Abstract
In a recent phase I clinical trial, a vaccine consisting of glypican-3 (GPC3)-derived CTL epitopes was found to be safe and induced measurable immune and clinical responses in patients with hepatocellular carcinoma (HCC). The aim of this study was to identify GPC3-derived long peptides (GPC3-LPs) carrying promiscuous HLA class II-restricted T helper (Th) cell epitopes. Using a computer algorithm, we predicted GPC3-LPs that can bind to promiscuous HLA class II molecules. Their antigenicity for induction of specific CD4+ T cells in healthy donors or patients with HCC, before and after vaccination with GPC3-SPs, was proven by IFNγ enzyme-linked immunospot assays. Natural processing of these epitopes was confirmed by the immune response of helper T cells to dendritic cells (DCs) loaded with GPC3 proteins. Cross-presentation capacity was assessed in vitro using human DCs and LPs encapsulated in liposomes and in vivo in HLA-A2 transgenic mice (Tgm). All five LPs could induce Th1 cells and were presented by several frequently occurring HLA class II molecules in vitro. Four of them were likely to be naturally processed. One of the LPs encapsulated in liposomes was well cross-presented in vitro; it cross-primed CTLs in HLA-A2 Tgm. LP-specific and HLA class II-restricted CD4+ T-cell responses were observed in 14 of 20 HCC patients vaccinated with GPC3-SPs. Repeated vaccinations enhanced GPC3-LP-specific responses in 8 of 13 patients with HCC. Moreover, the presence of the specific Th cell was correlated with prolonged overall survival (OS). GPC3-LPs can be useful for cancer immunotherapy.
Abbreviations
| APCs | = | antigen-presenting cells |
| DC | = | dendritic cell |
| ELISPOT | = | enzyme-linked immunospot assay |
| GM-CSF | = | granulocyte macrophage colony-stimulating factor |
| GPC3 | = | glypican-3 |
| HCC | = | hepatocellular carcinoma |
| HLA | = | human histocompatibility leukocyte antigen |
| IEDB | = | Immune-Epitope Database |
| IL | = | interleukin |
| LP | = | long peptide |
| mAb | = | monoclonal antibody |
| OS | = | overall survival |
| PBMCs | = | peripheral blood mononuclear cells |
| SP | = | short peptide |
| Tgm | = | transgenic mice |
| Th | = | CD4+ helper T cells |
| Th1 | = | T-helper type 1. |
Introduction
Current treatments of advanced HCC are at best minimally effective; the multikinase inhibitor sorafenib, considered the first-line treatment in patients with HCC,Citation1 extends the life span by only 3 months.Citation2 The efficacy against HCC recurrence after a liver transplant is weak,Citation3 and a high incidence of adverse drug reactions is observed in elderly patients.Citation4 These observations underscore the need for novel therapies for HCC, and combination therapies including an immunotherapy may fit the bill.
Tumor-associated antigens (TAA), especially cancer testis or oncofetal antigens, which are recognized by adaptive immune system, can be used as immunotherapeutic targets. One of such novel oncofetal antigens is membrane-bound heparan sulfate proteoglycan GPC3 previously identified by us using genome-wide cDNA microarray analysis;Citation5 GPC3 is overexpressed in HCCCitation6 and promotes the growth of such tumors through stimulating canonical Wnt signaling.Citation7 A phase I clinical trial of cancer immunotherapy for advanced HCC using two short peptides (SPs), A2-GPC3144–152 (A2-GPC3-SP) and A24-GPC3298–306 (A24-GPC3-SP),Citation8,9 showed that these peptide vaccines were well tolerated, induced measurable immune responses, and exhibited some antitumor efficacy. The clinical trial also showed that high frequency of GPC3-specific CTLs was correlated with prolonged OS in patients vaccinated with GPC3-SPs. A phase II clinical trial of adjuvant cancer immunotherapy (using the two above-mentioned GPC3-SPs) is underway for patients with HCC who received curative surgical treatment.
Inoculation with a GPC3-SP emulsified with incomplete Freund's adjuvant (IFA) could elicit a peptide-specific CTL response in patients;Citation8 however, OS was not convincingly prolonged. One possibility is that GPC3-SP can bind directly to HLA class I molecules expressed not only on professional antigen-presenting cells (APCs) but also on all nucleated cells; therefore, SPs may induce T-cell tolerance or anergy.Citation10,11 To overcome this drawback, an extended long peptide (LP) consisting of more than 23 amino acid residues can be used.Citation12 LPs may form a tertiary structure that may protect from exopeptidase-mediated degradation and are too long to be presented directly by HLA class I molecules; thus, they are internalized and cross-presented by professional APCs such as DCs obligated to be recognized by CTLs.Citation13
Depletion of CD4+ T cells abrogates protective immune response induced by a vaccine,Citation14 and an adoptive transfer therapy with CD4+ T cells specific to NY-ESO-1 antigen was shown to induce antitumor protection.Citation15 Moreover, induction of HIV-1-specific CD4+ T-cell responses augments HIV-specific CD8+ T-cell reactivity in patients with HIV-1 infection.Citation16
T helper type 1 (Th1) cells play a pivotal role in enhancing CTL-mediated antitumor immunity.Citation17 Th1 cells also help to induce and maintain lasting immune responses through multiple interactions with CTLs.Citation18 Direct antitumor or anti-angiogenic effects were also reported to be mediated by IFNγ-secreting Th1 cells.Citation19 Furthermore, Th1 cells make it possible for CTLs to enter a tumor site.Citation20 Therefore, for induction of effective antitumor immunity, it is important to identify the epitopes that can activate tumor-specific Th1 cells.
After injection of an LP, DCs can take up the LP, process it, and present all possible epitopes in the context of various HLA class I and class II molecules.Citation21 In line with this finding, GV1001, a promiscuous telomerase-derived Th cell epitope vaccine, increases the survival of cancer patients when it is combined with radiotherapy and chemotherapy.Citation22,23 Given the important role of both CD8+ and CD4+ T cells in antitumor immunity, an LP encompassing CTL epitope(s) may be regarded as a promising component of a vaccine.Citation21
In this study, we used a computer algorithm for prediction of HLA class II-binding GPC3-derived peptides and utilized this information for selection of promising promiscuous Th cell epitopes. We demonstrated that the predicted GPC3-LPs triggered Th1 responses in healthy donors carrying several frequent HLA-DR and DP alleles. GPC3-LPs-specific Th cell responses were observed in the majority of HCC patients vaccinated with GPC3-SPs, and prolonged OS was observed in patients with Th cell response.
Results
Prediction and selection of possible promiscuous HLA class II-binding GPC3-LPs
We selected five GPC3-LPs; GPC392–116 (LP1), GPC3137–161 (LP2), GPC3289–313 (LP3), GPC3386–412 (LP4), and GPC3556–576 (LP5), with overlapping high-consensus percentile ranks for multiple HLA class II molecules encoded by DPB1*05:01, DRB1*07:01, DRB1*08:03, DRB1*09:01, DRB1*13:02, or DRB1*15:02 alleles (See “Materials and Methods,” and Fig. S1). Two regions, GPC3-LP2 and GPC3-LP3, were identified proximal to known 9- or 10-mer CTL epitopes recognized by HLA-A2- or A24-restricted CTLs ( and Fig. S1B) and predicted to have high binding affinity to HLA class II molecules. The other three LPs (GPC3-LP1, GPC3-LP4, and GPC3-LP5) were predicted to have high binding affinity to HLA class II molecules but did not include known CTL epitope sequences.
