Abstract
The efficacy of antitumoral responses can be increased using combinatorial vaccine strategies. We recently showed that vaccination could be optimized by local administration of diverse molecular or bacterial agents to target and augment antitumoral CD8 T cells in the genital mucosa (GM) and increase regression of cervical cancer in an animal model. Non muscle-invasive bladder cancer is another disease that is easily amenable to local therapies. In contrast to data obtained in the GM, in this study we show that intravesical (IVES) instillation of synthetic toll-like receptor (TLR) agonists only modestly induced recruitment of CD8 T cells to the bladder. However, IVES administration of Ty21a, a live bacterial vaccine against typhoid fever, was much more effective and increased the number of total and vaccine-specific CD8 T cells in the bladder approximately 10 fold. Comparison of chemokines induced in the bladder by either CpG (a TLR-9 agonist) or Ty21a highlighted the preferential increase in complement component 5a, CXCL5, CXCL2, CCL8, and CCL5 by Ty21a, suggesting their involvement in the attraction of T cells to the bladder. IVES treatment with Ty21a after vaccination also significantly increased tumor regression compared to vaccination alone, resulting in 90% survival in an orthotopic murine model of bladder cancer expressing a prototype tumor antigen. Our data demonstrate that combining vaccination with local immunostimulation may be an effective treatment strategy for different types of cancer and also highlight the great potential of the Ty21a vaccine, which is routinely used worldwide, in such combinatorial therapies.
Abbreviations:
- BCG, Bacillus Calmette Guerin
- BMDC, bone marrow-derived dendritic cell
- C5a, complement component 5a
- ESL, E-selectin ligands
- IVAG, intravaginal
- IVES, intravesical
- GM, genital mucosa
- NMIBC, non-muscle invasive bladder cancer
- PBS, phosphate buffered saline
- PE, phycoerythrin
- PIC, poly (I:C)
- s.c., subcutaneously
- SEM, standard error of the mean
- TLR, toll-like receptor
- TUR, transurethral resection
Introduction
Although cancer vaccines have been considered a promising therapeutic approach, their general lack of clinical efficacy as a single therapy argues for the use of combinatorial protocols. Even when antitumor T cells are generated, trafficking to the tumor site may be limited.Citation1 This can be improved by using appropriate vaccine administration routes, allowing targeting of relevant tumor sites through specific mucosal homing or cancer site retention programs.Citation2-5 An alternative approach, irrespective of the immunization route, is to enhance T-cell attraction to the tumor site through the local application of selected chemokinesCitation6 or Toll-like receptor (TLR) agonists that are able to modify the expression of selectins, integrins, chemokines, and chemokine receptors.Citation7,8 Along this line, we recently reported that intravaginal (IVAG) administration of CpG (a TLR-9 agonist) resulted in the accumulation of CD8 T cells co-expressing CCR5 and CXCR3 chemokine receptors and E-selectin ligands (ESL), most probably through CpG-induced expression of CCL5, CXCL9, CXCL10, CXCL11, and/or E-selectinCitation9. Most importantly, in a murine model of cervical cancer, this strategy resulted in more efficient genital tumor regression than vaccination alone.Citation9 Moreover, we have also shown that live bacteria, such as Salmonella attenuated vaccine strains, are more potent immunostimulants than CpG for the recruitment of vaccine-specific CD8 T cells to the genital mucosa (GM) of mice.Citation10
Here, we explore how such therapeutic approaches can be extended to other cancers. Bladder cancer is a common urologic malignancy that is in part caused by smoking habits and exposure to industrial chemicals and has an increased incidence in the elderly population.Citation11 Seventy percent of bladder cancers are diagnosed as non muscle-invasive and are treated by transurethral resection (TUR). However, they have a high propensity to recur and/or progress to invasive cancer. Interestingly, the association between tuberculosis and a lower frequency of cancer has led to use of the Bacillus Calmette Guerin (BCG) vaccine against tuberculosis as a standard intravesical (IVES) treatment after TUR for high-risk non muscle-invasive bladder cancer (NMIBC) to reduce both recurrence and progression.Citation12,13 Repeated BCG treatments are however associated with significant side effects and treatment resistance, arguing for alternative or complementary therapies such as vaccination.Citation14 In the absence of a murine bladder tumor model expressing a tumor antigen relevant in humans, we have used an orthotopic model expressing E7 as a prototype tumor antigen and a cognate E7 vaccineCitation3 to explore the ability of either synthetic or bacterial IVES TLR agonists to increase CD8 T-cell recruitment to the bladder and improve bladder tumor regression.
Results
IVES instillation of CpG after subcutaneous E7 vaccination modestly increased the number of total and vaccine-specific CD8 T cells in the bladder but did not influence bladder tumor regression
Groups of C57BL/6 mice were subcutaneously (s.c.) immunized with a long synthetic E7 peptide together with adjuvantsCitation15 or additionally received an IVES instillation of CpG 5 d after immunization. Flow cytometric analysis of bladder cell suspensions at day 9 showed a significant (approximately 2-fold) increase in both total and vaccine-specific (TetE7+) CD8 T cells, but not in CD4 T cells (), compared to the group that did not receive IVES CpG instillation. In contrast, systemic T cell levels were not affected (). IFN-γ ELISPOT analysis confirmed the significant increase induced by IVES CpG (13 ± 2 [geometric mean ± standard error of the mean] IFN-γ secreting CD8 T cells/105 bladder cells, compared to 5 ± 1 in the absence of IVES immunostimulation, P < 0.01, ). This increase is, however, modest compared to the 5-fold increase we previously reported after IVAG CpG.Citation9 Furthermore, and in contrast to the data obtained in the GM,Citation9 successive IVES applications of CpG (days 6, 9, and 12) were not able to sustain a higher vaccine-specific CD8 T-cell response in the bladder (see day 15 in ). We further tested whether successive IVES CpG instillations after vaccination would enhance the regression of bladder tumors. For this purpose, mice were first IVES instilled with E7- and luciferase-expressing tumor cells (TC-1 luc) and at day 8, when bioluminescent tumors were detected, treated with vaccination alone or vaccination followed by IVES CpG treatment (3 doses at 5, 8 and 11 d after vaccination). IVES CpG alone had no effect on tumor regression compared to untreated mice. As expected, vaccination resulted in significant tumor regression and survival of 70% of the mice (P < 0.001), although no benefit of the additional IVES CpG treatment was observed ().
