myeloid cells that can contribute to tumor initiation and progression by a wide range of mechanisms.Citation2 In human breast cancer and most other solid tumors, high numbers of tumor-associated myeloid cells, along with high expression of the myeloid colony-stimulating factor −1 (CSF-1),Citation3 correspond to poor prognosis.
CSF-1 Dependent and Independent Tumor-associated Myeloid Cells
Due to the plasticity of macrophages, the complex spectrum of macrophage activation and the lack of cell surface markers that are distinct to subsets of macrophages,Citation4,5 a clear understanding of macrophage diversity and function within the tumor and during tumor progression cannot be delineated by immunohistochemistry and flow cytometry alone. In addition to these methods, we therefore utilized intravital microscopy, in combination with targeted therapy against CSF-1R, to provide unique insight into the cellular composition and real-time dynamics of the stromal microenvironment.Citation6 Our goal was to characterize the evolving phenotype of TAMs and their dynamic interplay with the microenvironment during tumor progression. We utilized the MMTV-PyMT/c-fms-EGFP mouse model of breast cancer, which mimics the stages of tumor progression observed in humans, and the IgG1 M279 blocking monoclonal antibody to the CSF-1R.
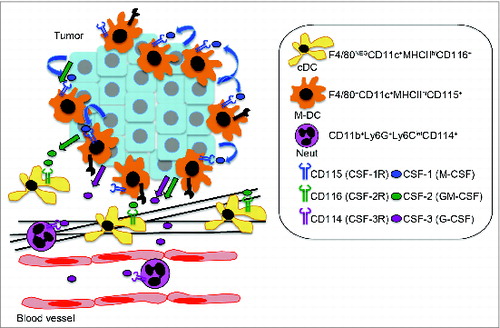
Using intravital imaging, we identified a c-fms-EGFP population of sessile myeloid cells that localized closely to the epithelium of tumor nodules and included subpopulations of cells with high endocytic and MMP proteolytic activity. Furthermore, we found these cells expressed macrophage cell surface markers F4/80 and CD115 (CSF-1R), along with classical DC markers CD11c and MHCII using flow cytometry. Accordingly, these cells were profoundly depleted with the anti-CSF-1R antibody M279. In addition, we observed CSF-1 independent myeloid cell populations in the stroma, which were identified as CD11c+ F480− MHCIIhi (classical DCs) and CD11c− F4/80− Gr1hi (neutrophils) cells by flow cytometry. Further detailed characterization of these populations in two recent studies showed the CD11c+ F480− MHCIIhi population is GM-CSF dependentCitation7 and the Gr1hi population, which consists predominantly of T cell-suppressive Ly6G+ Ly6Cint neutrophils, is G-CSF dependent,Citation8 revealing distinct regulation of these myeloid cell populations in the PyMT model of breast cancer ().
Figure 1. Proposed model for the distinct regulation of myeloid cell subpopulations in breast cancer. Tumor-derived hematopoietic growth factors CSF-1 (M-CSF), CSF-2 (GM-CSF), and CSF-3 (G-CSF) regulate distinct populations of myeloid cells in tumors. CSF-1-dependent F4/80+ CD11c+ MHCIIhi CD115+ myeloid cells, referred to as macrophage-dendritic cells (M-DCs), showed high endocytic and matrix metalloproteinase (MMP) proteolytic activity and localized close to the epithelium of tumor nodules. CSF-1-independent CD11b+ Ly6G+ Ly6Cint (neutrophils, CSF-3 dependent) and F4/80neg CD11c+ MHCIIhi (classical DCs, CSF-2 dependent) localized in the stroma.
CSF-1-dependent Regulation of Lung Metastasis During Early Tumor Progression
To determine whether depletion of CSF-1-dependent CD11c+ F4/80+ MHCIIhi myeloid cells was therapeutic, two treatment regimens were performed with M279 to evaluate efficacy of early (hyperplastic stage) and late (early carcinoma stage) treatment. We found that depletion of CSF-1R-depedent macrophages during early tumor progression modestly reduced primary tumor growth and lung metastases, while it only affected primary tumor growth during late stage tumor progression, suggesting CSF-1R-dependent cells promoted the establishment of metastatic lesions. Surprisingly, we did not detect an accumulation of macrophage- or DC-like cells in lungs in this study, but, in contrast, observed only an expansion of Gr1hi neutrophils. The modest delay in tumor growth and metastasis suggests that other stromal cells contribute to the maintenance of the tumor trophic microenvironment. Alternatively, given the diversity of myeloid cells that are depleted with anti-CSF-1R blockade, the relatively modest effect on tumor growth may also suggest that CSF-1R dependent myeloid cells may contribute both pro and antitumor functions.