Table 1. Identification of glypican-3 (GPC3)-derived and promiscuous HLA class II-restricted CD4+ T-cell epitopes encompassing cytotoxic T lymphocyte (CTL) epitopes
Identification of promiscuous GPC3-derived Th cell epitopes
To examine the immunogenicity of candidate peptide, CD4+ T cells isolated from PBMCs of healthy donors (HD; Genotypes of the HDs were given in and Table S1) were stimulated with GPC3-LPs at weekly intervals. After at least 3 rounds of stimulation we found GPC3-LP1-specific and IFNγ producing cells were induced from healthy donor HD10 (DRB1*07:01/13:02/DR53/DR52) and, healthy donor HD5 (DRB1*04:05/09:01/DR53) and these Th cell responses were restricted by HLA-DR ().
Figure 1. Induction of GPC3-LP-specific Th cells from healthy donors (HDs). (A–E) GPC3-specific Th cells were generated from healthy donors by stimulating isolated CD4+ T cells with GPC3-LPs as indicated. The generated Th cells were restimulated with autologous PBMCs pulsed with GPC3-LPs. The number of IFNγ-producing Th cells was analyzed using ELISPOT. Representative data from at least three independent experiments (all yielded similar results) are shown. The HLA class II genotype of the donor is indicated at the top of the panels. The underlined HLA class II alleles encode an HLA class II molecule presenting the peptides to Th cells from . The underlined mAb inhibited the Th cell response. (A) HLA-DR-restricted GPC3-LP1-specific Th cells were generated from PBMCs of an HLA-DRB1*07:01/13:02+ healthy donor (HD10, left panel) and from PBMCs of an HLA-DRB1*04:05/09:01+ healthy donor (HD5, right panel). (B) HLA-DR-restricted GPC3-LP2-specific Th cells were generated from PBMCs of HD10 (upper left panel), an HLA-DRB1*08:03/14:05+ healthy donor (HD4, lower left panel), and an HLA-DRB1*09:01/14:54+ healthy donor (HD11, lower right panel). HLA-DP-restricted GPC3-LP2-specific Th cells were generated from PBMCs of an HLA-DPB1*02:01/04:02+ healthy donor (HD5, upper right panel). (C) HLA-DR-restricted GPC3-LP3-specific Th cells were generated from PBMCs of HD10 (left panel) and HD5 (right panel). (D) HLA-DR-restricted GPC3-LP4-specific Th cells were generated from PBMCs of an HLA-DRB1*08:02/15:02+ healthy donor (HD3, left panel) and HD10 (right panel). (E) HLA-DR-restricted GPC3-LP5-specific Th cells were generated from HD10 (left panel) and HD5 (right panel).
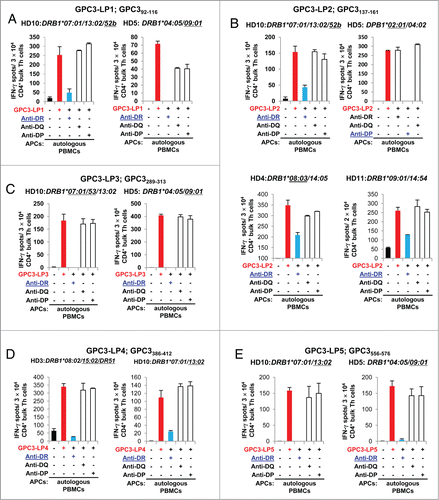
Figure 2 (See previous page). Exact identification of restriction HLA class II molecules of GPC3-specific Th cells. (A–E) GPC3-specific Th cells were generated from healthy donors (HDs) by stimulating magnetic bead-isolated CD4+ T cells with GPC3-LPs as shown in . The generated Th cells from healthy donors were then re-stimulated with autologous PBMCs, allogeneic PBMCs, or HLA class II-expressing L cells pulsed with individual GPC3 LPs. The number of IFNγ-producing cells was analyzed using an ELISPOT. Representative data from at least two independent experiments (yielded similar results) are shown. The HLA class II genotype of the donor is indicated at the top of the panels. The underlined HLA class II alleles encode HLA class II molecules presenting the peptides to Th cells. The underlined mAb inhibited the Th cell response. (A) HLA-DR52b- and DR9-restricted GPC3-LP1-specific Th cells were generated from PBMCs of healthy donors HD10 (left panel) and HD5 (right panel). (B) HLA-DR52b- and DP2-restricted GPC3-LP2-specific Th cells were generated from PBMCs of HD10 (left panel) and HD5 (right panel). (C) HLA-DR7/53- and DR9-restricted GPC3-LP3-specific Th cells were generated from PBMCs of HD10 (upper panel) and HD5 (lower panel). (D) HLA-DR15/51- and DR13-restricted GPC3-LP4-specific Th cells were generated from PBMCs of healthy donors HD3 (left panel) and HD10 (right panel). (E) HLA-DR13- and DR9-restricted GPC3-LP5-specific Th cells were generated from PBMCs of HD10 (left panel) and HD5 (right panel).
By doing similar experiments we are also able to generate GPC3-LP2 (), LP3 ( and Fig. S2A), LP4 () and LP5 ()-specific and IFNγ producing cells. GPC3-LP2-iduced Th cells were derived from HD3, HD4, HD5, HD10, and HD11. GPC3-LP3-iduced Th cells were derived from HD5, HD10, and HD11. GPC3-LP4-induced Th cells were derived from HD3 and HD10. GPC3-LP5-induced Th cells were derived from HD5 and HD10. GPC3-LP2-induced Th cells derived from HD5 were restricted by HLA-DP. Other GPC3-LPs-induced Th cells were restricted by HLA-DR. All the HLA allelic products that can present these five peptides are summarized in the . These peptides can be applicable to more than 70 % of the Japanese population (Table S2).
Exact identification of restriction HLA class II molecules of GPC3-specific Th cells
The bulk GPC3-LP1-specific Th cells from healthy donor HD10 (DRB1*07:0/13:02/DR53/DR52) specifically recognized RM3-DR52b cells () pulsed with GPC3-LP1 but not GPC3-LP1-pulsed L-DR7, L-DR13, L-DR53, and L-DR52a. The other bulk GPC3-LP1-specific Th cells derived from healthy donor HD5 (DRB1*04:05/09:01/DR53) specifically recognized L-DR9 cells pulsed with GPC3-LP1 but not respond to GPC3-LP1-pulsed L-DR4 or L-DR53 (). These results indicated that GPC3-LP1 was presented at least by HLA-DR52b and HLA-DR9.
To identify the restriction HLA class II molecule of the bulk GPC3-LP2-specific T cells that were derived from healthy donor HD10 (DRB1*07:0/13:02/DR53/DR52), we generated a Th cell clone (Th-clone). Th-clone specifically recognized the GPC3-LP2-pulsed RM3-DR52b (HLA-DRB3*02:02) and allogeneic PBMCs from two HLA-DR13+DR7− healthy donors but did not respond to L-DR7, L-DR13 and L-DR52a ( and S2B). These results suggested that GPC3-LP2 could be presented by HLA-DR52b.