Figure 1. IVES CpG after E7 immunization modestly increased the number of E7-specific and total CD8 T cells in bladder but did not influence bladder tumor regression. Groups of female C57BL/6 mice were immunized s.c. with the E7 vaccine. Five days later, mice were either left untreated (φ, white diamond) or received IVES with 1 (day 6, black square) or 3 (day 6, 9 and 12, white square) instillations of 100 μg CpG. Bladder cell suspensions were prepared 3 d after the last IVES CpG and analyzed by flow cytometry (A) or by IFN-γ ELISPOT assay (B). Representative flow cytometric analysis of bladder cells is shown in the upper panel of (A) and the percentage of TetE7+CD8+ T cells/total cells is indicated in parentheses. Horizontal bars represent mean percent in panel (A) and geometric mean percent in panel (B). Significant differences between groups of mice are indicated by *P < 0.05 and ** P < 0.01. Two groups of tumor-bearing mice were left untreated (black triangles) or received IVES CpG (black asterisk), while 2 other groups received s.c. E7 vaccine alone (white diamonds) or followed by IVES CpG (black square) (C). Significant differences in mouse survival between each treatment and control are indicated by ** P = 0.0012 and *** P = 0.0003 following an adjusted log-rank test.
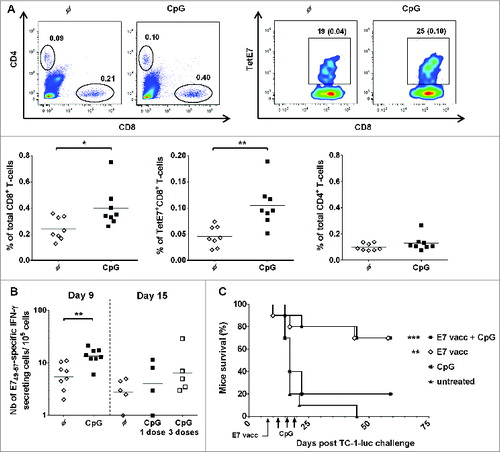
Table 1. CD8 T-cell responses in the spleen of E7-vaccinated mice after challenge with different IVES immunostimulants
Immunostimulation by IVES Ty21a live bacteria after vaccination greatly increased the number of total and vaccine-specific CD8 T cells in bladder
As the bladder may be more responsive to other TLR agonists, we decided to test the effects of a synthetic TLR-3 agonist, poly (I:C) (PIC), and 2 bacterial vaccines (BCG, which is used in NMIBC immunotherapy, and Ty21a, the live attenuated Salmonella enterica serovar Typhi vaccine against typhoid fever), as we had previously shown that IVAG-attenuated Salmonella strains increased the number of effector vaccine-specific CD8 T cells in the GM.Citation10 Groups of E7-vaccinated mice received IVES PIC, BCG, or Ty21a 5 d after vaccination. IFN-γ ELISPOT analysis of bladder cell suspensions () showed a significant (4- to 5-fold) increase in vaccine-specific effector CD8 T-cells following treatment with PIC (20 ± 4 IFN-γ–secreting CD8 T cells/105 bladder cells) and BCG (15 ± 5) compared to vaccination alone (4 ± 1, P < 0.01 and P < 0.05, respectively). Interestingly, however, Ty21a bacteria resulted in a greater than 10-fold increase in the number of vaccine-specific effector CD8 T cells in bladder (58 ± 9 IFN-γ–secreting CD8 T cells/105 bladder cells; P < 0.0001). Live Ty21a bacteria were required as heat-killed Ty21a bacteria were less efficient (24 ± 3 IFN-γ–secreting CD8 T cells/105 bladder cells , P < 0.01 by Student t test). Flow cytometric analysis of IVES Ty21a paralleled the ELISPOT data with an approximately 10-fold increase in both total and vaccine-specific CD8 T-cells (P < 0.001), confirming that the increase in IFN-γ–secreting cells measured by ELISPOT corresponded to enhanced recruitment of CD8 T cells ().
Figure 2. Increase in E7-specific IFN-γ–secreting cells in the bladder by different IVES immunostimulants after E7 immunization. Groups of female C57BL/6 mice were immunized s.c. with the E7 vaccine. Five days later, mice were either left untreated (φ, white diamond) or received IVES PIC (black circles), BCG (black triangles), live Ty21a (black squares), or heat-killed Ty21a (white squares). After a further 3 days, bladder cell suspensions were prepared and analyzed by IFN-γ ELISPOT assay. Horizontal bars represent geometric mean percent. Significant differences from control mice are indicated by *P < 0.05, **P < 0.01, or ****P < 0.0001.
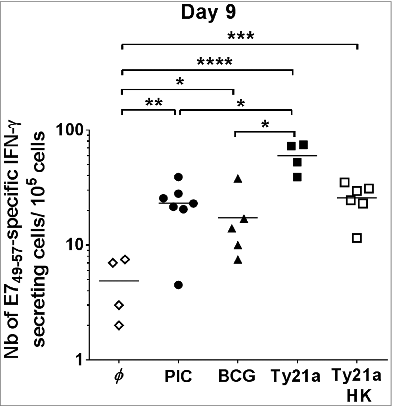
Figure 3. IVES Ty21a after E7 immunization strongly increased the number of T cells in bladder. Groups of naïve (white symbols) or s.c. E7-immunized (black symbols) female C57BL/6 mice received IVES Ty21a 5 days after vaccination in 1 (day 6, black square) or 3 (day 6, 9, and 12, black circles) instillations, or were left untreated (φ, diamonds). Bladder cell suspensions prepared 3 d after the last treatment were analyzed by flow cytometry (A) or by IFN-γ ELISPOT (B) assay. A representative flow cytometric analysis of bladder cells from vaccinated mice is shown in (A) (upper panel) and the percentage of TetE7+CD8+ T cells is indicated in parentheses. Horizontal bars represent geometric mean percent. Significant differences between groups of mice are indicated by *P < 0.05 or **P < 0.01, ***P < 0.001, or ****P < 0.0001.
