Abstract
In our previous studies, we have shown that patients with serous ovarian carcinoma in advanced surgical stage disease have a particularly poor prognosis if they carry the HLA-A*02 genotype. This represent a stronger prognostic factor than loss or downregulation of the MHC class I heavy chain (HC) on tumor cells. In this study, we investigated the expression of the non-classical, immune tolerogenic HLA -G and -E on the tumor cells along with the infiltration of immune cells in the tumor microenvironment. FFPE primary tumors from 72 patients with advanced stages of serous adenocarcinoma and metastatic cells present in ascites fluid from 8 additional patients were included in this study. Both expression of HLA-G and aberrant expression of HLA-E were correlated to a significant worse prognosis in patients with HLA-A*02, but not with different HLA genotypes. Focal cell expression of HLA-G correlated to a site-specific downregulation of classical MHC class I HC products and aberrant HLA-E expression, showing a poor survival. HLA-G was more frequently expressed in metastatic cells than in primary tumor lesions and the expression of HLA-G inversely correlated with the frequency of tumor infiltrating immune cells. All these parameters can contribute together to identify and discriminate subpopulations of patients with extremely poor prognosis and can give them the opportunity to receive, and benefit of individually tailored treatments.
Abbreviations
| APC | = | Antigen presenting cells |
| BP | = | better prognosis |
| β2-m | = | β2-microglobulin |
| BSA | = | bovine serum albumin |
| CTL | = | cytotoxic T lymphocyte |
| EOC | = | epithelial ovarian cancer |
| FFPE | = | formalin fixed paraffin-embedded |
| HLA genotype | = | (the constitutional allele characteristics) |
| HR | = | hazard ratio |
| HLA | = | human leukocyte antigen |
| IHC | = | immunohistochemistry |
| FIGO | = | International Federation of Gynecology and Obstetrics |
| MHC | = | major histocompatibility complex |
| HC | = | heavy chain |
| mAb | = | monoclonal antibody |
| NK | = | natural killer cells |
| NKT | = | natural killer T cell |
| HLA-otherwise | = | non-HLA-A*02 |
| PCR | = | polymerase chain reaction |
| TMA | = | Tissue microarray |
| TBS | = | tris buffered saline |
| TA | = | tumor antigen |
| TIL | = | tumor infiltrating lymphocytes |
| WP | = | worst prognosis |
| Treg | = | regulatory T-cell. |
Introduction
Tumor cells and the host's immune system are constantly interacting in a battlefield that we are only beginning to understand. The outcome of the battle is evident in patient's survival but can also partly explain the difference in response to treatment and time to relapse and progression.Citation1 The view is multifactorial, taking the patients genetics, the properties of tumor cells and the composition of the tumor microenvironment into account.
In short, there are three essential phases in host and tumor cells interactions, elimination, equilibrium and escape.Citation2 The innate immune system, such as NK cells, can eliminate incipient malignant cells, but are silenced when tumor cells expresses the non-classical HLA class I antigens such as HLA-G and -E.Citation3 Cytotoxic T lymphocytes (CTL) are dependent on classical MHC class I presentation of tumor antigen (TA) for the induction of a CTL based tumor specific adaptive immune response in the equilibrium phase.Citation4
Tumor infiltrating lymphocytes (TIL) also includes tolerogenic cells such as helper T-lymphocytes (CD4+), and regulatory T-lymphocytes (Tregs).Citation5 Tumor cells that escape from immune surveillance are evolved by immune selection and progress to malignant clones.Citation6 They may also gain other tumor-derived factors facilitating progression and metastasis.Citation7,8
Many groups have tried to initiate immune responses by introducing TAs to specific CTLs as anti-tumor vaccines in development of new therapeutic strategies.Citation9 However, the results of these clinical trials have not been as rewarding as expected. This could be explained by the link between loss of classical HLA class I and tumor progression that has been demonstrated in several clinical studies.Citation10-12
We have studied, previously, the relationship between classical HLA class I expression, HLA genotype and prognosis in patients with ovarian carcinoma.Citation1,13-17 We found that the correlation was only relevant in patients with advanced stage of serous carcinoma and HLA-A*02 genotype.
Our findings are well corroborated by others in several malignant diseases.Citation14,18-20 In addition to the classical MHC class I, there are three genes in close proximity within the class I region on chromosome 6 encoding for the non-classical class I genes, HLA-E, -F and -G. Each is hypothesized to have its unique function in cell-to-cell interaction and immune response.Citation21
Upregulation of HLA-G is one of the more recently described escape mechanisms a tumor can develop.Citation22 HLA-G is a tolerogenic molecule, which means that when expressed it protects the cell from destruction by killers of the immune system.Citation23 It is characterized by a restricted expression in especially fetal cytotrophoblasts, giving the fetus immune privilege from the maternal alloimmune response, but also some other exclusive immune privileged sites such as thymus, cornea, pancreas, erythroid and endothelial precursors.Citation24-26
The expression of the HLA-G gene is regulated at the epigenetic, transcriptional and post-transcriptional levelsCitation27 and HLA-G can be expressed at the cell surface, secreted, or incorporated into tumor-derived exosomes.Citation28,29 Its presence has been demonstrated in various solid tumors and haematological malignancies. In most studies, the increased expression of HLA-G has been linked to a worse clinical outcomeCitation30,31 with exception of improved prognosis in hematological malignancies.Citation22
HLA-G mediates inhibition of immune cell functions both directly and indirectly. Directly by inhibiting the activity of APC, NK and CTL through binding with inhibitory receptors present on immune cellsCitation27 leading to T cell anergy but also by inducing differentiation of CD4+ andCD8+ T cells into suppressor T cells, altering the balance of the immune system from cytotoxic to tolerogenic.