M1 and M2 Characterization Should Be Avoided
While CSF-1 stimulation as a single agent polarizes macrophages away from an antigen presentation phenotype and induces a different gene expression profile than GM-CSF, CSF-1 dependent macrophages should not be classified as M2 polarized, since their heterogeneity is likely regulated by the microenvironment.Citation4 Furthermore, while TAMs have been termed M2 or alternatively activated macrophages (AAMs), IL-4−/− PyMT mice had normal proportions of TAMs (characterized as CD11blo MHCIIhi VCAM+ cells that highly expressed itgax, the gene that encodes CD11c) and TAM differentiation was intact in the absence of lymphocytes, suggesting TAMs are not the same as classical AAMs.Citation9 Thus, it has been suggested that macrophage heterogeneity should not be simplified on a linear scale between M1 and M2, but should be described in terms of the diversity of points on a color wheel.Citation4
Future Therapy With CSF-1 Blockade in Cancer
CSF-1 blockade depletes macrophage populations in various tumor models, but the effects as a single agent therapy on primary tumor growth is variable.Citation3 This variability may result from the heterogeneity and relative proportions of macrophage subpopulations in different tumor types and their role(s) in disease progression. Of interest, CSF-1 blockade in combination with radiation, chemotherapy, and immunotherapy has shown synergistic effects.Citation3 However, one study reported a neutrophil-dependent enhanced metastasis with CSF-1 blockade.Citation10 A better understanding of the complex interplay between tumor-associated myeloid cells and disease progression in different tumor types is warranted and should provide predictive and prognostic value.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by a grant from the National Cancer Institute (R01 CA057621).
References
- Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell 2010; 18:884-901; PMID:20627072; http://dx.doi.org/10.1016/j.devcel.2010.05.012
- Hagerling C, Casbon AJ, Werb Z. Balancing the innate immune system in tumor development. Trends Cell Biol 2014; 25(4):214-220;; PMID:25444276; http://dx.doi.org/10.1016/j.tcb.2014.11.001
- Laoui D, Van Overmeire E, De Baetselier P, Van Ginderachter JA, Raes G. Functional Relationship between Tumor-Associated Macrophages and Macrophage Colony-Stimulating Factor as Contributors to Cancer Progression. Front Immunol 2014; 5:489; PMID:25339957; http://dx.doi.org/10.3389/fimmu.2014.00489
- Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 2012; 119:1810-20; PMID:22186992; http://dx.doi.org/10.1182/blood-2011-09-379214
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014; 41:14-20; PMID:25035950; http://dx.doi.org/10.1016/j.immuni.2014.06.008
- Lohela M, Casbon AJ, Olow A, Bonham L, Branstetter D, Weng N, Smith J, Werb Z. Intravital imaging reveals distinct responses of depleting dynamic tumor-associated macrophage and dendritic cell subpopulations. Proc Natl Acad Sci USA 2014; 111:E5086-95; PMID:25385645; http://dx.doi.org/10.1073/pnas.1419899111
- Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 2014; 26:638-52; PMID:25446897; http://dx.doi.org/10.1016/j.ccell.2014.09.007
- Casbon AJ, Reynaud D, Park C, Khuc E, Gan DD, Schepers K, Passegué E, Werb Z. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci USA 2015; 112(6):E566-75 PMID:25624500; http://dx.doi.org/10.1073/pnas.1424927112
- Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, Pamer EG, Li MO. The cellular and molecular origin of tumor-associated macrophages. Science 2014; 344:921-5; PMID:24812208; http://dx.doi.org/10.1126/science.1252510
- Swierczak A, Cook AD, Lenzo JC, Restall CM, Doherty JP, Anderson RL, Hamilton JA. The promotion of breast cancer metastasis caused by inhibition of CSF-1R/CSF-1 signaling is blocked by targeting the G-CSF receptor. Cancer Immunol Res 2014; 2:765-76; PMID:25005824; http://dx.doi.org/10.1158/2326-6066.CIR-13-0190