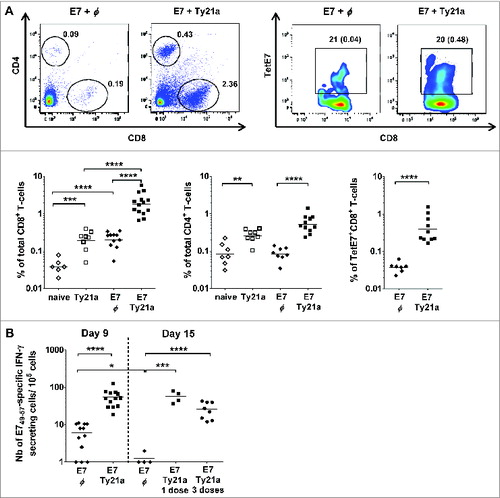
IVES Ty21a in the absence of vaccination induced an approximately 4-fold increase in total CD8 T cells in the bladder. Interestingly, a synergistic effect was observed between local immunostimulation and systemic vaccination with respect to the local recruitment of CD8 T cells; vaccination alone augmented the total number of CD8 T cells in the bladder approximately 3-fold, whereas combination with IVES Ty21a resulted in a 40-fold increase (mean of ∼2% of total CD8 T cells compared to 0.05% in naïve bladder). In contrast to CpG and PIC,Citation9 IVES Ty21a also induced a modest 3-fold increase in CD4 T-cells, either alone or following vaccination (), but once again IVES immunostimulation had no systemic effect (). The increased number of vaccine-specific effector CD8 T cells in bladder was maintained at least until day 15 after a single IVES Ty21a dose (49 ± 8/105 bladder cells at day 9, compared to 55 ± 10 at day 15) and successive IVES Ty21 resulted in a similar outcome (see ).
IVES Ty21a immunostimulation preferentially induced C5a, CXCL5, CXCL2, CCL8, and CCL5
CD8 T cells accumulating in the GM upon IVAG CpG treatment were shown to co-express CXCR3 and CCR5 as well as ESL.Citation9 We first examined the levels of these chemokine receptors and ESL on the T cells that were attracted to the bladder upon IVES CpG or Ty21a (). Our data show that ESL was expressed on approximately 40% and 20% of CD4 and CD8 T cells in the bladder, respectively, and that both frequencies were significantly increased to 50–60% by IVES CpG or IVES Ty21a, suggesting that E-selectin may not be involved in preferential recruitment of CD8 T cells versus CD4 T cells by either IVES CpG or Ty21a in the bladder. CCR5 and CXCR3 were expressed on less than 25% of the CD4 T cells and were not significantly affected by IVES CpG or Ty21a. These chemokine receptors were expressed by approximately 40% of the CD8 T cells, with a slightly increased frequency after IVES CpG although this was not significant (40–55% for CCR5 and 45–55% for CXCR3) and was not affected by Ty21a. In all cases, less than 10% of the CD4 T cells co-expressed CCR5 and CXCR3, whereas IVES CpG slightly increased the frequency of CCR5+CXCR3+CD8 T cells (37% compared to 27% in unstimulated or IVES Ty21-treated bladder), which may account for the modest recruitment of CD8 T cells upon IVES CpG (). This suggests that some CXCR3/CCR5 ligand chemokines (CCL5, CXCL9, CXCL10, and/or CXCL11) may be induced by CpG. Knowledge of chemokine levels in the bladder is limited to chemokine expression data obtained upon repeated IVES instillation of BCG or lactobacillusCitation16 or upon IVES instillation of lipopolysaccharide.Citation17 To gain more insight, we used a chemokine protein array to determine the levels of 25 mouse chemokines 24 h after IVES instillation of phosphate buffered saline (PBS), CpG, or Ty21a (see ). All tested chemokines could be detected in the bladder at variable levels over background (mean ± SD, 8.3 ± 3 pixels in PBS control animals). Interestingly, all of the ligands of CXCR3 and CCR5 were detected at low levels (9 to 12 pixels), with only CXCL10 being increased by IVES CpG (1.8-fold) but not by Ty21a, which is in agreement with the above findings. In addition, CCL6 and CCL9/10 were similarly induced by CpG and Ty21a (1.5- and 4- to 5-fold, respectively), suggesting that these chemokines are not involved in the specific recruitment of CD8 T cells upon IVES Ty21a. In contrast, complement component 5/5a (C5a), CXCL5, and CXCL2a were preferentially increased following Ty21a immunostimulation (1.4- to 2-fold). When considering the effect of IVES Ty21a on vaccination, comparison of the chemokine levels () confirmed an increase in C5a and CXCL5 by IVES Ty21a, and also showed an increase in CCL8 and CCL5 (1.5- to 1.7-fold). We therefore examined the expression of receptors of C5a (C5aR), CXCL5 and CXCL2 (CXCR2), CCL8 and CCL5 (CCR1, for CCR5) on CD4 and CD8 T cells in the bladder (). Our data show that fewer than 10% of either CD4 or CD8 T cells expressed these receptors; this proportion decreased upon IVES Ty21a with the exception of the percentage of C5aR-expressing CD4 T cells, which increased non-significantly (˜2-fold) to 35%. This suggests that the chemokines that are increased by IVES Ty21a may not directly mediate preferential attraction of CD8 T cells to the bladder.