Indirectly, for example, inhibition can be mediated by trogocytosis.Citation32 This is a recently described mechanism where whole fragments of cell membrane including associated molecules such as HLA-G, are transferred from one cell and incorporated into another cells membrane altering the function of the recipient cell. If the recipient cell is an immune cell, it would give auto-signal to stop proliferation and cytotoxic effects. This is a very rapid and effective tumor-driven immune evasion mechanism where tumors dictate the conditions for the immune system in their immediate surroundings.
Indeed, HLA-G expression in tumors is heterogeneous and focal. It has been shown in vitro, that only 10% HLA-G-expressing tumor cells in a tumor are sufficient to protect also HLA-G negative tumor cells from CTL mediated destruction.Citation33
HLA-E interacts directly with receptors on NK cells and inhibits NK cell-mediated lysis.Citation34,35 The role of HLA-E is unique since surface expression requires conserved nonamer peptides from the signal sequences of other MHC class I molecules including HLA-A, -B, -C and -G but not HLA-F. HLA-E is expressed in most human healthy tissues,Citation36 but show a weak surface expression. However, in human tumors it is often up-regulated, especially when classical MHC class I is down-regulated and free β2-m is available, a common situation in malignancies.Citation37
Since the HLA-E/β2-m association is weaker than classical MHC class I/β2-m, HLA-E favors from the relative competition when classical MHC class I is down regulated.Citation37
The effect of HLA-E on NK cells seems to be dependent on the origin of the peptide presented.Citation38,39 Even very small changes in the peptide conformation will affect the recognition by the NK cell receptors and possibly change the effector cell function.Citation40 Leader peptides derived from HLA-G but also HLA-A2 can stabilize the surface expression of HLA-E and thus enhance the inhibition of NK cytolysis.Citation41
The purpose of this study is to determine whether changes in the expression of the non-classical HLA class I HLA-G and -E on tumor cells, and in the repertoire of tumor infiltrating immune cells are associated with the negative prognostic relationship of HLA-A*02 genotype of patients with serous adenocarcinoma of the ovary in stage III–IV.
Results
Patient population
All patients in this study were diagnosed with high-grade serous ovarian carcinoma in advanced surgical stages (). Fifty-four patients were diagnosed in stage III and 18 in stage IV. Forty-two patients carried the HLA-A*02 genotype and thus fulfilled the criteria for worst prognosis grouping. In the HLA-A*02 genotype group 35% were diagnosed in stage IV compared to only 10% in the HLA-otherwise group (p > 0.001). There was also a difference in success ratio of radical surgery between the groups with only seven percent radical surgery in HLA-A*02 patients compared to 20% (p > 0.001) in HLA-otherwise patients. It is also noteworthy that only three patients (7%) received radiotherapy and all of them were HLA-A*02 positive.
Table 1. Patients' characteristics
Non classical MHC class I expression in tumor tissue
Immunohistochemical expression of HLA-G HC was detected in fourteen cases in the total cohort (). They were equally distributed between worst and better prognosis group with seven cases in each group. Thirty-one patients in the worst prognosis group showed aberrant staining for HLA-E, scored as 1 or 3, compared to only 19 in the other group.
Table 2. Cumulative survival according to patients' HLA-A genotype and HLA-G and E tumor cells expresssion
Association of HLA-G expression with patients' survival
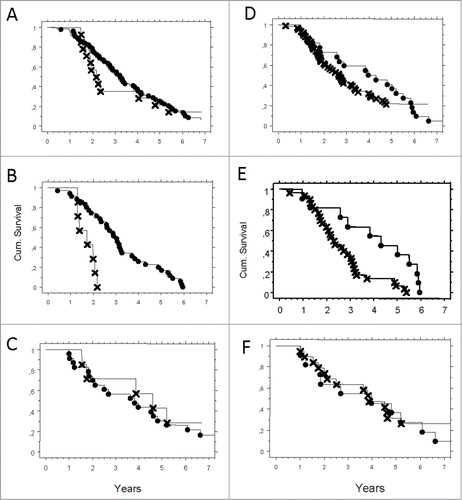
HLA-G expression coincided with lower probability of survival (). Cumulative survival at 5 y was 14% compared to 22% for HLA-G negative cases.
Figure 1. (A–F) Probability of survival correlated to expression of HLA-G and HLA-E, presented by Kaplan–Meier. Pathologic (X) or normal expression (•) in serous adenocarcinoma tumor cells. (A) HLA-G in the total cohort N.S. (B) HLA-G in worst prognosis group (HLA-A*02) p = 0.0003. (C) HLA-G in otherwise group (HLA-OW) N.S. (D) HLA-E in the total cohort p = 0.003. (E) HLA-E in worst prognosis group (HLA-A*02) p = 0.0003. (F) HLA-E in better prognosis group (HLA-OW) N.S.
However, when we correlated HLA-G positivity to the different prognostic subgroups, the impact on survival could only be confirmed in HLA-A*02 genotype patients with zero cumulative survival at 5 y ().