The Th-clone from GPC3-LP2-specific T cells that were generated from healthy donor HD5 (DPB1*02:01/04:02) specifically recognized L-DP2 cells and allogeneic PBMCs carrying a shared HLA-DP2 molecule (pulsed with GPC3-LP2) but not RM3-DP4 cells or allogeneic PBMCs negative for HLA-DP2 ( and S2C). We confirmed that GPC3-LP2 induced HLA-DP2-restricted Th cells. GPC3-LP2 generated HLA-DR8 (DRB1*08:03)-restricted Th cells; this result was assessed using both allogeneic PBMCs and L-cell transfectant serving as APCs (Fig. S2D and S2E). Thus, GPC3-LP2 appeared to be a promiscuous Th cell epitope presented by several frequently occurring HLA class II moleculesCitation24,25 including HLA-DR52b, HLA-DP2, HLADR8, HLA-DR9/14, and HLA-DR8/15 ().
Because the GPC3-LP3-specific bulk Th cells derived from healthy donor HD10 (DRB1*07:0/13:02/DR53/DR52) did not recognize allogeneic GPC3-LP3-pulsed PBMCs isolated from 2 HLA-DR13+ healthy donors (HD7 and HD9) and HLA-DR52 is linked with HLA-DR13 allele, we concluded that GPC3-LP3 generated HLA-DR7- or DR53-restricted Th cells in HD10 (). The GPC3-LP3-specific bulk Th cells from healthy donor HD5 (DRB1*04:05/09:01/DR53) were specifically activated by L-DR9 cells pulsed with GPC3-LP3 in an HLA-DR-dependent manner but not GPC3-LP3-pulsed L-DR4 or L-DR53 cells (), indicating that GPC3-LP3 was presented by HLA-DR9.
We established a GPC3-LP4-reactive Th-clone from bulk Th cells generated from healthy donor HD3 (DRB1*08:02/15:02). We then used allogeneic PBMCs as APCs to determine restriction by shared HLA-DR molecules. We assessed that GPC3-LP4 generated HLA-DR15- or DR51-restricted Th-clone (). We also found that GPC3-LP4 induced HLA-DR13-restricted Th cells () from healthy donor HD10 (DRB1*07:01/13:02/DR53 /DR52) because this Th-clone specifically recognized L-DR13 but not L-DR7 pulsed with GPC3-LP4.
GPC3-LP5-reactive Th-clone from healthy donor HD10 (DRB1*07:01/13:02/DR53 /DR52) could recognize L-DR13 () but not L-DR7, L-DR53, L-DR52a, or RM3-DR52b cells pulsed with GPC3-LP5. Another GPC3-LP5-reactive Th-clone from healthy donor HD5 (DRB1*04:05/09:01/DR53) could recognize L-DR9 but not L-DR4 or L-DR53 cells pulsed with GPC3-LP5. Thus, we suggest that GPC3-LP5 generated HLA-DR13- and HLA-DR9-restricted Th cells ().
GPC3-LPs-induced Th cells secrete Th1 type cytokines in response to stimulation with the cognate peptide
For the characterization of the Th cells reactive to the GPC3-LPs, we measured various cytokines secreted by the Th cells in response to stimulation with the cognate peptide-pulsed autologous PBMCs. GPC3-LP1-, GPC3-LP2- and, GPC3-LP4-specific Th cell clones that were derived from healthy donor HD10 produced a large amount of IFNγ, TNF-α, IL-2, GM-CSF, and MIP1β after re-stimulation with cognate peptides; this finding suggested that GPC3-LPs have the ability to induce Th cells with Th1 polarization characteristics (Fig. S3).
Possible natural processing and presentation of GPC3-LPs by DCs
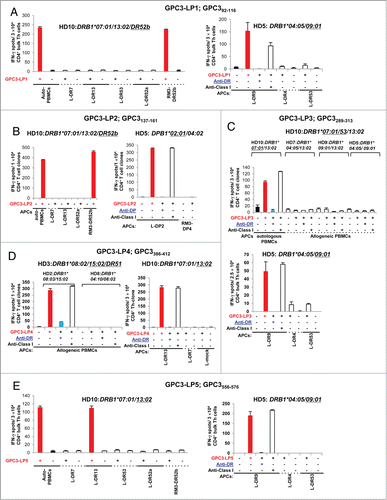
We proceeded to test whether DCs take up and process the GPC3 protein to stimulate GPC3-LP-specific Th cells that were generated by stimulation with synthetic LPs. DCs that were loaded with the recombinant GPC3 protein were used as APCs in IFNγ ELISPOT.Citation26,27 Four GPC3-LPs (GPC3-LP1, −2, −4, and −5)-reactive Th cell clones derived from healthy donor HD10 (DRB1*07:01/13:02/DR53/DR52) efficiently recognized DCs loaded with the GPC3 protein but did not recognize control protein-loaded DCs, indicating that these epitopes could be naturally processed from the GPC3 protein ().
Figure 3. Natural processing and presentation of GPC3-LPs by DCs loaded with the recombinant human GPC3 protein. (A) An HLA-DR52b (HLA-DRB3*02:02)-restricted and GPC3-LP1-specific Th clone that was derived from healthy donor HD10 recognized autologous DCs loaded with the recombinant human GPC3 protein. Representative data from two independent experiments that were performed in duplicate (yielded similar results) are shown. (B) An HLA-DR52b-restricted GPC3-LP2-specific Th clone that was derived from HD10 recognized autologous DCs loaded with the recombinant human GPC3 protein. (C) An HLA-DR13-restricted and GPC3-LP4-specific Th clone that was derived from HD10 recognized autologous DCs loaded with the recombinant human GPC3 protein. Representative data from three independent experiments that were performed in duplicate (all yielded similar results) are shown. (D) An HLA-DR13-restricted and GPC3-LP5-specific Th cell clone that was derived from healthy donor HD10 recognized autologous DCs loaded with the recombinant human GPC3 protein.
In vitro cross-presentation of SPs by human DCs loaded with GPC3-LP2
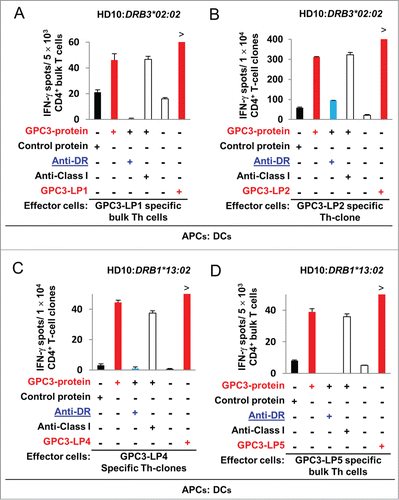
We evaluated the ability of GPC3-LP2 to stimulate A2-GPC3-SP-specific CTLs by means of IFNγ ELISPOTs as described in Materials and Methods. As shown in , A2-GPC3-SP-specific bulk CTLs that were derived from an HLA-A2+ donor specifically produced IFNγ in response to stimulation with DCs loaded with GPC3-LP2 encapsulated in liposomes but not DCs loaded with liposomes plus GPC3-LP or control LP encapsulated in liposomes. The specific IFNγ production was markedly inhibited by the anti-HLA class I mAb but not by the anti-HLA-DR mAb, suggesting that the A2-GPC3-SP-reactive CTLs were stimulated through the cross-presentation of GPC3-LP2 by DCs in vitro.