Figure 4. Expression of chemokine receptors and ESL on T cells from bladder upon IVES immunostimulation. Groups of female C57BL/6 mice were immunized s.c. with the E7 vaccine. Five days later, mice were either left untreated (circles) or received IVES CpG (triangles) or Ty21a (squares). Bladder cell suspensions prepared 3 d after immunostimulation were analyzed by flow cytometry. Expression of chemokines and ESL among CD4 T cells (black symbols) or among CD8 T cells (white symbols) is shown. ND: not detectable. Horizontal bars represent geometric mean percent. Significant differences versus control mice are indicated by **P < 0.01, ***P < 0.001, or ****P < 0.0001.
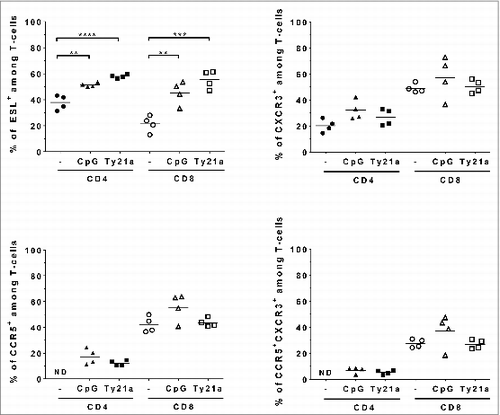
Table 2 Chemokine levelsFootnote in bladder tissue upon IVES immunostimulation
Table 3 Chemokine levelsFootnote in bladder tissue upon vaccination and IVES immunostimulation
Table 4 T cells expressing the indicated chemokine receptor in the bladder of E7-vaccinated mice that were IVES challenged with Ty21a
IVES Ty21a immunostimulation after vaccination significantly improved bladder tumor regression and mouse survival
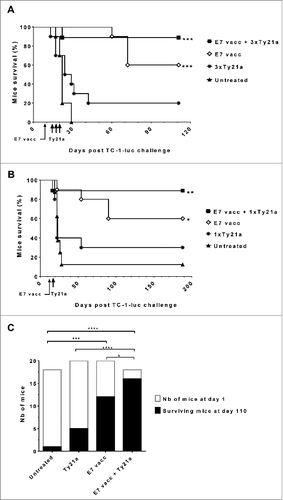
Finally, we evaluated the therapeutic impact of IVES Ty21a immunostimulation on bladder tumor regression. In the first experiment, groups of 10 bladder tumor-bearing mice received 3 consecutive IVES Ty21a doses at days 13, 16, and 19 alone or after vaccination (day 8), vaccination alone, or were left untreated (). Our data show that both vaccination alone and the combinatorial treatment were significantly effective, resulting in 60% and 90% survival respectively (P < 0.01 compared to untreated mice, adjusted log-rank test). In contrast, IVES Ty21a treatment alone was not significantly effective. Because our ELISPOT data showed similar vaccine-specific CD8 T cell recruitment upon a single IVES Ty21a administration, we further tested this protocol in a second experiment. Interestingly, the results roughly paralleled the previous experiment, resulting in 60% survival after vaccination alone and 90% survival with the combinatorial treatment (), confirming that a single IVES Ty21a immunostimulation after vaccination is sufficient to increase antitumor T cells locally and provide almost full tumor regression. Importantly, our data show that, irrespective of the number of doses of IVES Ty21a used, the combinatorial treatment was significantly more efficient than vaccination alone (P < 0.05 by Chi square test, ).
Figure 5. IVES Ty21a immunostimulation after immunization significantly improved bladder tumor regression and mouse survival. Eight groups of 8–10 tumor-bearing mice were either left untreated (black triangles) or received IVES Ty21a (black asterisk, by 3 instillations in A or 1 instillation in B), or s.c. E7 vaccine alone (white diamonds) or followed by Ty21a (black squares, 3 doses in A and 1 dose in B). Mouse survival curves are shown for each treatment (A and B). Significant differences in mouse survival between each treatment and the control are indicated by *P < 0.017, **P < 0.003, and ***P < 0.0003 following adjusted log-rank test. Data from both tumor protection assays were compiled and survival at day 110 is shown in (C). Significant differences between groups are indicated by *P < 0.05, ***P < 0.001, and **** P < 0.0001 following χ2 test.
Discussion
Despite intensive research, immunotherapy of cancer remains highly challenging and combination approaches are becoming more and more appealing to improve clinical outcomes. Using a preclinical model of bladder cancer, we show that IVES immunostimulation with Ty21a bacteria, but not CpG, after tumor antigen vaccination efficiently recruits vaccine-specific CD8 T cells to the bladder, resulting in tumor regression and 90% survival of the mice.
In contrast to the GM, the bladder turned out to be less responsive to the synthetic TLR-9 agonist CpG, at least in terms of induction of chemokines. The modest effect observed on CD8 T-cell recruitment to the bladder was associated with the induction of fewer and different chemokines by CpG in the bladder (1.5- to 4-fold increase in CCL6, CCL9/10, and CXCL10) compared to the GM, in which 10- to 70-fold increases in CCL2, CCL3, CCL4, CXCL2, and CXCL10 were observed 24 h after IVAG CpGCitation18 whereas CCL9 and CCL6 were not affected or only poorly induced (1.6-fold at the RNA level for CCL6Citation19). In contrast to CXCL10, which may be involved in the recruitment of CXCR3-expressing CD8 T cells, CCL6 and CCL9 may attract CCR1-expressing macrophages or dendritic cells as shown in the inflamed lungCitation20 or in the bladder upon IVES BCG treatment.Citation16 This may account for the vaccination-unrelated tumor regression induced by IVES CpG or BCG in the MB49-bladder tumor model,Citation21 as well as by Ty21a (our unpublished data), which also induced CCL6 and CCL9 (). In turn, and in comparison to CpG, Ty21a uniquely induced C5a, CXCL5, and CXCL2, whereas CCL8 and CCL5 are further induced following vaccination. C5a (through the C5aR receptor) and CXCL5/CXCL2 (through the CXCR2 receptor) are primarily chemoattractants for neutrophils,Citation22,23 although they are also involved in T-cell migration to the intestinal mucosaCitation24 and to the brain,Citation25,26 whereas CCL5 and CCL8 can attract monocytes, NK cells, and activated T cells (through CCR5 and CCR1 receptors).Citation27-29 However, our data show a lower or similar percentage of C5aR-, CXCR2-, CCR1-, and CCR5-expressing CD8 T cells in the bladder with or without IVES Ty21a immunostimulation, suggesting an indirect effect of the cognate chemokines on the preferential increase in CD8 T cells in the bladder.