Association of HLA-E expression with patients' survival
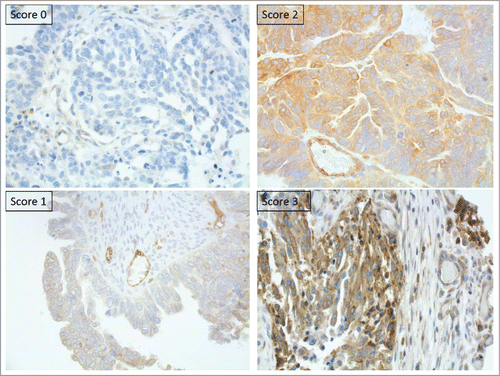
The probability of survival was better when HLA-E staining intensity was equal to normal tissue, score 2. All aberrant expressions showed impaired survival and no difference could be seen between score 0, 1 or 3.
Again, when we stratified for prognostic subgroups, the effect on survival could only be seen in HLA-A*02 genotype patients but not in the other genotypes ()
Correlation of HLA-G and -E expression with that of classical MHC class I in primary tumor lesions
HLA-G positivity was only found in small foci, staining a fraction of the tumor cells. When scrutinizing these small areas for HLA-E and classical MHC class I HC expression we noticed that all but one case showed aberrant HLA-E expression, mainly score 3 and concordant loss of classical MHC class I expression in that specific area. () However, prognosis was only determined by HLA-A*02 genotype. ()
Expression of HLA-G and -E in primary tumor lesions and metastatic cells
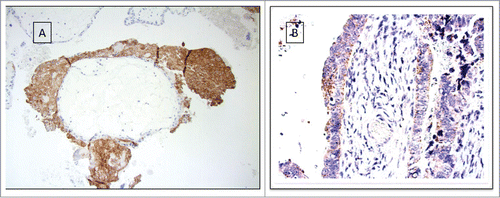
We had access to ascites from eight patients with serous adenocarcinoma in advanced stages and obtained solid tumor tissue from ovarian site at initial debulking surgery. In most cases, we also had solid tumor tissue from distant sites, like omentum, for comparison. In seven out of the eight patients we could detect a positive HLA-G staining pattern in metastatic cells, but not in primary tumor lesions. All accessible metastatic lesions showed aberrant HLA-E expression and all but one patient had aberrant expression of HLA-E in tumor from the primary site. This same patient did not have any detectable HLA-G positive cells in metastatic cells. ()
Table 3. Characteristic of malignant cells obtained from ascites
Tumor infiltrating immune cells
The whole cohort was analyzed for infiltrating CD8+ and FOXp3+ immune cells and correlated to HLA-G and -E expression. The data was further divided for different HLA-A genotypes. Fifty percent (36) of the total cohort had tumor infiltrating CD8+ T cells and 31% (22) had infiltrating FOXp3+ Tregs. Positive HLA-G expression correlated to absence of both types of immune cells. Aberrant HLA-E expression correlated to infiltrating FOXp3 Tregs but not to CD8+ T lymphocytes. HLA-A02* genotype solidified the correlation for HLA-G and lack of immune cells but did not have any significant influence on HLA-E and immune cell correlation. ()
Table 4. Immunocomptentent infiltrating cells
Association of tumor infiltrating immune cells and HLA-G expression with patients' survival
The influence of tumor infiltrating immune cells on survival was entirely dependent on the patients HLA-A*02 status ().
Figure 4. Cumulative survival by Kaplan–Meier analysis. HLA-A*02 patients with HLA-G positive tumor cells and lack of CD8+ lymphocytes (X), compared to HLA-A otherwise, HLA-G negative tumor cells and presence of CD8+ lymphocytes (•);. p = 006.
Patients with HLA-A*02 genotype, positive HLA-G immunohistochemical expression and no detected immune cells had the worst outcome.
Patients with other HLA-genotype, negative for HLA-G and presence of immune cells, especially CD8+, had the best outcome.
Discussion
HLA-G and E are the key modulators of immune responses. They interfere with the actions of CD8+ T cells as well as natural killer (NK) cell cytotoxicity promoting successful tumor escape.Citation22,42
Our previous studies have established that HLA-A*02 genotype is a strong poor prognostic factor in both high grade, serous adenocarcinoma of the ovary and malignant melanoma, however only in advanced stage disease.Citation13,43,Citation44 We have also observed that the presence or absence of HLA-A*02 genotype is the defining factor of the poor prognostic impact of classical MHC class I downregulation. Our conclusion was that HLA-A*02 may orchestrate important aspects of immune escape and immune selection. Our aim in this study was to investigate the correlation between HLA-A*02 and other relevant prognostic features involved in immune recognition and immune tolerance, namely the role played by HLA-G and -E. Our results have definitively shown that the HLA-A*02 genotype dictates the worse prognosis correlated to HLA-G and -E expression. Conflicting findings on the correlation between non-classical MHC class I expression and prognosis in other studies might have several explanations, the most important factor being that the genotype hasn't been tested. However, there are also other mechanisms to take into consideration, such as posttranslational modifications of HLA-G. This might increase HLA-G cell-surface expressions through affinity regulations of peptide loading in the antigen presenting machineryCitation45 giving an effect that is unexpected or not measurable. Function of HLA-G is affected also by the microenvironment in which it is expressed and results between different tumor types and/or studies, should be evaluated in its context.Citation22,28,Citation31
Different results could be related also to the methods used to examine the tumor material.
Different research groups have performed analysis applying diverse antibodies, some of these known to cross-react with classical MHC class I moleculesCitation46 Citation47,48 or not recognizing all seven different isotypes of HLA-G receptors, the four membrane-bound and three soluble that have different biological functions.Citation49 In our study we intentionally used the MEM-G/1 which is a well studied monoclonal antibody that reacts with all isoforms of the HC of the HLA-G molecule. Another possible methodological reason could be related to the use of TMA (tissue micro array)-material. Indeed, in serous adenocarcinoma of the ovary and in other tumor tissues, Often, HLA-G is expressed only focally and consequently important information might therefore be lost in TMA-based cohorts.