Figure 4. DCs induced efficient cross-presentation of GPC3-LP2 to A2-GPC3144–152-SP-specific and HLA-A2-restricted CTLs in vitro and cross-priming in HLA-A2 Tgm in vivo. (A) A2-GPC3144–152-SP-specific CTLs that were derived from healthy donor HD5 (HLA-A2+ and HLA-DP2+) were stimulated in vitro with autologous DCs pulsed with GPC3-LP2 encapsulated in liposomes (Lip-GPC3-LP2), IMP3507–527-LP encapsulated in liposomes (Lip-control LP), liposomes plus soluble GPC3-LP2 (Lip + GPC3-LP2), or liposomes alone (Lip). Representative data of three independent experiments (all yielded similar results) are shown. (B–C) HLA-A2 Tgm was immunized with A2-GPC3144–152-SP (A2-GPC3-SP-IFA-PBS), GPC3-LP2 (LP2-IFA-PBS), or PBS emulsified in incomplete Freund's adjuvant (IFA; IFA-PBS). Seven days after the second immunization, murine CD4+/CD8+ T cells were isolated from the pooled inguinal lymph nodes and were stimulated ex vivo with BMDCs pulsed with GPC3-LP2 or GPC3-LP5 (control LP) and A2-GPC3144–152-SP, A2-CDCA1-SP, or A2-HIV-SP. The numbers of IFNγ-producing murine CD4+/CD8+ T cells were assessed using an ex vivo ELISPOT. Representative data from 2∼4 independent experiments (2–3 mice in each group) that were performed in duplicate or triplicate (all yielded similar results) are shown. (B) GPC3-LP2 immunization induced an enhanced SP-specific CTL response in comparison with GPC3-A2-SP immunization in vivo when an equimolar dose of the peptide was used. (C) An immune response of GPC3-LP2-specific CD4+ Th cells isolated from the same pooled inguinal lymph nodes.
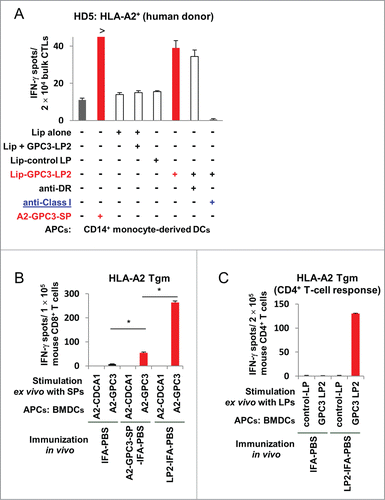
An in vivo cross-priming assay using HLA-A2 Tgm
The ability of GPC3-LP2 to prime A2-GPC3-SP-specific CTLs was examined using HLA-A2 Tgm. The CD8+ T cells from HLA-A2 Tgm that were vaccinated with GPC3-LP2 produced IFNγ specifically in response to ex vivo stimulation with bone marrow DCs pulsed with the A2-GPC3-SP (). These results suggest that after uptake of GPC3-LP2, APCs can cross-prime A2-GPC3-SP-specific CTLs in vivo in HLA-A2 Tgm. In the isolated CD8+ cells, the number of A2-GPC3-SP-specific CTLs as estimated by an IFNγ ELISPOT was increased in the mice immunized with GPC3-LP2 compared to mice immunized with A2-GPC3-SP ().
The CD4+ T cells that were isolated from HLA-A2 Tgm immunized with GPC3-LP2 produced IFNγ specifically in response to stimulation with murine BMDCs pulsed with the GPC3-LP2 () but not with the control (GPC3-LP5). These results suggested that GPC3-LP2 could also prime GPC3-LP2-specific and probably I-Ab-restricted Th cells in vivo in HLA-A2 Tgm.
The number of GPC3-LP-specifc CD4+ T cells is increased in HCC patients vaccinated with GPC3-SPs
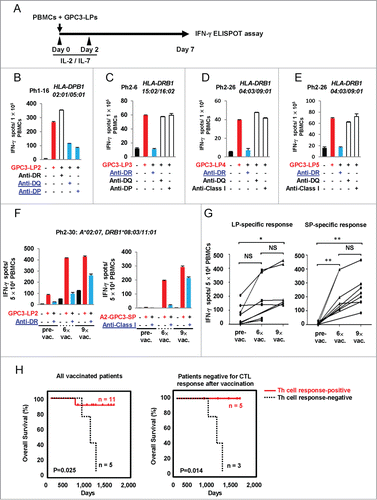
Cancer patients who are vaccinated with restricted epitopes often mount a T-cell response to epitopes not present in the vaccine.Citation15 To assess the induction of GPC3-LPs-specific Th cell response in cancer patients, we stimulated PBMCs isolated from HCC patients vaccinated with A2-GPC3-SP or A24-GPC3-SP. The patients' characteristics are summarized in Table S3. After seven-day in vitro stimulation of PBMCs with GPC3-LPs, the frequency of individual GPC3-LPs-specifc T cells was measured (). The responses were considered positive when the number of IFNγ-secreting cells was increased at least two-fold above the negative control. GPC3-LP-specific immune responses were observed in 14 of the 20 vaccinated patients (only representative four data was shown in , rest of the data was shown in Fig. S5 and Table S3). GPC3-LP-specific IFNγ production by T cells was significantly inhibited by addition of an anti-HLA-class II mAb ( and data not shown) but not an anti-HLA-class I mAb. These results demonstrated that IFNγ was produced by GPC3-LP-specific CD4+ T cells. We compared these responses in patients' PBMCs collected just before the first vaccination, the seventh vaccination and the tenth vaccination, and found that LP-specific CD4+ T-cell responses were induced, maintained or enhanced in 8 of 13 vaccinated patients after repeated vaccinations (, Fig. S5 and Table S4). Th cell response was observed in 11 of 16 patients who enrolled in a phase II clinical trial. OS was significantly prolonged in patients with specific Th cell response compared to patients with negative Th response ( left and Table S5). We also compared OS between GPC3-LPs-specific Th cell response-positive and -negative patients who didn't mount vaccine specific CTL response, and found that OS was significantly prolonged in Th response-positive group ( right and Table S5).
Figure 5 (See previous page). The presence of GPC3-LP-specific Th cells in the PBMCs of patients with HCC who were vaccinated with a GPC3-SP. (A–H). Frozen PBMCs that were derived from HCC patients vaccinated with GPC3-SP (Tables S3 and Table S4) were stimulated with a mixture of GPC3-LP1, −2, −3, −4, and −5 plus IL-2 and IL-7 in vitro. After 7 days, the frequency of individual GPC3-LP-specific T cells was assessed using an ELISPOT. Th cell responses specific to GPC3-LP2 (B), LP3 (C), LP4 (D), and LP5 (E) were observed in 14 of the 20 HCC patients tested. HLA class II restriction of the GPC3-LP-specific Th cells was determined by means of a blocking assay using monoclonal antibodies specific to HLA-DR, -DQ, or -DP. (F) The number of LP-specific CD4+ T cells increased after repeated SP vaccinations. (G) Both GPC3-LP- and SP-specific responses were assessed in 13 HCC patients vaccinated with GPC3-SPs. The results represent specific IFNγ spots after subtraction of the background response. The PBMCs were isolated from the same patient at different time points as follows: just before the first vaccination with SPs (pre-vac), before the seventh vaccination (6×-vac), and before the tenth vaccination (9×-vac). (H) Kaplan–Meir curves for OS. Patients with GPC3-specific Th cell responses vs. patients with no-response (left panel) and patients with GPC3-specific Th cell responses vs. patients without response among patients negative for CTL induction (right panel).
Discussion
In our previous studies, although a patient showed strong specific CTL responses along with tumor lysis after vaccination with GPC3-SPs,Citation28 no complete response was observed when GPC3-SP was used as monotherapy for advanced HCC.Citation8 Maintenance of the memory CTLs induced by GPC3-SPs can be improved by the help of tumor-specific CD4+ Th cells.Citation18 Therefore in this study, we sought to identify GPC3-derived LPs capable of inducing both CTL and Th1 cells.
