Abstract
For cancer vaccines, tumor antigen availability is currently not an issue due to technical advances. However, the generation of optimal immune stimulation during vaccination is challenging. We have recently demonstrated that tumor cell-derived microparticles (MP) can function as a new form of potent cancer vaccine by efficiently activating type I interferon pathway in a cGAS/STING dependent manner.
In response to endogenous or exogenous stimuli, cells change their cytoskeleton, leading to encapsulation of cytosolic and nuclear contents by cellular membrane to form vesicles with 0.1–1 μm in size, called MP, which are subsequently released into the extracellular space.Citation1 Recently, we have demonstrated that tumor cell-derived microparticles (T-MP) can serve as a safe and efficient carrier to deliver chemotherapeutic drugs to tumor cells, whereas their capacity as a novel vaccine platform remains to be determined.Citation2 In another related study, we demonstrated that MPs released by Listeria monocytogenes-infected macrophages could transfer L. monocytogenes antigens to dendritic cells (DCs), leading to the induction of protective adaptive immune responses against the bacteria,Citation3 suggesting that MPs might be employed as a powerful vehicle for antigen delivery and DC targeting. On the basis of those early findings, we wonder whether T-MPs could function as novel cell-free cancer vaccines.
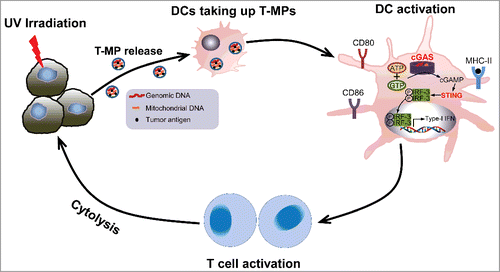
Cancer vaccines are highly unique and attractive approaches to boost our immune system, generating antitumor immunity. Success of tumor vaccines relies on providing both tumor antigens and innate signals to DC, stimulating tumor antigen-specific T cells to recognize and lyse tumor cells. Tumor cells offer a broader repertoire of tumor-associated antigens to reduce the possibility of immune escape and development of resistance. Currently, several irradiated live whole tumor cell vaccines are entering phase I and II clinical trials.Citation4 However, whole tumor cell vaccines seem to lack appropriate innate signals, even if the exogenous GM-CSF gene is incorporated. T-MPs, derivatives of whole tumor cells, cover the similar tumor antigen repertoires, but they are different from whole tumor cells owing to their unique capability of triggering innate activation. T-MPs harbor abundant DNA fragments of both genomic and mitochondrial origins, which are capable of activating type I IFN pathway inside DC.Citation5 Type I interferon recently is highlighted to play a crucial role in the induction of protective antitumor immunity.Citation6 IFN-α is recognized as a powerful inducer of the activation of DCs and IFN-α-conditioned DCs represent promising DC candidates for the development of therapeutic cancer vaccines.Citation7 Moreover, data from pilot clinical trials support the concept of using IFN-α as an enhancer to boost patients' response to cancer vaccines.Citation8 Recent studies identified that cyclic GMP-AMP (cGAMP) synthase (cGAS) as one of the key cytosolic DNA sensors that induces type I IFN production by synthesizing the second messenger cGAMP and subsequently activating TBK1, IRF3 in a STING-dependent manner.Citation9 In our recent study, knocking down either cGAS or STING by siRNAs resulted in the diminution of T-MP-induced type I interferon production by DCs, suggesting that DNA fragments in T-MPs are intrinsic innate signals to stimulate DCs producing type I interferon. This type I IFN is responsible for T-MP-induced DC maturation via upregulation of CD80, CD86, and MHCII.Citation5 In our study, we found that DCs rarely took up apoptotic tumor cells but highly effectively took up T-MPs, probably due to the appropriate size of T-MPs. Following taking up T-MPs, DCs underwent maturation, presented tumor antigen peptides, and stimulated T cell development toward Th1 effector cells. In several different murine tumor models, T-MPs without exception elicited protective immunity against tumor cell challenge. However, these effects were ablated in both CD4+ and CD8+ cell-depleted mice as well as in nude mice, indicating that T-MP-induced antitumor immunity is T cell dependent. Moreover, T-MP-loaded DCs were capable of effectively suppressing the growth of established tumors, although T-MPs by themselves did not show such effect. Furthermore, experiments using T-MPs derived from allogeneic tumor cell lines demonstrated that the shared tumor antigens can be cross-presented by DCs, offering a possibility to simplify clinical application of T-MP-based vaccination therapy. This intrinsic immunogenic property makes T-MPs as an ideal vaccination platform ().
Figure 1. T-MPs function as novel cell-free cancer vaccines. Upon UV irradiation, tumor cells release MPs that contain tumor antigens and DNA fragments. After being taken up by DCs, DNAs of T-MPs induce the expression of type I IFN by activating the cGAS/STING pathway. Type I IFN, in turn, enhances DC maturation by upregulating CD80, CD86, and MHCII. Armed DCs then activate tumor-specific T cells, leading to cytolysis of tumor cells.