We investigated the expression of HLA-G in both solid tumors and ascites from patients in stage III/IV. The intensity of HLA-G staining, distributed in all positive tumors, was focal and relatively weak.
Of great interest is the finding that tumor cells in ascites (considered as metastatic tissue) and/or solid metastatic tumor tissue the number of cases with positive HLA-G staining expression was considerably higher than in solid tumor from the primary site. The presence of HLA-G in the primary tumor tissue studied in our relatively limited cohort indicates that HLA-G is more frequently upregulated in late stage disease. This is in concordance with findings by other groups.Citation50 The positive cells, found in primary tumors, could represent a clone with relatively greater metastatic potential than the surrounding neighbors could. It is reasonable to believe that the HLA-G positive cells modify the microenvironment in favor of progression and metastasis.
This is further supported by reports of HLA-G molecules being transferred to neighbor tumor cells or infiltrating immune cells through “trogocytosis” and the following transformation of immune surveillance to immune tolerance.Citation22
HLA-G has a dual role in the tumor cells due to multiple active structures of HLA-G that adhere to different receptors. There are also reports on HLA-G regulation on vascular remodeling. This might in theory indicate additional effects on tumor growth and metastatic potential.Citation51
HLA-E is, in contrast to HLA-G, expressed in normal tissue and upregulation in tumors is frequently described. However, we also noticed that many samples showed decreased staining. We made use of an evaluation method where the staining intensity of HLA-E in tumor cells was compared to staining intensity of normal cells. We found a correlation to impaired survival for all aberrant staining, both decreased and increased. This confirms results from other studies.Citation30,31. That increased staining implicates an upregulation of HLA-E and thus enhanced inhibition of NK cytolysis is well described.Citation49 However, loss of HLA-E expression also seems to inflict a worse OS. Some authors speculate that a HLA-E as well as HLA-G can play dual roles in cancer, but these still needs to be elucidated.Citation52
We also found correlation between expression of HLA-G, increased staining intensity of HLA-E and loss of classical MHC class I expression in the HLA-A*02 population. This subgroup had a very poor prognosis of approximately 18 months. Our results support the finding that HLA-E benefits from classical MHC class I downregulation in the competition for β2-m (38). It also countenances the notion that the surface expression of HLA-E is stabilized by HLA-G derived peptides. (42–43)Citation53
The most important finding in our study is that the impaired effect on survival for both HLA-G and –E expression was dependent on HLA-A*02 genotype. The importance of the HLA-A*02 genotype was also supported by the influence of tumor infiltrating immune cells.
In conclusion, serous ovarian cancer is a deadly disease with poor outcome in general. Most patients receive indiscriminate treatment. In this study, we introduce the analysis of HLA-G and –E, in relation to the classical MHC class I expression, T-cell infiltration and HLA genotype as determinant prognostic variables. Consequently, we emphasize the need of a multifactorial approach in the care of these patients. When genetics, histology, surgical stage, tumor microenvironment and molecular alterations are put together, it is possible to discriminate subpopulations and identify patients with particular poor prognosis. This gives the opportunity to adjust, actually, the treatment individually. Further studies with focus on the genetics and epigenetics are necessary to understand the mechanisms and find leads toward new therapeutic strategies.
Material and Methods
Patients
Out from our previous study of 162 patients admitted to the Gynecological Oncology Unit at the Karolinska University Hospital Department of Oncology between 1995 and 2004, we extracted a specific subgroup of 72 patients with serous adenocarcinoma and advanced surgical stage. Patient information and histopathology samples were accessed with approval of the Regional Ethic Committee in Stockholm #2007/1:5# and #2003-507#. The diagnosis of epithelial ovarian cancer (EOC) of serous type was confirmed by histopathology of formalin fixed paraffin-embedded (FFPE) tumor specimens. Grade (high or low), date of diagnosis and International Federation of Gynecology and Obstetrics (FIGO) stage (I–IV) were recorded and re-evaluated. HLA genotype and analysis of classical HLA class I HC were already performed in the previous study.Citation13
Patients groups
In previous findings we identified a subgroup of patients with particularly poor prognosis, namely, serous adenocarcinoma, surgical stage III–IV and HLA-A*02 genotype. We called this the worst prognosis group. In this study, we extracted all patients with EOC of serous histology in advanced surgical stage and then grouped them by HLA-A genotype into HLA-A*02 genotype (worst prognosis) or HLA-A of other genotypes (better prognosis).
DNA extraction and HLA genotyping
DNA analysis was used for HLA genotyping. The DNA was extracted from peripheral blood mononuclear cells from blood samples from consented patients or from FFPE material from deceased patients. The methods were described previously.Citation17
Collection of ascitic fluid for metastatic cells
To investigate a possible correlation between HLA-G and -E expression and metastatic potential we analyzed ascitic fluid from eight patients with advanced cancer disease. The ascites was collected during initial debulking surgery. All patients were diagnosed with EOC of serous histology. Only two of the patients carried the HLA-A*02 genotype. From the liquid, cells were harvested by centrifugation (800 g, 10 min, followed by two washing steps at 450 g for 8 min) and cryopreserved until further analysis.
Immunophenotyping of thawed tumor cells was performed by flow cytometry and confirmed by IHC on formalin fixed paraffin embedded (FFPE) cell pellets of the same sample. We also collected corresponding FFPE solid tumors for immunohistochemical comparison. In available cases, we collected tumor tissue from both primary sites, defined as the site with the greatest tumor mass and from distant lesions fulfilling the criteria of at least stage III disease.
