Abstract
Peritoneal carcinomatosis (PC) is a metastatic disease of primary tumors localized in the abdomen. Although this disease is considered a terminal condition, recent treatments combining surgery with heated intra-peritoneal chemotherapy (HIPEC) significantly increase patient survival. We have determined that the protective effect of HIPEC is partially linked to the induction of an efficient anticancer immune response.
Abdominal organs are covered and protected by a transparent membrane called the peritoneum. Primary tumors of the digestive tract such as colorectal and ovarian cancer frequently metastasize to this membrane and cause PC. PC was for long considered as a terminal disease, with a median survival of 24 mo and treated palliatively.Citation1 Treatment consisting of surgery with hyperthermia has been combined with high doses of chemotherapy (HIPEC, Hyper-thermic intra-peritoneal chemotherapy), and the combination therapy significantly increases the overall survival of selected PC patients to 63 mo.Citation1
Our understanding of how HIPEC can protect PC patients is still rudimentary. It was originally hypothesized that the protection was mediated by the ability of HIPEC to kill tumor cells. The cell killing resulted in eradication of cancerous cells from the abdominal cavity and led to local control of cancer progression. However, several studies have indicated that the way the tumor cells die directly influences the response of the immune system.Citation2 We hypothesize the protective effect of HIPEC could be partially mediated by its ability to kill tumor cells in an immunogenic way, causing an efficient anticancer immune response.Citation3
The current HIPEC procedure combines high doses of chemotherapy (mitomycin c, Mc for PC of colorectal origin) with hyperthermia (heat shock, HS). The peritoneal plasma barrier limits the systemic exposure to chemotherapy. The barrier reduces the systemic toxicity and permits the intraperitoneal administration of higher doses of chemotherapy than the maximum tolerated dose for intravenous administration. There are no studies investigating the respective role of HS, Mc or HIPEC (HS + Mc) on cell death and anticancer immune response. Therefore, we established an in vitro model of HIPEC using the murine colon carcinoma cell line CT26. This cell line is syngeneic to Balb/c mice. CT26 cells were killed with Mc or in HIPEC conditions and then incubated with dendritic cells (DCs) derived from bone marrow Balb/c monocytes. We found that both Mc and HIPEC treatments caused a similar activation of DCs. We then verified that in both cases, the activated DCs could activate T cells in a tumor antigen-dependent manner.Citation4 We next performed vaccination experiments to determine whether the Mc or HIPEC-induced T cell activation was sufficient to induce an efficient anticancer immune response.Citation4 Syngeneic immuno-competent Balb/c mice were subcutaneously injected with PBS or with either Mc- or HIPEC-treated CT26 cells into one flank. The mice were then challenged by injecting viable CT26 cells into the other flank 1 week later. The mice injected with either Mc- or HIPEC-treated CT26 cells exhibited a vigorous antitumor immune response in vivo under conditions that led the control mice to develop tumors within 10 d after challenge. After re-challenge with live tumor cells, the vaccinated mice did not develop tumors for 40 d. These findings indicate the establishment of a permanent antitumor immune response.