Our key findings are as follows, (1) Five GPC3-LPs capable of eliciting promiscuous HLA class II-restricted Th1 cell responses were identified and four of them are likely to be naturally processed from the GPC3 protein by DCs in vitro. (2) GPC3-LP2, which bears a natural HLA-A2-restricted CTL epitope, was cross-presented effectively when encapsulated in newly developed liposomes. GPC3-LP2 alone was also cross-presented to prime specific CTLs in vivo in HLA-A2 Tgm. (3) Immunization of HLA-A2 Tgm with GPC3-LP2 encompassing A2-GPC3-SP was more effective in eliciting SP-specific CTLs in comparison with immunization with A2-GPC3-SP alone. (4) Th cell responses were also observed in HCC patients repeatedly vaccinated with GPC3-SP and they strongly correlated with the prolonged OS of the patients.
CD4+ T cell help was needed to mount an effective secondary CD8+ T cell response.Citation29 This help may be mediated through costimulatory signals induced by CD40/CD40L interaction necessary to activate APC or through signals induced by cytokines including Il-2, IL-7 and IL-15 for the maintenance of memory T cells.Citation18 In our study, we found increased number of SP-specific CTLs in mice that were immunized with GPC3-LP2 compared to mice immunized with SP alone. A part of this augmented CTL response may be attributed to the help of the CD4+ T cell response, because GPC3-LP2 stimulated a specific murine CD4+ T cell response.
The use of LPs increases a possibility that any DC taking up LPs stimulate both CD4+ T cells and CD8+ T cells.Citation21,30–34 LPs can be taken up, processed into SPs and presented to CD8+ T cells by DC rather than nonprofessional APC, and this can evoke a stronger and more effective CTL response. Such DC-focused antigen presentation may be another mechanism that caused increased CTL response in mice immunized with GPC3-LP2.
In cancer patients treated with immunotherapy, growth arrest and cancer regression correlated with the presence of tumor-specific, IFNγ producing CD4+ cells.Citation35 One of the plausible mechanisms of Th1 cells mediated tumor regression could be cellular senescence, because IFNγ and TNF produced by Th1 cells can induce senescence in numerous murine and human cancer cells.Citation36 As most of the Th cells induced by GPC3-LPs were found to secrete a large amount of Th1 type cytokines including IFNγ and TNF-α (Fig. S3), we speculate that the use of GPC3-LPs will be beneficial for immunotherapy of HCC.
Cancer patients vaccinated with a restricted epitope often mount a T-cell response to peptides that were not included in the vaccine, a phenomenon being called “epitope spreading.”Citation37 We observed that GPC3-LPs-specific Th cell responses were induced, maintained or enhanced in HCC patients by repeated GPC3-SP vaccinations. Therefore, the GPC3-LPs-specific Th cell response might be induced through epitope spreading. So far no correlation was observed between responses to SPs and responses to LPs among the patients possibly due to the low number of patients investigated and the heterogeneity in magnitude of epitope spreading among patients. However, when OS of HCC patients was assessed, on the basis of GPC3-LPs-specific Th cell response we observed a strong correlation between prolonged OS and positive Th cell response.
MGlu-PG-modified liposomes are taken up efficiently by DCs and reportedly deliver entrapped ovalbumin molecules into the cytosol.Citation38 These liposomes are regarded as a promising system to deliver antigens. The pH-sensitive liposomes were prepared by surface modification of egg yolk phosphatidylcholine liposomes with pH-sensitive poly (glycidol) derivatives containing MGlu-PG. In our in vitro experiments we didn't observe cross presentation of soluble GPC3-LP2. We thought this was due to inefficient delivery of the LPs into HLA class I-mediated antigen processing pathway in APC. However, the use of LP encapsulated in our liposome resulted in efficient cross presentation probably through efficient delivery of LP to cytoplasm of DC. Although in our in vivo cross priming experiments we didn't use LP encapsulated in liposome, we observed sufficient cross priming.
One possible reason is that we used IFA as an adjuvant in in vivo settings. IFA causes accumulation of mature DC.Citation39 Mature DCs present the IFA-embedded peptide and cross prime CTLs in vivo in HLA-A2 Tgm.
MHC class II proteins are highly polymorphic.Citation40 Thus, it is advisable to use a peptide or a cocktail of peptides vaccine that can be presented by multiple HLA class II molecules, so that the vaccine can be applied to a large number of patients. However, several common HLA-DR types share largely overlapping peptide repertoires bound by HLA-DRCitation41 due to which broad coverage of population could be achieved with a relatively limited number of peptides in a vaccine. Although we checked immunogenicity in a small number of healthy donors, five GPC3-LPs used in the present study are expected to be applicable to the majority of the Japanese population (Table S2).Citation42
Among the various clinical and histopathological markers, intratumoral T cell infiltration is one of the reliable predictive indicators for prolonged survival.Citation43 So far there is no correlation between CD4+ infiltration and OS of the HCC patients vaccinated with GPC3-SP (data not shown). More detailed quantitative and qualitative analysis of the infiltrated GPC3-specific T cells is necessary to elucidate the role of immune infiltrate in the patients with a longer survival. The analysis of direct recognition of autologous tumor cells by patients' GPC3-LPs-specific Th cells is also necessary to evaluate their capacity of direct killing.
Taken together, we propose the use of vaccines consisting of GPC3-LPs bearing both CD4+ and CD8+ T-cell epitopes that possesses many promises to improve the GPC3 peptide-based cancer immunotherapy for HCC.
Materials and Methods
Clinical trials of GPC3-SPs vaccination in HCC patients
A phase I clinical trial was approved by the Ethics Committee of the National Cancer Center and was conducted from February 2007 to November 2009. The trial was registered in the University Hospital Medical Information Network Clinical Trial Registry (UMIN-CTR number, 000001395). Patients with advanced or metastatic HCC were enrolled after providing written informed consent as described previously.Citation8 A phase II trial (UMIN-CTR number 000002614) was conducted for patients with HCC who received curative surgical treatment from 2010 to 2013 (manuscript in preparation). Unlike the phase I trial, the phase II trial was a single-arm study without dose escalation of the GPC3 peptide, and 3 mg of the GPC3 peptide was used for each vaccination. In the phase II clinical trial, 10 times vaccinations were performed: the initial 6 times every 2 weeks and the last 4 times every 2 mo. The primary endpoints were 1- and 2-year recurrence rates. The secondary endpoints were safety and immunological responses.
Cell lines
Mouse fibroblast cell lines (L cells), genetically engineered to express DP5 (DPA1*02:02/DPB1*05:01, L-DP5) and DR4 (DRB1*04:05, L-DR4), DR8 (DRB1*08:03, L-DR8), DR13 (DRB1*13:02, L-DR13), or DR15 (DRB1*15:02, L-DR15) were used as APCs. These L cells were maintained in vitro in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum. L cells expressing DR7 (DRB1*07:01, L-DR7), DR13 (DRB1*13:01, L-DR13), DR52a (DRB3*01:01, L-DR52a), DR52b (DRB3*02:02, RM3-DR52b), DR15 (DRB1*15:01, L-DR15), DP2 (DPA1*01:03/DPB1*02:01, L-DP2), and DP4 (DPA1*01:03/DPB1*04:01, RM3-DP4) were kindly provided by Dr. Alessandro Sette of La Jolla Institute for Allergy and Immunology, California, USA.Citation44
Prediction and selection of possible promiscuous HLA class II-binding GPC3-LPs
To predict possible promiscuous HLA class II-binding human GPC3-derived peptides, the amino acid sequence of the human GPC3 protein was analyzed using a computer algorithm, http://tools.immuneepitope.org/mhcii/).Citation45 We defined the score of percentile rank less than 10 as indicating stronger binding affinity to HLA class II molecules and selected the regions which were predicted to have high binding affinity to multiple frequently observed HLA class II molecules (encoded by DPB1*05:01, DRB1*07:01, DRB1*08:03, DRB1*09:01, DRB1*13:02, or DRB1*15:02 alleles. We also considered the regions that were proximal to known 9- or 10-mer CTL epitopes recognized by HLA-A2- or A24-restricted CTLs (Fig. S1B) and predicted to have strong binding affinity to HLA class II molecules. We avoid the regions that have potential glycosylation sites.