Monoclonal antibodies for immunohistochemistry and flow cytometry
The monoclonal antibodies MEM-E/02 and MEM-G/1 are specific and recognize the HC of HLA-E and HLA-G of human origin.Citation27,54 HC10 and HCA have been described in detail previously.Citation55 Citation43
The mAb anti-CD8+ (Clone C8/144B (Dako) reacts with the cytoplasmic domain of the CD8+ domain of α-chain of the CD8+ molecule expressed by cytotoxic T cells as well as thymocytes and NK-cells. Anti-FOX3P is a specific marker of natural T regulatory cells (nTregs).
The following antibodies were used for flow cytometry: HLA-A2 (BB7.2, Fitc, BD), EpCAM (EBA-1, PerCP-Cy5.5, BD), HLA-E (3D12HLA-E, PE, eBioscience), HLA-G (87G, PerCP-eFluor710, ebioscience), Her2 (Neu 24.7 PE, BD), MHCI (W6/32, Fitc, BD), MHCII (G46–6, Fitc, BD).
Immunohistochemical staining and analysis
Tissue sections were stained according to protocol previously describedCitation43
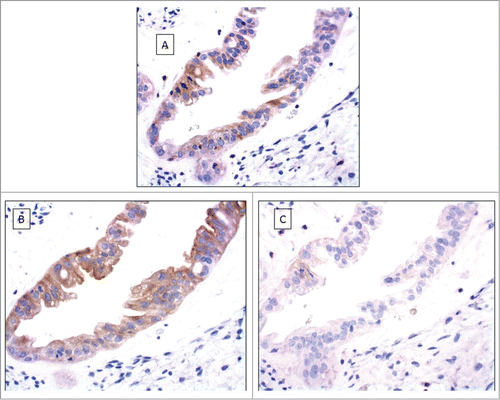
Human placenta was used as positive control. The stained slides were evaluated for the presence or absence of HLA-G expression scored as positive or negative. All of the positive cases were only weakly positive and stained only a small fraction of tumor cells (Fig. S1).
HLA-E was evaluated as absent, less intense than, equal to or more intense than normal tissue using score 0-3. Score 2 was regarded as normal expression whereas 0.1 and 3 were regarded as aberrant. Since all lesions showed a very heterogeneously staining pattern each slide was given two scores, the first for the dominating cell population and the second for the subordinate cell population (Fig. S2). The quantification of intratumoural CD8+ T cells were counted in high power fieldsCitation56 and divided into four scoring groups; 0 = 0 T cells, 1 = ≤5 T cells, 2 = 6–19 T cells and 3 = ≥20 T cells. T regs were recorded as present or absent.
Flow cytometry
Extracellular staining was performed in 96-well v-bottom plate for 20 min at 4 degrees. Washing and antibody dilution was carried out using PBS + 1% human serum albumin (HSA, Octopharma, Stockholm, Sweden). Cells were acquired using an LSRII flow cytometer (BD Biosciences, San Jose, USA) and analyzed using FlowJo analysis software (Treestar, Ashland, USA).
Statistics
The χ2 test was used to examine patient characteristics for discrete categorical variables or factors. Time to death from any cause was defined as the primary end point in the study. Survival time for deceased patients was calculated using the date of first diagnosis to the date of death. For surviving patients, the survival time was calculated from the date of first diagnosis to the date of the last clinical follow-up. HLA-A*02 genotype was scored as 1 and HLA-otherwise as 0. Cumulative survival plots and time-to-event Kaplan-Meier curves were constructed with the log-rank test applied to detect univariate differences between groups. Diagnostic data were collected during the year 1995–2004 and were censored at 2013. All analyses were performed with program StatView for Windows, SAS Institute Inc.. Version 5.0.1.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
KONI_A_1052213_supplementary_material.zip
Download Zip (12.5 MB)Acknowledgments
The statistics was performed under the supervision of Dr. Hemming Johansson, Dept. of Oncology-Karolinska University Hospital, Stockholm, Sweden: [email protected]. We thank: Mrs Inger Bodin, Mr Martin Tagel and the personnel at the Pathology Unit for assistance with the histological samples.