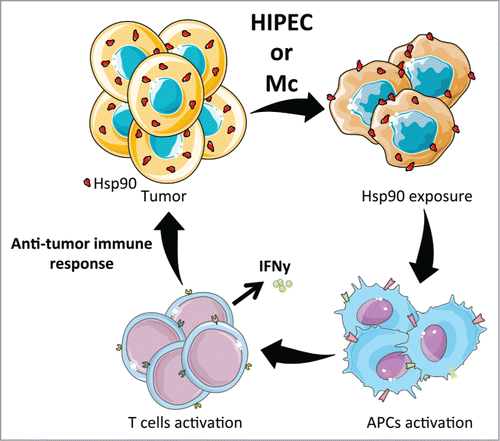
To address how Mc or HIPEC treatment caused antitumor vaccination, we investigated the role of Heat Shock Proteins (HSPs). HSPs are cytosolic proteins that can be exposed to the plasma membrane in very specific conditions. Once exposed at the membrane, these proteins participate in the anticancer immune response.Citation5,6 We used HSP inhibitors and blocking antibodies to further demonstrate that Mc- and HIPEC-mediated antitumor vaccination was mediated by HSP90 exposure on the cell surface of the dying cells (Fig. 1).Citation3
The main insights of our work include (i) the concept that Mc and HIPEC are equivalent in the way they induce an anticancer immune response. Knowing that the addition of HIPEC to cytoreductive surgery increases the risk of morbidity and mortality,Citation7 the favorable effect of hyperthermia over the high doses of chemotherapy used in this procedure worth being investigated in patients. (ii) Several HSP90 inhibitors are currently in oncological clinical trials.Citation8 These inhibitors might sensitize tumor cells to chemotherapy. However, they also might have negative effects on the induction of an anticancer immune response. This possibility should be carefully considered during the development of inhibitors. Finally, (iii) we demonstrate that HIPEC induces an efficient anticancer immune response. We think this result is an important discovery because it may represent the rationale for using immuno-modulating approaches in the treatment of PC. If patients relapse after HIPEC procedures, their therapeutic options are very limited. Our work suggests that the combination of HIPEC with immunotherapy might increase the anticancer immune response and enhance overall survival. For example, we highlight the chimeric antibody catumaxomab because it binds to three different types of cells: tumor cells, T cells and antigen presenting cells (APCs). The effects of Catumaxomab were tested in PC patients. The antibody was safe and well tolerated.Citation9 Therefore, combining immunotherapy with HIPEC could improve patient survival. However, because hyperthermia may destroy the antibody, such immunotherapy should be given after the HIPEC procedure is completed.
In conclusion, the results of this study should inspire further research into new alternative treatments for PC patients.
Figure 1. HIPEC and Mc induce an anticancer immune response. Both HIPEC and Mc treatments lead to HSP90 exposition on the cell surface of dying tumor cells. The exposure of HSP90 promotes DCs and T cells activation and induces an immune response against cancer cells. Figures were created using Servier Medical Art (www.servier.com).
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.
References
- Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F, Classe JM, Ferron G, Guilloit JM, Meeus P, Goéré D et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 2009; 27:681-5; PMID:19103728; http://dx.doi.org/10.1200/JCO.2008.19.7160
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31:51-72; PMID:23157435; http://dx.doi.org/10.1146/annurev-immunol-032712-100008
- Zunino B, Rubio-Patino C, Villa E, Meynet O, Proics E, Cornille A, Pommier S, Mondragón L, Chiche J, Bereder JM et al. Hyperthermic intraperitoneal chemotherapy leads to an anticancer immune response via exposure of cell surface heat shock protein 90. Oncogene 2015; PMID:25867070; http://dx.doi.org/10.1038/onc.2015.82
- Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 2014; 3:e955691; PMID:25941621; http://dx.doi.org/10.4161/21624011.2014.955691
- Murshid A, Gong J, Calderwood SK. The role of heat shock proteins in antigen cross presentation. Front Immunol 2012; 3:63; PMID:22566944; http://dx.doi.org/10.3389/fimmu.2012.00063
- Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood 2007:4839-45; PMID:17299090; http://dx.doi.org/10.1182/blood-2006-10-054221
- Klaver YL, Hendriks T, Lomme RM, Rutten HJ, Bleichrodt RP, de Hingh IH. Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery for peritoneal carcinomatosis in an experimental model. Br J Surg 2010; 97:1874-80; PMID:20806291; http://dx.doi.org/10.1002/bjs.7249
- Kim YS, Alarcon SV, Lee S, Lee MJ, Giaccone G, Neckers L, Trepel JB. Update on Hsp90 inhibitors in clinical trial. Curr Top Med Chem 2009; 9:1479-92; PMID:19860730; http://dx.doi.org/10.2174/156802609789895728
- Goere D, Gras-Chaput N, Auperin A, Flament C, Mariette C, Glehen O, Zitvogel L, Elias D. Treatment of gastric peritoneal carcinomatosis by combining complete surgical resection of lesions and intraperitoneal immunotherapy using catumaxomab. BMC Cancer 2014; 14:148; PMID:24589307; http://dx.doi.org/10.1186/1471-2407-14-148