Bacteria were recognized as potential immunotherapeutic treatments against cancer more than 100 years ago by William Coley.Citation30 Later, the association between tuberculosis and a lower frequency of cancer led to use of the BCG vaccine against tuberculosis as a standard IVES treatment for NMIBC.Citation13 In our setting, we showed that IVES BCG after vaccination also increased the number of effector vaccine-specific CD8 T cells in the bladder, which prompted us to design a clinical trial in which vaccination with a tumor vaccine and IVES BCG are combined (NCT01498172 in ClinicalTrials.gov). This currently ongoing trial is aimed to assess the safety of the therapeutic combination as well as potential enhancement of innate and/or vaccine-specific T-cell responses both systemically and locally in the bladder. Our present findings, however, demonstrate the superior efficacy of Ty21a bacteria as an IVES immunostimulant. Both BCG, which are gram-positive bacteria, and Salmonella, which are gram-negative bacteria, can provide TLR-4Citation31,32 and TLR-9Citation33,34 agonists. BCG also contains an agonist of TLR-2, whereas Salmonella may engage TLR-5 through flagellin.Citation35 Interestingly, mucosal application of Salmonella flagellin induced CXCL2 in the lung,Citation36 suggesting that the increase in CXCL2 observed in the bladder upon IVES Ty21a may be mediated by TLR-5. In addition to TLR, it is noteworthy that Salmonellae enterica encode 2 type 3 secretion systems that allow the secretion of effector proteins able to trigger proinflammatory host responses and their injection into host cells.Citation37 It is possible that this secretion system may also contribute to T-cell recruitment in the bladder since live Ty21a were more efficient than heat-killed bacteria, although the TLR-agonist activities of the latter should be similar, or even increased in the case of flagellin.Citation38
NMIBC is the most prevalent form of bladder cancer, which in turn represents the second most common urogenital cancer with a lifetime risk of 1 in 26 for men and 1 in 87 for women in the United States. The high recurrence/progression rate of NMIBC contributes considerably to the high socioeconomic burden associated with the management of these patients.Citation39 Ty21a is a vaccine strain against typhoid fever (Vivotif®) that has been used for decades by the oral route with an excellent safety recordCitation40 and deserves investigation in NMIBC patients. Our data suggest that this vaccine should be added to the list of TLR agonists to be tested in oncologic indications.Citation41
Materials and Methods
Immunization and IVES immunostimulation of mice
C57BL/6 wild-type female mice aged 7 to 10 weeks (Charles River) were used in compliance with ethical directives of the Swiss veterinary authorities. The E734–98 and E749–57 peptides were chemically synthesized by the Protein and Peptide Chemistry Facility of the Institute of Biochemistry (UNIL, Lausanne, Switzerland). Mice were immunized s.c. with the E7 vaccineCitation42 (50 μg E734-98, 0.4 μg Heat Labile enterotoxin, kindly provided by Berna-Biotech, Bern, Switzerland and 10 μg CpG, [#1826; 5′-TCCATGACGT TCCTGACGTT-3′, Coley Pharmaceutical Group]). IVES immunostimulation was performed by urethral catheterization of deeply anesthetized mice using Introcan 24G/3/4 (Braun, Melsungen, Germany) and instillation of 50 μL/mouse of 100 μg CpG, 100 μg PIC (Sigma-Aldrich or Invivogen), 2–8 × 107CFU of BCG (OncoTICE), 1–5 × 108 CFU of attenuated Salmonella enterica serovar Typhi Ty21a bacteriaCitation43 (Berna-Biotech) grown to mid-log phase (OD600 < 0.7) in Luria-Bertani broth (Difco BD).Citation44
Preparation of murine cells
Mice were sacrificed by CO2 inhalation and the spleen and bladder were harvested. Single-cell suspensions were obtained as previously described.Citation45 Briefly, splenocytes were obtained by mechanical dissociation, whereas the bladders were minced and digested stepwise with 0.5 mg/mL thermolysin (Roche) and 1 mg/mL collagenase/dispase (Roche).Citation3 Bone marrow-derived dendritic cells (BMDCs) were isolated using bone marrow cells collected from tibias and femurs of female C57BL/6 mice and cultured in the presence of 150 U/mL recombinant granulocyte-macrophage-colony stimulating-factor (R and D Systems).Citation2
IFN-γ ELISPOT assay
IFN-γ ELISPOT assays were performed as describedCitation45 using Multiscreen-HA 96-well plates (MAHA S4510, Millipore) anti–IFN-γ monoclonal antibody (R4-6A2, Beckton Dickinson PharMingen), biotinylated anti–IFN-γ monoclonal antibody (XMG1.2, Beckton Dickinson PharMingen), and Streptavidin-AP (Roche). In brief, 3 × 104 BMDCs/well, used as antigen-presenting cells, were incubated for 1 h in duplicate with 1 μg/mL of H-2Db restricted E749–57 peptide or medium alone (control wells) before addition of 1 × 105 bladder cells and incubation for 16–24 h. E7-specific responses were defined as the number of IFN-γ spots/105cells in the E7-stimulated wells minus the number of IFN-γ spots/105 cells in the control wells (<3 spots/well).