Funding
This study was supported by grants from the Cancer Society in Stockholm and the King Gustaf V Jubilee Fund and the Swedish Cancer Society, the Karolinska Institute/Stockholm County ALF grant and the Deutsche Forschungsgemeinschaft GRK 1591 (DFG) Se-585-10-1 (BS) and the Deutsche Krebshilfe (BS).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Anderson ARA, Weaver AM, Cummings PT, Quaranta V. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell 2006; 127:905-15; PMID:17129778; http://dx.doi.org/10.1016/j.cell.2006.09.042
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004; 22:329-60; PMID:15032581; http://dx.doi.org/10.1146/annurev.immunol.22.012703.104803
- Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat Rev Cancer 2012; 12:210-9; PMID:22362216; http://dx.doi.org/10.1038/nrc3186
- Serrano A, Castro-Vega I, Redondo M. Role of gene methylation in antitumor immune response: implication for tumor progression. Cancers 2011; 3:1672-90; PMID:24212778; http://dx.doi.org/10.3390/cancers3021672
- Janikashvili N, Bonnotte B, Katsanis E, Larmonier N. The dendritic cell-regulatory T lymphocyte crosstalk contributes to tumor-induced tolerance. Clin Dev Immunol 2011; 2011:14; http://dx.doi.org/10.1155/2011/430394
- Paschen A, Méndez RM, Jimenez P, Sucker A, Ruiz-Cabello F, Song M, Garrido F, Schadendorf D. Complete loss of HLA class I antigen expression on melanoma cells: a result of successive mutational events. Int J Cancer 2003; 103:759-67; http://dx.doi.org/10.1002/ijc.10906
- Campoli M, Ferrone S. Tumor escape mechanisms: potential role of soluble HLA antigens and NK cells activating ligands. Tissue Antigens 2008; 72:321-34; PMID:18700879; http://dx.doi.org/10.1111/j.1399-0039.2008.01106.x
- Browning M, Dunnion D. HLA and cancer: implications for cancer immunotherapy and vaccination. Eur J Immunogenet 1997; 24:293-312; PMID:9306099; http://dx.doi.org/10.1111/j.1365-2370.1997.tb00025.x
- Sioud M. Does our current understanding of immune tolerance, autoimmunity, and immunosuppressive mechanisms facilitate the design of efficient cancer vaccines? Scand J Immunol 2009; 70:516-25; PMID:19906192; http://dx.doi.org/10.1111/j.1365-3083.2009.02326.x
- Garrido F, Cabrera T, Aptsiauri N. “Hard” and “soft” lesions underlying the HLA class I alterations in cancer cells: implications for immunotherapy. Int J Cancer 2010; 127:249-56; PMID:20178101; http://dx.doi.org/10.1002/ijc.25270#
- Seliger B. Molecular mechanisms of MHC class I abnormalities and APM components in human tumors. Cancer Immunol Immunother 2008; 57:1719-26; PMID:18408926; http://dx.doi.org/10.1007/s00262-008-0515-4
- Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol 2006; 90:1-50; PMID:16730260; http://dx.doi.org/10.1016/S0065-2776(06)90001-7
- Gamzatova Z, Villabona L, van der Zanden H, Haasnoot GW, Andersson E, Kiessling R, Seliger B, Kanter L, Dalianis T, Bergfeldt K et al. Analysis of HLA class I-II haplotype frequency and segregation in a cohort of patients with advanced stage ovarian cancer. Tissue Antigens 2007; 70:205-13; PMID:17661908; http://dx.doi.org/10.1111/j.1399-0039.2007.00875.x
- Helgadottir H, Andersson E, Villabona L, Kanter L, van der Zanden H, Haasnoot GW, Seliger B, Bergfeldt K, Hansson J, Ragnarsson-Olding B et al. The common Scandinavian human leucocyte antigen ancestral haplotype 62.1 as prognostic factor in patients with advanced malignant melanoma. Cancer Immunol Immunother 2009; 58:1599-608; PMID:19214504; http://dx.doi.org/10.1007/s00262-009-0669-8
- De Petris L, Bergfeldt K, Hising C, Lundqvist A, Tholander B, Pisa P, van der Zanden HG, Masucci G. Correlation between HLA-A2 gene frequency, latitude, ovarian and prostate cancer mortality rates. Med Oncol 2004; 21:49-52; PMID:15034213; http://dx.doi.org/10.1385/MO:21:1:49
- Norell H, Carlsten M, Ohlum T, Malmberg KJ, Masucci G, Schedvins K, Altermann W, Handke D, Atkins D, Seliger B et al. Frequent loss of HLA-A2 expression in metastasizing ovarian carcinomas associated with genomic haplotype loss and HLA-A2-restricted HER-2/neu-specific immunity. Cancer Res 2006; 66:6387-94; PMID:16778217; http://dx.doi.org/10.1158/0008-5472.CAN-06-0029
- Villabona L, Leon Rodriguez DA, Andersson EK, Seliger B, Dalianis T, Masucci GV. A novel approach for HLA-A typing in formalin-fixed paraffin-embedded-derived DNA. Mod Pathol 2014; 27:1296-305; PMID:24504073; http://dx.doi.org/10.1038/modpathol.2013.210
- So T, Takenoyama M, Sugaya M, Yasuda M, Eifuku R, Yoshimatsu T, Osaki T, Yasumoto K. Unfavorable prognosis of patients with non-small cell lung carcinoma associated with HLA-A2. Lung Cancer 2001; 32:39-46; PMID:11282427; http://dx.doi.org/10.1016/S0169-5002(00)00204-X