Synthetic peptides and recombinant proteins
Two human GPC3-derived SPs presented by HLA-A2 (A2-GPC3144–152; A2-GPC3-SP) or HLA-A24 (A24-GPC3298–306; A24-GPC3-SP), and five GPC3-LPs described above were synthesized by MBL (Nagoya, Japan; purity >95%; Fig. S1B). An HIV-related SP (A2-HIV) and a CDCA1-derived SP (A2-CDCA1) that binds to HLA-A2 were used as negative control SPs.Citation27,46 In some experiments, IMP3507–527-LP served as a control LP. The recombinant full-length GPC3 protein was purchased from R&D Systems (Minneapolis, USA; purity >90%) and was reconstituted as a 1 mg/mL solution in PBS containing 0.2% fetal calf serum. The recombinant human full-length CDCA1 protein was used as a control.Citation46 The liposomes loaded with GPC3-LP2 and IMP-3507–527-LP (as a control) were prepared as described previously.Citation47
Generation of antigen-specific CD4+ T cells from healthy donors
The protocols for isolation and use of peripheral blood mononuclear cells (PBMCs) from healthy donors were approved by the Institutional Review Board of Kumamoto University. We obtained PBMCs from 11 healthy donors with written informed consent. Genotyping of HLA-A, DRB1, and DPB1 alleles was performed at the HLA Laboratory (Kyoto, Japan; Table S1). Induction of antigen-specific CD4+ T cells was performed as described previously.Citation33 Briefly, CD4+ T cells were purified from PBMCs via positive selection with magnetic microbeads (Miltenyi Biotec, Auburn, CA, USA).Citation48 Monocyte-derived DCs were generated from CD14+ cells by means of in vitro cultivation,Citation26 and were used as APCs to induce antigen-specific CD4+ T cells.Citation30 In some instances, T cells were cloned by limiting dilution for further analysis.Citation49
Assessment of T-cell responses to peptides and proteins
The immune responses of Th cells to APCs pulsed with peptides (10 µg/mL) or proteins (10 µg/mL) were assessed using IFNγ enzyme-linked immunospot assays (ELISPOTs; BD Biosciences).Citation46 We analyzed the frequency of peptide-specific and IFNγ producing CD4+ T cells per 3 × 104 of bulk CD4+ T cells after stimulation with peptide-pulsed PBMCs (3 × 104) or 1 × 104 of bulk CD4+ T cells after stimulation with peptide-pulsed and HLA-DR-expressing L cells (5 × 104/well) or RM3 (5 × 104/well).Citation33
The in vitro cross-presentation assay
Yuba et al.Citation38 developed pH-sensitive 3-methylglutarylated poly (glycidol) (MGLu-PG)-modified liposomes containing the tumor antigen to enhance the efficiency of cross-presentation by DCs. To assess the cross-presentation of GPC3-LP2, we utilized DCs pulsed with GPC3-LPs encapsulated in liposomes. Briefly, a peptide (0.22 µmol) that was dissolved in N, N-dimethylformamide or deionized water (5 mg/mL) was added to a dry thin membrane of EYPC/CHexPG-PE (97/3, mol/mol; 6.25 µmol), and then the solvent was removed under vacuum for >3 h. The resulting lipid-peptide mixture was resuspended in PBS (500 µL) with 2-min sonication using a bath-type sonicator, yielding a suspension of liposomes that incorporated the peptide. The liposome suspension was further hydrated by freezing and thawing and was extruded through a polycarbonate membrane with a pore size of 100 nm. The liposome suspension was centrifuged at 55,000 rpm for 1.5 h at 4°C twice to remove the free peptide from the liposomes. The lipid and peptide concentrations were measured by means of Phospholipids C (Wako) and Micro BCA Protein assays (Thermo Scientific), respectively.
Immature DCs were prepared from positively isolated CD14+ cells. CD14+ cells were cultured in the presence of IL-4 (10 ng/mL) and granulocyte macrophage colony-stimulating factor (GM-CSF, 100 ng/mL). Immature DCs were harvested on day 5 and pulsed with an LP encapsulated in liposomes (equivalent to an LP at 20 µg/mL) for 4 h. We counted the number of IFNγ-producing-A2-GPC3144–152-SP-specific bulk CTLs in response to DCs loaded with GPC3-LP2 encapsulated in liposomes using an ELISPOT. Stimulation with SP-pulsed DCs was used as a positive control; un-pulsed DCs, DCs pulsed with liposomes alone, liposomes mixed with soluble GPC3-LP2, and DCs pulsed with IMP3502–527-LP encapsulated in liposomes served as negative controls.
In vivo cross priming and induction of LP-specific murine CD4+ T cells
HLA-A2 (HHD) Tgm were kindly provided by Dr. F.A. Lemonnier.Citation50 The mice were subcutaneously injected (at 7-day intervals) at the tail base with equimolar (50 µM,) solutions of GPC3-LP2 or A2-GPC3-SP emulsified in IFA.Citation30
The cytokine assay
GPC3-LPs-specific bulk Th cells or Th cell clones (3 × 104/well) were cultured in the presence of cognate peptide-pulsed autologous PBMCs in 96-well plates. After 24 h, the culture supernatants were collected, and indicated cytokine concentrations were measured using the Bio-Plex System (Bio-Rad).
Assessment of GPC3-LP- or SP-specific CD4+ or CD8+ T-cell responses in HCC patients immunized with A2 or A24-GPC3-SP
After thawing the frozen PBMCs that were isolated from patients with HCC, we cultured the cells in the presence of a mixture of five or individual GPC3-LPs (10 μg/mL each) in a final volume of 2 mL of the AIM-V medium (supplemented with 5% human decomplemented plasma) at 37°C (2 × 106/well, 24-well plates). IL-2 and IL-7 were added on days 0 and 2. After 1 week of cell culture, the cells were collected, washed, and cultured in ELISPOT plates (5–10 × 104/well) with the individual GPC3-LP (or GPC3-SP) or control LP (or SP) for 18 h. The numbers of GPC3-LP- and SP-specific CD4+ or CD8+ T cells were estimated as described previously.Citation31
Statistical analysis
We compared the means using 1-way ANOVA (bar graphs and scatter dot graph). OS rates were analyzed by the Kaplan–Meir method. The significance of these parameters was estimated with the log-rank test. Differences with a p value < 0.05 were considered statistically significant.
Disclosure of Potential Conflicts of Interest
Yasuharu Nishimura is supported by funding from OncoTherapy Science, Inc.. Tetsuya Nakatsura is supported by funding from Ono Pharmaceutical Co., Ltd.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
1062209_supplemental_files.zip
Download Zip (663.3 KB)Acknowledgments
The authors thank T. Ikeda for helpful technical advices.