Tetramer and T-cell labeling
T-cell staining was performed as previously describedCitation2 using phycoerythrin (PE)-conjugated E749–57 and L1165–173 (as a control) H-2Db-restricted tetramers (TetE7 and TetL1, respectively, from TC-Metrix), allophycocyanine (APC)-labeled CD8α (clone 53–6.7) and fluorescein isothiocyanate (FITC)-labeled CD4 (clone RM4-5) (both from eBioscience). TetE7+CD8+ T cell percentages among total cells were calculated after subtraction of the background measured with control TetL1 (<5 events/bladder). Staining for chemokine receptors and ESL was performed using a 10-color flow cytometry protocol as previously described.Citation9 The monoclonal anti-mouse antibodies used were: PE/TXRD-anti-CD45/LCA (I3/2.3), PerCPCy5.5-anti-CD3 (17A2), AlexaFluor700-anti-CD4 (GK1.5), AlexaFluor488-anti-CCR5 (HM-CCR5), PE-Cy7-anti-CXCR3 (CXCR3-173), PE-Cy7-anti-C5aR (20/70) (Biolegend); APC-, PE/TXRD- or PE-Cy7-anti-CD8a (53–6.7, eBioscience); APC-anti-CXCR2 (242216), R and D Systems); PerCPCy5.5-anti CCR1 (C-20, Santa Cruz Biotechnology). For ESL staining, cells were incubated with 2 μg of E-selectin/human IgG-Fc chimera (R and D Systems) and detected with an AlexaFluor647-anti-human IgG Fcγ−specific antibody (Jackson Immunoresearch). Dead cells were excluded by a Live/Dead Fixable Aqua Dead Cell staining kit (Invitrogen, Life Technologies).
Cell acquisition and analysis were performed using FACS Calibur (BD Biosciences) or Gallios Flow Cytometer (Beckman Coulter) and CellQuest Pro software Version 4.0.1 (BD Biosciences) or FlowJo software (Tree Star), respectively.
Orthotopic bladder tumor model
TC-1 cells are primary mouse lung epithelial cells that were transduced with retroviral vectors expressing the oncogenes HaRas and HPV16 E6 and E7.Citation46 TC-1 cells expressing luciferase were then generated by lentiviral infection.Citation47 Bladder tumors were established as previously described.Citation3 Briefly, deeply anesthetized mice were instilled with 2.5 × 105 TC-1–luc cells by urethral catheterization. Tumor growth was monitored by bioluminescence 15 minutes after intraperitoneal injection of D-luciferin (Promega; 150 μg/g of body weight) in the Xenogen imaging system (Xenogen/IVIS Caliper Life Science, kindly provided by the cellular imaging facility, CIF/UNIL, Lausanne, Switzerland).
Chemokine array
Bladders were recovered 24 h after IVES immunostimulation with PBS, CpG, or Ty21a alone, or 5 d after E7 vaccination for Ty21a, and homogenized in 500 μL PBS with protease inhibitors (10 μg/mL aprotinin from bovine lung, 10 μg/mL leupeptin hemisulfate salt, and 10 μg/mL pepstatin A; all from Sigma-Aldrich). TritonX-100 (final concentration 1%, Sigma-Aldrich) was added after homogenization, and after 2 freeze-thaw cycles the samples were centrifuged at 10,000 g for 5 min to remove debris. Protein concentration was assessed using the BCA protein assay (Thermo Scientific). Chemokines were detected using the Proteome Profiler Array: Mouse Chemokine Array kit (R and D Systems), according to the manufacturer's instructions. Briefly, 150 μg of protein (pooled from 3 bladders, 50 μg each) was used for the assay. Detection of chemokine levels was performed using ImageJ software (NIH) and expressed as mean pixel density. Significant detection was determined as greater than the mean + 3 SD of pixel density of negative spots. Differences in the increased chemokine levels between the different conditions were considered significant when greater than or equal to the 99% confidence interval of the mean fold increases examined.
Statistical analysis
Statistical analyses were performed using Prism 5.00 for Windows (GraphPad software). Single comparisons were performed by the Mann-Whitney test or by Student t test after log10 normalization. Multiple comparisons were performed using one-way ANOVA and the Tukey Multiple Comparison Test. Other statistical tests are indicated in the text or figure legends.
Disclosure of Potential Conflicts of Interest
Denise Nardelli Haefliger is an inventor on patent PCT/IB2009/051372: “Method and Vaccine for optimizing the specific immune responses”. The remaining authors declared no conflict of interest.
Funding
This work was supported by the Swiss Cancer League (KFS 2808-08-2011) and the Swiss National Science Foundation (#31003A-135109 and 320030-153201, as well as 310030-130812 to PR).
References
- Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, Spranger S. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol 2013; 25:268-76; PMID:23579075; http://dx.doi.org/10.1016/j.coi.2013.02.009
- Decrausaz L, Domingos-Pereira S, Duc M, Bobst M, Romero P, Schiller JT, Jichlinski P, Nardelli-Haefliger D. Parenteral but not mucosal immunization is able to induce regression of human papillomavirus associated genital tumors. Int J Cancer 2011; 129:762-72; PMID:21384340; http://dx.doi.org/10.1002/ijc.25973
- Domingos-Pereira S, Derré L, Warpelin-Decrausaz L, Haefliger JA, Romero P, Jichlinski P, Nardelli-Haefliger D Intravaginal and subcutaneous immunization induced vaccine-specific CD8 T-cells and tumor-regression in the bladder. J Urol. 2014; 191: 814-22.