- Rogentine CN, Jr., Dellon AL, Chretien PB. Prolonged disease-free survival in bronchogenic carcinoma associated with HLA-Aw19 and HLA-B5. A two-year prospective study. Cancer 1977; 39:2345-7; PMID:872033; http://dx.doi.org/10.1002/1097-0142(197706)39:6%3c2345::AID-CNCR2820390605%3e3.0.CO;2-W
- Tisch M, Kyrberg H, Weidauer H, Mytilineos J, Conradt C, Opelz G, Maier H. Human leukocyte antigens and prognosis in patients with head and neck cancer: results of a prospective follow-up study. Laryngoscope 2002; 112:651-7; PMID:12150518; http://dx.doi.org/10.1097/00005537-200204000-00011
- Menier C, Saez B, Horejsi V, Martinozzi S, Krawice-Radanne I, Bruel S, Le Danff C, Reboul M, Hilgert I, Rabreau M et al. Characterization of monoclonal antibodies recognizing HLA-G or HLA-E: new tools to analyze the expression of nonclassical HLA class I molecules. Hum Immunol 2003; 64:315-26; PMID:12590976; http://dx.doi.org/10.1016/S0198-8859(02)00821-2
- Rouas-Freiss N, Moreau P, LeMaoult J, Carosella ED. The dual role of HLA-G in cancer. J Immunol Res 2014; 2014:359748; PMID:24800261; http://dx.doi.org/10.1155/2014/359748
- Crisa L, McMaster MT, Ishii JK, Fisher SJ, Salomon DR. Identification of a thymic epithelial cell subset sharing expression of the class Ib HLA-G molecule with fetal trophoblasts. J Exp Med 1997; 186:289-98; PMID:9221758; http://dx.doi.org/10.1084/jem.186.2.289
- Le Discorde M, Moreau P, Sabatier P, Legeais JM, Carosella ED. Expression of HLA-G in human cornea, an immune-privileged tissue. Hum Immunol 2003; 64:1039-44; PMID:14602233; http://dx.doi.org/10.1016/j.humimm.2003.08.346
- Cirulli V, Zalatan J, McMaster M, Prinsen R, Salomon DR, Ricordi C, Torbett BE, Meda P, Crisa L. The class I HLA repertoire of pancreatic islets comprises the nonclassical class Ib antigen HLA-G. Diabetes 2006; 55:1214-22; PMID:16644675; http://dx.doi.org/10.2337/db05-0731
- Menier C, Rabreau M, Challier JC, Le Discorde M, Carosella ED, Rouas-Freiss N. Erythroblasts secrete the nonclassical HLA-G molecule from primitive to definitive hematopoiesis. Blood 2004; 104:3153-60; PMID:15284117; http://dx.doi.org/10.1182/blood-2004-03-0809
- Carosella ED, Moreau P, Le Maoult J, Le Discorde M, Dausset J, Rouas-Freiss N. HLA-G molecules: from maternal-fetal tolerance to tissue acceptance. Adv Immunol 2003; 81:199-252; PMID:14711057; http://dx.doi.org/10.1016/S0065-2776(03)81006-4
- Rouas-Freiss N, Moreau P, Menier C, LeMaoult J, Carosella ED. Expression of tolerogenic HLA-G molecules in cancer prevents antitumor responses. Semin Cancer Biol 2007; 17:413-21; PMID:17881247; http://dx.doi.org/10.1016/j.semcancer.2007.07.003
- Riteau B, Faure F, Menier C, Viel S, Carosella ED, Amigorena S, Rouas-Freiss N. Exosomes bearing HLA-G are released by melanoma cells. Hum Immunol 2003; 64:1064-72; PMID:14602237; http://dx.doi.org/10.1016/j.humimm.2003.08.344
- Kleinberg L, Florenes VA, Skrede M, Dong HP, Nielsen S, McMaster MT, Nesland JM, Shih Ie M, Davidson B. Expression of HLA-G in malignant mesothelioma and clinically aggressive breast carcinoma. Virchows Arch 2006; 449:31-9; PMID:16541284; http://dx.doi.org/10.1007/s00428-005-0144-7
- Rouas-Freiss N, Moreau P, Ferrone S, Carosella ED. HLA-G proteins in cancer: do they provide tumor cells with an escape mechanism? Cancer Res 2005; 65:10139-44; PMID:16287995; http://dx.doi.org/10.1158/0008-5472.CAN-05-0097
- Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol 2003; 4:815; PMID:12942076; http://dx.doi.org/10.1038/ni0903-815
- Carosella ED, Favier B, Rouas-Freiss N, Moreau P, Lemaoult J. Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood 2008; 111:4862-70; PMID:18334671; http://dx.doi.org/10.1182/blood-2007-12-127662
- Tomasec P, Braud VM, Rickards C, Powell MB, McSharry BP, Gadola S, Cerundolo V, Borysiewicz LK, McMichael AJ, Wilkinson GW. Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science 2000; 287:1031; PMID:10669413; http://dx.doi.org/10.1126/science.287.5455.1031.
- Lee N, Llano M, Carretero M, Ishitani A, Navarro F, Lopez-Botet M, Geraghty DE. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci U S A 1998; 95:5199-204; PMID:9560253; http://dx.doi.org/10.1073/pnas.95.9.5199
- Wei XH, Orr HT. Differential expression of HLA-E, HLA-F, and HLA-G transcripts in human tissue. Hum Immunol 1990; 29:131-42; PMID:2249951; http://dx.doi.org/10.1016/0198-8859(90)90076-2
- Marin R, Ruiz-Cabello F, Pedrinaci S, Mendez R, Jimenez P, Geraghty DE, Garrido F. Analysis of HLA-E expression in human tumors. Immunogenetics 2003; 54:767-75; PMID:12618909
- Hoare HL, Sullivan LC, Clements CS, Ely LK, Beddoe T, Henderson KN, Lin J, Reid HH, Brooks AG, Rossjohn J. Subtle changes in peptide conformation profoundly affect recognition of the non-classical MHC class I molecule HLA-E by the CD94-NKG2 natural killer cell receptors. J Mol Biol 2008; 377:1297-303; PMID:18339401; http://dx.doi.org/10.1016/j.jmb.2008.01.098