Funding
This research was supported by MEXT Grant-in-Aid for Scientific Research on Innovative Areas, Grant Number 22133005; JSPS KAKENHI, Grant Number 24300334 and 15H04311; a research grant from the Princess Takamatsu Cancer Research Fund, No.10–24215; funding from OncoTherapy Science; and in part by the Scholarship of the Graduate School of Medical Sciences, Kumamoto University, Japan.
References
- Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020-2; PMID:21374666; http://dx.doi.org/10.1002/hep.24199
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378-90; PMID:18650514; http://dx.doi.org/10.1056/NEJMoa0708857
- Mancuso A, Mazzarelli C, Perricone G, Zavaglia C. Sorafenib efficacy for treatment of HCC recurrence after liver transplantation is an open issue. J Hepatol 2014; 60:681; PMID:24216445; http://dx.doi.org/10.1016/j.jhep.2013.10.030
- Morimoto M, Numata K, Kondo M, Hidaka H, Takada J, Shibuya A, Kobayashi S, Ohkawa S, Okuse C, Morita S et al. Higher discontinuation and lower survival rates are likely in elderly Japanese patients with advanced hepatocellular carcinoma receiving sorafenib. Hepatol Res 2011; 41:296-302; PMID:21348907; http://dx.doi.org/10.1111/j.1872-034X.2011.00778.x
- Okabe H, Satoh S, Kato T, Kitahara O, Yanagawa R, Yamaoka Y, Tsunoda T, Furukawa Y, Nakamura Y. Genome-wide analysis of gene expression in human hepatocellular carcinomas using cDNA microarray: identification of genes involved in viral carcinogenesis and tumor progression. Cancer Res 2001; 61:2129-37; PMID:11280777.
- Nakatsura T, Yoshitake Y, Senju S, Monji M, Komori H, Motomura Y, Hosaka S, Beppu T, Ishiko T, Kamohara H et al. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun 2003; 306:16-25; PMID:12788060; http://dx.doi.org/10.1016/S0006-291X(03)00908-2
- Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res 2005; 65:6245-54; PMID:16024626; http://dx.doi.org/10.1158/0008-5472.CAN-04-4244
- Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K, Konishi M et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res 2012; 18:3686-96; PMID:22577059; http://dx.doi.org/10.1158/1078-0432.CCR-11-3044
- Komori H, Nakatsura T, Senju S, Yoshitake Y, Motomura Y, Ikuta Y, Fukuma D, Yokomine K, Harao M, Beppu T et al. Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma. Clin Cancer Res 2006; 12:2689-97; PMID:16675560; http://dx.doi.org/10.1158/1078-0432.CCR-05-2267
- Toes RE, Blom RJ, Offringa R, Kast WM, Melief CJ. Enhanced tumor outgrowth after peptide vaccination. Functional deletion of tumor-specific CTL induced by peptide vaccination can lead to the inability to reject tumors. J Immunol 1996; 156:3911-8; PMID:8621930
- Toes RE, Offringa R, Blom RJ, Melief CJ, Kast WM. Peptide vaccination can lead to enhanced tumor growth through specific T-cell tolerance induction. Proc Natl Acad Sci U S A 1996; 93:7855-60; PMID:8755566; http://dx.doi.org/10.1073/pnas.93.15.7855
- Srinivasan M, Domanico SZ, Kaumaya PT, Pierce SK. Peptides of 23 residues or greater are required to stimulate a high affinity class II-restricted T cell response. Eur J Immunol 1993; 23:1011-6; PMID:8386663; http://dx.doi.org/10.1002/eji.1830230504
- Sercarz EE, Maverakis E. Mhc-guided processing: binding of large antigen fragments. Nat Rev Immunol 2003; 3:621-9; PMID:12974477; http://dx.doi.org/10.1038/nri1149
- Kayaga J, Souberbielle BE, Sheikh N, Morrow WJ, Scott-Taylor T, Vile R, Chong H, Dalgleish AG. Anti-tumour activity against B16-F10 melanoma with a GM-CSF secreting allogeneic tumour cell vaccine. Gene Ther 1999; 6:1475-81; PMID:10467372; http://dx.doi.org/10.1038/sj.gt.3300961
- Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med 2008; 358:2698-703; PMID:18565862; http://dx.doi.org/10.1056/NEJMoa0800251
- Lichterfeld M, Kaufmann DE, Yu XG, Mui SK, Addo MM, Johnston MN, Cohen D, Robbins GK, Pae E, Alter G et al. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J Exp Med 2004; 200:701-12; PMID:15381726; http://dx.doi.org/10.1084/jem.20041270
- Chamoto K, Tsuji T, Funamoto H, Kosaka A, Matsuzaki J, Sato T, Abe H, Fujio K, Yamamoto K, Kitamura T et al. Potentiation of tumor eradication by adoptive immunotherapy with T-cell receptor gene-transduced T-helper type 1 cells. Cancer Res 2004; 64:386-90; PMID:14729649; http://dx.doi.org/10.1158/0008-5472.CAN-03-2596
- Bevan MJ. Helping the CD8+ T-cell response. Nat Rev Immunol 2004; 4:595-602; PMID:15286726; http://dx.doi.org/10.1038/nri1413
- Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood 2001; 97:192-7; PMID:11133760; http://dx.doi.org/10.1182/blood.V97.1.192
- Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res 2010; 70:8368-77; PMID:20940398; http://dx.doi.org/10.1158/0008-5472.CAN-10-1322
- Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer 2008; 8:351-60; PMID:18418403; http://dx.doi.org/10.1038/nrc2373
- Brunsvig PF, Kyte JA, Kersten C, Sundstrom S, Moller M, Nyakas M, Hansen GL, Gaudernack G, Aamdal S. Telomerase peptide vaccination in NSCLC: a phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin Cancer Res 2011; 17:6847-57; PMID:21918169; http://dx.doi.org/10.1158/1078-0432.CCR-11-1385
- Kyte JA, Gaudernack G, Dueland S, Trachsel S, Julsrud L, Aamdal S. Telomerase peptide vaccination combined with temozolomide: a clinical trial in stage IV melanoma patients. Clin Cancer Res 2011; 17:4568-80; PMID:21586625; http://dx.doi.org/10.1158/1078-0432.CCR-11-0184
- Saito S, Ota S, Yamada E, Inoko H, Ota M. Allele frequencies and haplotypic associations defined by allelic DNA typing at HLA class I and class II loci in the Japanese population. Tissue Antigens 2000; 56:522-9; PMID:11169242; http://dx.doi.org/10.1034/j.1399-0039.2000.560606.x
- Mack SJ, Bugawan TL, Moonsamy PV, Erlich JA, Trachtenberg EA, Paik YK, Begovich AB, Saha N, Beck HP, Stoneking M et al. Evolution of Pacific/Asian populations inferred from HLA class II allele frequency distributions. Tissue Antigens 2000; 55:383-400; PMID:10885559; http://dx.doi.org/10.1034/j.1399-0039.2000.550501.x
- Harao M, Hirata S, Irie A, Senju S, Nakatsura T, Komori H, Ikuta Y, Yokomine K, Imai K, Inoue M et al. HLA-A2-restricted CTL epitopes of a novel lung cancer-associated cancer testis antigen, cell division cycle associated 1, can induce tumor-reactive CTL. Int J Cancer 2008; 123:2616-25; PMID:18770861; http://dx.doi.org/10.1002/ijc.23823