Besides MPs, tumor cells also release the extracellular microenvironment membrane vesicles of endosomal origin called exosomes, which are 30–100 nm in size, much smaller than MPs. Intriguingly, although tumor cell-derived exosomes contains tumor antigens expressed in the parental tumor cells, they seem not to be suitable for in vivo immune priming or tumor vaccine design. A number of reports indicate that tumor cell-derived exosomes can confer immune suppression through different mechanisms, raising concerns about the use of exosomes as tumor vaccines.Citation10 Compared with exosomes or tumor lysates, T-MPs generate much better protective immunity, and T-MP loaded onto DC enhanced the infiltration of CD8+ T cells and the production of IFNγ, leading to significant inhibition of tumor growth. Collectively, these findings suggest that although T-MPs and exosomes share common tumor antigens, T-MPs are different from exosomes by their distinct innate activating properties, largely due to the intrinsic DNA signals in T-MPs.
In summary, we demonstrate that T-MPs not only cover tumor antigen spectrums but also carry potential innate signals, making them ideal candidates for developing novel prophylactic and therapeutic cancer vaccines. The identification of DNA component in T-MP to activate cGAS/STING signaling pathway provides a molecular basis for clinical applications of T-MPs. Innate DNA components in T-MPs engage the cGAS/STING pathway for induction of type I IFN, which potently enhances DC maturation, T cells activation and tumor rejection. Interaction between T-MPs and DCs needs to be further delineated in future studies. We have observed that T-MPs accumulated in lysosome of DCs, but how T-MPs release DNAs to trigger type I IFN production is not fully understood. It remains a challenge to unveil how MPs released by tumor cells regulate tumor microenvironment or even host immune system in vivo.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by National Basic Research Program of China (2014CB542100), National Science Fund for Distinguished Young Scholars of China (81225021), National Natural Science Foundation of China (81472653), and the Special Fund of Health Public Welfare Profession of China (201302018).
References
- Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res 2010; 107:1047-57; PMID:21030722; http://dx.doi.org/10.1161/circresaha.110.226456
- Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, Lv M, Li D, Katirai F, Shen GX et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun 2012; 3:1282; PMID:23250412; http://dx.doi.org/10.1038/ncomms2282
- Zhang Y, Zhang R, Zhang H, Liu J, Yang Z, Xu P, Cai W, Lu G, Cui M, Schwendener RA et al. Microparticles released by Listeria monocytogenes-infected macrophages are required for dendritic cell-elicited protective immunity. Cell Mol Immunol 2012; 9:489-96; PMID:23064105; http://dx.doi.org/10.1038/cmi.2012.33
- Chiang CL, Benencia F, Coukos G. Whole tumor antigen vaccines. Semin Immunol 2010; 22:132-43; PMID:20356763; http://dx.doi.org/10.1016/j.smim.2010.02.004
- Zhang H, Tang K, Zhang Y, Ma R, Ma J, Li Y, Luo S, Liang X, Ji T, Gu Z et al. Cell-free tumor microparticle vaccines stimulate dendritic cells via cGAS/STING signaling. Cancer Immunol Res 2015; 3(2):196-205; PMID:25477253; http://dx.doi.org/10.1158/2326-6066
- Schiavoni G, Mattei F, Gabriele L. Type I interferons as stimulators of DC mediated cross-priming: impact on anti-tumor response. Front Immunol 2013; 4:483; PMID:24400008; http://dx.doi.org/10.3389/fimmu.2013.00483
- Rizza P, Moretti F, Capone I, Belardelli F. Role of type I interferon in inducing a protective immune response: perspectives for clinical applications. Cytokine Growth Factor Rev 2014; 26(2):195-201; PMID:25466627; http://dx.doi.org/10.1016/j.cytogfr.2014.10.002
- Di Pucchio T, Pilla L, Capone I, Ferrantini M, Montefiore E, Urbani F, Patuzzo R, Pennacchioli E, Santinami M, Cova A et al. Immunization of stage IV melanoma patients with Melan-A/MART-1 and gp100 peptides plus IFN-alpha results in the activation of specific CD8(+) T cells and monocyte/dendritic cell precursors. Cancer Res 2006; 66:4943-51; PMID:16651452; http://dx.doi.org/10.1158/0008-5472.CAN-05-3396
- Cai X, Chiu YH, Chen ZJ. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell 2014; 54:289-96; PMID:24766893; http://dx.doi.org/10.1016/j.molcel.2014.03.040
- Yang C, Robbins PD. The roles of tumor-derived exosomes in cancer pathogenesis. Clin Dev Immunol 2011; 2011:842849; PMID:22190973; http://dx.doi.org/10.1155/2011/842849