- Braud VM, Allan DS, O'Callaghan CA, Soderstrom K, D'Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 1998; 391:795-9; PMID:9486650; http://dx.doi.org/10.1038/35869
- Malmberg KJ, Levitsky V, Norell H, de Matos CT, Carlsten M, Schedvins K, Rabbani H, Moretta A, Soderstrom K, Levitskaya J et al. IFN-gamma protects short-term ovarian carcinoma cell lines from CTL lysis via a CD94/NKG2A-dependent mechanism. J Clin Invest 2002; 110:1515-23; PMID:12438449; http://dx.doi.org/10.1172/JCI0215564
- Marchesi M, Andersson E, Villabona L, Seliger B, Lundqvist A, Kiessling R, Masucci GV. HLA-dependent tumour development: a role for tumour associate macrophages? J Transl Med 2013; 11:247; PMID:24093459; http://dx.doi.org/10.1186/1479-5876-11-247
- Pietra G, Romagnani C, Manzini C, Moretta L, Mingari MC. The emerging role of HLA-E-restricted CD8+ T lymphocytes in the adaptive immune response to pathogens and tumors. J Biomed Biotechnol 2010; 2010:907092; PMID:20634877; http://dx.doi.org/10.1155/2010/907092
- Andersson E, Villabona L, Bergfeldt K, Carlson JW, Ferrone S, Kiessling R, Seliger B, Masucci GV. Correlation of HLA-A02* genotype and HLA class I antigen downregulation with the prognosis of epithelial ovarian cancer. Cancer Immunol Immunother 2012; 61:1243-53; PMID:22258792; http://dx.doi.org/10.1007/s00262-012-1201-0
- Gamzatova Z, Villabona L, Dahlgren L, Dalianis T, Nillson B, Bergfeldt K, Masucci GV. Human leucocyte antigen (HLA) A2 as a negative clinical prognostic factor in patients with advanced ovarian cancer. Gynecol Oncol 2006; 103:145-50; PMID:16542716; http://dx.doi.org/10.1016/j.ygyno.2006.02.004
- Park B, Lee S, Kim E, Chang S, Jin M, Ahn K. The truncated cytoplasmic tail of HLA-G serves a quality-control function in post-ER compartments. Immunity 2001; 15:213-24; PMID:11520457; http://dx.doi.org/10.1016/S1074-7613(01)00179-0
- Gooden M, Lampen M, Jordanova ES, Leffers N, Trimbos JB, van der Burg SH, Nijman H, van Hall T. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8+ T lymphocytes. Proc Natl Acad Sci 2011; 108:10656-61; PMID:3127933; http://dx.doi.org/10.1073/pnas.1100354108
- Polakova K, Kuba D, Russ G. The 4H84 monoclonal antibody detecting beta2m free nonclassical HLA-G molecules also binds to free heavy chains of classical HLA class I antigens present on activated lymphocytes. Hum Immunol 2004; 65:157-62; PMID:14969770; http://dx.doi.org/10.1016/j.humimm.2003.10.005
- Polakova K, Bennink JR, Yewdell JW, Bystricka M, Bandzuchova E, Russ G. Mild acid treatment induces cross-reactivity of 4H84 monoclonal antibody specific to nonclassical HLA-G antigen with classical HLA class I molecules. Hum Immunol 2003; 64:256-64; PMID:12559628; http://dx.doi.org/10.1016/S0198-8859(02)00777-2
- Kochan G, Escors D, Breckpot K, Guerrero-Setas D. Role of non-classical MHC class I molecules in cancer immunosuppression. Oncoimmunology 2013; 2:e26491; PMID:24482746; http://dx.doi.org/10.4161/onci.26491
- Lin A, Chen HX, Zhu CC, Zhang X, Xu HH, Zhang JG, Wang Q, Zhou WJ, Yan WH. Aberrant human leucocyte antigen-G expression and its clinical relevance in hepatocellular carcinoma. J Cell Mol Med 2010; 14:2162-71; PMID:19799650; http://dx.doi.org/10.1111/j.1582-4934.2009.00917.x
- McCormick J, Whitley GS, Le Bouteiller P, Cartwright JE. Soluble HLA-G regulates motility and invasion of the trophoblast-derived cell line SGHPL-4. Hum Reprod 2009; 24:1339-45; PMID:19223288; http://dx.doi.org/10.1093/humrep/dep026
- Morandi F, Airoldi I, Pistoia V. IL-27 driven upregulation of surface HLA-E expression on monocytes inhibits IFN-gamma release by autologous NK cells. J Immunol Res 2014; 2014:938561; PMID:24741633; http://dx.doi.org/10.1155/2014/938561
- Clements CS, Kjer-Nielsen L, Kostenko L, Hoare HL, Dunstone MA, Moses E, Freed K, Brooks AG, Rossjohn J, McCluskey J. Crystal structure of HLA-G: a nonclassical MHC class I molecule expressed at the fetal-maternal interface. Proc Natl Acad Sci U S A 2005; 102:3360-5; PMID:15718280; http://dx.doi.org/10.1073/pnas.0409676102
- Menier C, Prevot S, Carosella ED, Rouas-Freiss N. Human leukocyte antigen-G is expressed in advanced-stage ovarian carcinoma of high-grade histology. Hum Immunol 2009; 70:1006-9; PMID:19660509; http://dx.doi.org/10.1016/j.humimm.2009.07.021
- Perosa F, Luccarelli G, Prete M, Favoino E, Ferrone S, Dammacco F. Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol 2003; 171:1918-26; PMID:12902494; http://dx.doi.org/10.4049/jimmunol.171.4.1918
- Han LY, Fletcher MS, Urbauer DL, Mueller P, Landen CN, Kamat AA, Lin YG, Merritt WM, Spannuth WA, Deavers MT et al. HLA class I antigen processing machinery component expression and intratumoral T-Cell infiltrate as independent prognostic markers in ovarian carcinoma. Clin Cancer Res 2008; 14:3372-9; PMID:18519766; http://dx.doi.org/10.1158/1078-0432.CCR-07-4433