- Nardelli-Haefliger D, Dudda JC, Romero P. Vaccination route matters for mucosal tumors. Sci Transl Med 2013; 5:172fs4; PMID:23408051; http://dx.doi.org/10.1126/scitranslmed.3005638
- Sandoval F, Terme M, Nizard M, Badoual C, Bureau MF, Freyburger L, Clement O, Marcheteau E, Gey A, Fraisse G, et al. Mucosal Imprinting of Vaccine-Induced CD8+ T Cells Is Crucial to Inhibit the Growth of Mucosal Tumors. Sci Transl Med 2013; 5:172ra20; PMID:23408053; http://dx.doi.org/10.1126/scitranslmed.3004888
- Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 2012; 491:463-7; PMID:23075848; http://dx.doi.org/10.1038/nature11522
- Matsushima H, Yamada N, Matsue H, Shimada S. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J Immunol 2004; 173:531-41; PMID:15210814; http://dx.doi.org/10.4049/jimmunol.173.1.531
- Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C). J Immunol 2005; 174:992-1002; PMID:15634923; http://dx.doi.org/10.4049/jimmunol.174.2.992
- Domingos-Pereira S, Decrausaz L, Derre L, Bobst M, Romero P, Schiller JT, Jichlinski P, Nardelli-Haefliger D. Intravaginal TLR agonists increase local vaccine-specific CD8 T cells and human papillomavirus-associated genital-tumor regression in mice. Mucosal Immunol 2013; 6:393-404; PMID:22968420; http://dx.doi.org/10.1038/mi.2012.83
- Decrausaz L, Pythoud C, Domingos-Pereira S, Derré L, Jichlinski P, Nardelli-Haefliger D. Intravaginal live attenuated Salmonella increase local anti-tumor vaccine-specific CD8 T clles. Oncoimmunology 2013; 2:e22944; PMID:23483225
- Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 2005; 66:4-34; PMID:16399414; http://dx.doi.org/10.1016/j.urology.2005.07.062
- Ellen A, Schenk-Braat M, Bangma CH. Immunotherapy for superficial bladder cancer. Cancer Immunol Immunother 2005; 54:414-23; PMID:15565330; http://dx.doi.org/10.1007/s00262-004-0621-x
- Herr HW, Morales A. History of bacillus Calmette-Guerin and bladder cancer: an immunotherapy success story. J Urol 2008; 179:53-6; PMID:17997439; http://dx.doi.org/10.1016/j.juro.2007.08.122
- Chevalier MF, Nardelli-Haefliger D, Domingos-Pereira S, Jichlinski P, Derre L. Immunotherapeutic strategies for bladder cancer. Hum Vaccin Immunother 2014; 10:977-81; PMID:24384699
- Decrausaz L, Revaz V, Bobst M, Corthesy B, Romero P, Nardelli-Haefliger D. Induction of human papillomavirus oncogene-specific CD8 T-cell effector responses in the genital mucosa of vaccinated mice. Int J Cancer 2010; 126:2469-78; PMID:19816937
- Seow SW, Rahmat JN, Bay BH, Lee YK, Mahendran R. Expression of chemokine/cytokine genes and immune cell recruitment following the instillation of Mycobacterium bovis, bacillus Calmette-Guerin or Lactobacillus rhamnosus strain GG in the healthy murine bladder. Immunology 2008; 124:419-27; PMID:18217952; http://dx.doi.org/10.1111/j.1365-2567.2007.02792.x
- Saban MR, Hellmich H, Nguyen NB, Winston J, Hammond TG, Saban R. Time course of LPS-induced gene expression in a mouse model of genitourinary inflammation. Physiol Genomics 2001; 5:147-60; PMID:11285368
- Tengvall S, Lundqvist A, Eisenberg RJ, Cohen GH, Harandi AM. Mucosal administration of CpG oligodeoxynucleotide elicits strong CC and CXC chemokine responses in the vagina and serves as a potent Th1-tilting adjuvant for recombinant gD2 protein vaccination against genital herpes. J Virol 2006; 80:5283-91; PMID:16699008; http://dx.doi.org/10.1128/JVI.02013-05
- Lindqvist M, Nookaew I, Brinkenberg I, Samuelson E, Thorn K, Nielsen J, Harandi AM. Unraveling molecular signatures of immunostimulatory adjuvants in the female genital tract through systems biology. PLoS One 2011; 6:e20448; PMID:21666746; http://dx.doi.org/10.1371/journal.pone.0020448
- Rose CE, Jr., Lannigan JA, Kim P, Lee JJ, Fu SM, Sung SS. Murine lung eosinophil activation and chemokine production in allergic airway inflammation. Cell Mol Immunol 2010; 7:361-74; http://dx.doi.org/10.1038/cmi.2010.31
- Mangsbo SM, Nanalga C, Essand M, Loskog A, Totterman TH. CpG therapy is superior to BCG in an otrhotopic bladder cancer model and generates CD4+ T -cell immunity. J Immunother 2008; 31:34-42; PMID:18157010; http://dx.doi.org/10.1097/CJI.0b013e3181587d29
- Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol 2005; 23:821-52; PMID:15771587; http://dx.doi.org/10.1146/annurev.immunol.23.021704.115835
- Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci 2008; 13:2400-7; PMID:17981721; http://dx.doi.org/10.2741/2853
- Ohtsuka Y, Lee J, Stamm DS, Sanderson IR. MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut 2001; 49:526-33; PMID:11559650; http://dx.doi.org/10.1136/gut.49.4.526
- Nataf S, Davoust N, Ames RS, Barnum SR. Human T cells express the C5a receptor and are chemoattracted to C5a. J Immunol 1999; 162:4018-23; PMID:10201923
- Liu YJ, Guo DW, Tian L, Shang DS, Zhao WD, Li B, Fang WG, Zhu L, Chen YH. Peripheral T cells derived from Alzheimer's disease patients overexpress CXCR2 contributing to its transendothelial migration, which is microglial TNF-alpha-dependent. Neurobiol Aging 2010; 31:175-88; PMID:18462836; http://dx.doi.org/10.1016/j.neurobiolaging.2008.03.024