- Tomita Y, Harao M, Senju S, Imai K, Hirata S, Irie A, Inoue M, Hayashida Y, Yoshimoto K, Shiraishi K et al. Peptides derived from human insulin-like growth factor-II mRNA binding protein 3 can induce human leukocyte antigen-A2-restricted cytotoxic T lymphocytes reactive to cancer cells. Cancer Sci 2011; 102:71-8; PMID:21087352; http://dx.doi.org/10.1111/j.1349-7006.2010.01780.x
- Sawada Y, Yoshikawa T, Fujii S, Mitsunaga S, Nobuoka D, Mizuno S, Takahashi M, Yamauchi C, Endo I, Nakatsura T. Remarkable tumor lysis in a hepatocellular carcinoma patient immediately following glypican-3-derived peptide vaccination: An autopsy case. Hum Vaccin Immunother 2013; 9:1228-33; PMID:23466818; http://dx.doi.org/10.4161/hv.24179
- Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 2003; 300:339-42; PMID:12690202; http://dx.doi.org/10.1126/science.1083317
- Tomita Y, Yuno A, Tsukamoto H, Senju S, Kuroda Y, Hirayama M, Irie A, Kawahara K, Yatsuda J, Hamada A et al. Identification of promiscuous KIF20A long peptides bearing both CD4+ and CD8+ T-cell epitopes: KIF20A-specific CD4+ T-cell immunity in patients with malignant tumor. Clin Cancer Res 2013; 19:4508-20; PMID:23714729; http://dx.doi.org/10.1158/1078-0432.CCR-13-0197
- Tomita Y, Yuno A, Tsukamoto H, Senju S, Yoshimura S, Osawa R, Kuroda Y, Hirayama M, Irie A, Hamada A et al. Identification of CDCA1-derived long peptides bearing both CD4+ and CD8+ T-cell epitopes: CDCA1-specific CD4+ T-cell immunity in cancer patients. Int J Cancer 2014; 134:352-66; PMID:24734272; http://dx.doi.org/10.1002/ijc.28376
- Nishimura Y, Tomita Y, Yuno A, Yoshitake Y, Shinohara M. Cancer immunotherapy using novel tumor-associated antigenic peptides identified by genome-wide cDNA microarray analyses. Cancer Sci 2015; 106(5):505–11; PMID:25726868; http://dx.doi.org/10.111/cas.12650
- Tomita Y, Yuno A, Tsukamoto H, Senju S, Kuroda Y, Hirayama M, Imamura Y, Yatsuda J, Sayem MA, Irie A et al. Identification of immunogenic LY6K long peptide encompassing both CD4 and CD8 T-cell epitopes and eliciting CD4 T-cell immunity in patients with malignant disease. Oncoimmunology 2014; 3:e28100; PMID:25340007; http://dx.doi.org/10.4161/onci.28100
- Yoshitake Y, Fukuma D, Yuno A, Hirayama M, Nakayama H, Tanaka T, Nagata M, Takamune Y, Kawahara K, Nakagawa Y et al. Phase II clinical trial of multiple peptide vaccination for advanced head and neck cancer patients revealed induction of immune responses and improved OS. Clin Cancer Res 2015; 21:312-21; PMID:25391695; http://dx.doi.org/10.1158/1078-0432.CCR-14-0202
- Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med 2009; 361:1838-47; PMID:19890126; http://dx.doi.org/10.1056/NEJMoa0810097
- Braumuller H, Wieder T, Brenner E, Assmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013; 494:361-5; PMID:23376950; http://dx.doi.org/10.1038/nature11824
- Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol 2002; 2:85-95; PMID:11910899; http://dx.doi.org/10.1038/nri724
- Yuba E, Harada A, Sakanishi Y, Watarai S, Kono K. A liposome-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials 2013; 34:3042-52; PMID:23374704; http://dx.doi.org/10.1016/j.biomaterials.2012.12.031
- Harris RC, Chianese-Bullock KA, Petroni GR, Schaefer JT, Brill LB, 2nd, Molhoek KR, Deacon DH, Patterson JW, Slingluff CL, Jr. The vaccine-site microenvironment induced by injection of incomplete Freund's adjuvant, with or without melanoma peptides. J Immunother 2012; 35:78-88; PMID:22130163; http://dx.doi.org/10.1097/CJI.0b013e31823731a4
- Kappes D, Strominger JL. Human class II major histocompatibility complex genes and proteins. Annu Rev Biochem 1988; 57:991-1028; PMID:3140715; http://dx.doi.org/10.1146/annurev.bi.57.070188.005015
- Southwood S, Sidney J, Kondo A, del Guercio MF, Appella E, Hoffman S, Kubo RT, Chesnut RW, Grey HM, Sette A. Several common HLA-DR types share largely overlapping peptide binding repertoires. J Immunol 1998; 160:3363-73; PMID:9531296
- Nakajima F, Nakamura J, Yokota T. Analysis of HLA haplotypes in Japanese, using high resolution allele typing. MHC 2001; 8:1-32.
- Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pages F et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011; 29:610-8; PMID:21245428; http://dx.doi.org/10.1200/JCO.2010.30.5425
- McKinney DM, Southwood S, Hinz D, Oseroff C, Arlehamn CS, Schulten V, Taplitz R, Broide D, Hanekom WA, Scriba TJ et al. A strategy to determine HLA class II restriction broadly covering the DR, DP, and DQ allelic variants most commonly expressed in the general population. Immunogenetics 2013; 65:357-70; PMID:23392739; http://dx.doi.org/10.1007/s00251-013-0684-y
- Wang P, Sidney J, Kim Y, Sette A, Lund O, Nielsen M, Peters B. Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinformatics 2010; 11:568; PMID:21092157; http://dx.doi.org/10.1186/1471-2105-11-568
- Tomita Y, Imai K, Senju S, Irie A, Inoue M, Hayashida Y, Shiraishi K, Mori T, Daigo Y, Tsunoda T et al. A novel tumor-associated antigen, cell division cycle 45-like can induce cytotoxic T-lymphocytes reactive to tumor cells. Cancer Sci 2011; 102:697-705; PMID:21231984; http://dx.doi.org/10.1111/j.1349-7006.2011.01865.x
- Yuba E, Kono Y, Harada A, Yokoyama S, Arai M, Kubo K, Kono K. The application of pH-sensitive polymer-lipids to antigen delivery for cancer immunotherapy. Biomaterials 2013; 34:5711-21; PMID:23639528; http://dx.doi.org/10.1016/j.biomaterials.2013.04.007
- Inoue M, Senju S, Hirata S, Ikuta Y, Hayashida Y, Irie A, Harao M, Imai K, Tomita Y, Tsunoda T et al. Identification of SPARC as a candidate target antigen for immunotherapy of various cancers. Int J Cancer 2010; 127:1393-403; PMID:20063317; http://dx.doi.org/10.1002/ijc.25160
- Tabata H, Kanai T, Yoshizumi H, Nishiyama S, Fujimoto S, Matsuda I, Yasukawa M, Matsushita S, Nishimura Y. Characterization of self-glutamic acid decarboxylase 65-reactive CD4+ T-cell clones established from Japanese patients with insulin-dependent diabetes mellitus. Hum Immunol 1998; 59:549-60; PMID:9757911; http://dx.doi.org/10.1016/S0198-8859(98)00050-0
- Pascolo S, Bervas N, Ure JM, Smith AG, Lemonnier FA, Perarnau B. HLA-A2.1-restricted education and cytolytic activity of CD8+ T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J Exp Med 1997; 185:2043-51; PMID:9182675; http://dx.doi.org/10.1084/jem.185.12.2043