- Gong X, Gong W, Kuhns DB, Ben-Baruch A, Howard OM, Wang JM. Monocyte chemotactic protein-2 (MCP-2) uses CCR1 and CCR2B as its functional receptors. J Biol Chem 1997; 272:11682-5; PMID:9115216; http://dx.doi.org/10.1074/jbc.272.18.11682
- Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med 1998; 338:436-45; PMID:9459648; http://dx.doi.org/10.1056/NEJM199802123380706
- Ruffing N, Sullivan N, Sharmeen L, Sodroski J, Wu L. CCR5 has an expanded ligand-binding repertoire and is the primary receptor used by MCP-2 on activated T cells. Cell Immunol 1998; 189:160-8; PMID:9790730; http://dx.doi.org/10.1006/cimm.1998.1379
- McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J 2006; 26:154-8; PMID:16789469
- Li Q, Cherayil BJ. Role of Toll-like receptor 4 in macrophage activation and tolerance during Salmonella enterica serovar Typhimurium infection. Infect Immun 2003; 71:4873-82; PMID:12933828; http://dx.doi.org/10.1128/IAI.71.9.4873-4882.2003
- Mendez-Samperio P, Belmont L, Miranda E. Mycobacterium bovis BCG Toll-like receptors 2 and 4 cooperation increases the innate epithelial immune response. Arch Med Res 2008; 39:33-9; PMID:18067993; http://dx.doi.org/10.1016/j.arcmed.2007.06.019
- von Meyenn F, Schaefer M, Weighardt H, Bauer S, Kirschning CJ, Wagner H, Sparwasser T. Toll-like receptor 9 contributes to recognition of Mycobacterium bovis Bacillus Calmette-Guerin by Flt3-ligand generated dendritic cells. Immunobiology 2006; 211:557-65; PMID:16920494; http://dx.doi.org/10.1016/j.imbio.2006.05.004
- Magnusson M, Tobes R, Sancho J, Pareja E. Cutting edge: natural DNA repetitive extragenic sequences from gram-negative pathogens strongly stimulate TLR9. J Immunol 2007; 179:31-5; PMID:17579017; http://dx.doi.org/10.4049/jimmunol.179.1.31
- Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol 2001; 167:1882-5; PMID:11489966; http://dx.doi.org/10.4049/jimmunol.167.4.1882
- Honko AN, Mizel SB. Mucosal administration of flagellin induces innate immunity in the mouse lung. Infect Immun 2004; 72:6676-9; PMID:15501801; http://dx.doi.org/10.1128/IAI.72.11.6676-6679.2004
- Hapfelmeier S, Stecher B, Barthel M, Kremer M, Muller AJ, Heikenwalder M, Stallmach T, Hensel M, Pfeffer K, Akira S, et al. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J Immunol 2005; 174:1675-85; PMID:15661931; http://dx.doi.org/10.4049/jimmunol.174.3.1675
- Salamone GV, Petracca Y, Fuxman Bass JI, Rumbo M, Nahmod KA, Gabelloni ML, Vermeulen ME, Matteo MJ, Geffner JR, Trevani AS. Flagellin delays spontaneous human neutrophil apoptosis. Lab Invest 2010; 90:1049-59; PMID:20368700; http://dx.doi.org/10.1038/labinvest.2010.77
- Barocas DA, Globe DR, Colayco DC, Onyenwenyi A, Bruno AS, Bramley TJ, Spear RJ. Surveillance and treatment of non-muscle-invasive bladder cancer in the USA. Adv Urol 2012; 2012:421709; PMID:22645607; http://dx.doi.org/10.1155/2012/421709
- Begier EM, Burwen DR, Haber P, Ball R, Vaccine Adverse Event Reporting System Working G. Postmarketing safety surveillance for typhoid fever vaccines from the Vaccine Adverse Event Reporting System, July 1990 through June 2002. Clin Infect Dis 2004; 38:771-9; PMID:14999618; http://dx.doi.org/10.1086/381548
- Aranda F, Vacchelli E, Obrist F, Eggermont A, Galon J, Sautes-Fridman C, Cremer I, Henrik Ter Meulen J, Zitvogel L, Kroemer G, et al. Trial Watch: Toll-like receptor agonists in oncological indications. Oncoimmunology 2014; 3:e29179; PMID:25083332; http://dx.doi.org/10.4161/onci.29179
- Decrausaz L, Revaz V, Bobst M, Corthésy B, Romero P, Nardelli-Haefliger D. Induction of Human Papillomavirus oncogene-specific CD8 T cell effector responses in the genital mucosa of vaccinated mice. Int J Cancer 2010; 126:2469-78; PMID:19816937
- Germanier R, Fürer E. Isolation and characterization of galE mutant Ty 21a of Salmonella typhi. A candidate strain for a live, oral typhoid vaccine. J Infect Dis 1975; 131:553-8; PMID:1092768; http://dx.doi.org/10.1093/infdis/131.5.553
- Fraillery D, Baud D, Pang SY, Schiller J, Bobst M, Zosso N, Ponci F, Nardelli-Haefliger D. Ty21a expressing Human papillomavirus type 16 L1 as a potential live Salmonella vaccine against cervical cancer and typhoid fever. Clin Vaccine Immunol 2007; 14:1285-95; PMID:17687110; http://dx.doi.org/10.1128/CVI.00164-07
- Revaz V, Debonneville A, Bobst M, Nardelli-Haefliger D. Monitoring of vaccine-specific gamma interferon inductionin in genital mucosa of mice by real-time reverse-transcription-PCR. Clin Vacc Immunol 2008; 5:757-64; PMID:18367582; http://dx.doi.org/10.1128/CVI.00392-07
- Lin KY, Guarnieri FG, Staveleyocarroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class ii presentation of tumor antigen. Cancer Res 1996; 56:21-6; PMID:8548765
- Decrausaz L, Goncalves A-R, Domingos-Pereira S, Pythoud C, Stehle JC, Schiller J, Jichlinski P, Nardelli-Haefliger D. A novel mucosal orthotopic murine model of Human papillomavrius-associated genital cancers. Int J Cancer 2011; 128:2105-13; PMID:20635385; http://dx.doi.org/10.1002/ijc.25561
