ABSTRACT
Practical experience with cancer vaccines combined with accumulated knowledge of the complex interactions between cancer and immune system rationalize the combinatorial use of immune adjuvants for better efficacy. We recently described a novel adjuvant system based on the costimulatory SA-4-1BBL and TLR4 agonist MPL that has desired therapeutic and safety profiles.
Abbreviations
| Ab | = | antibody |
| APCs | = | antigen-presenting cells |
| MPL | = | monophosphoryl lipid A |
| PRRs | = | pattern recognition receptors |
| streptavidin-4-1BBL | = | SA-4-1BBL |
| TAAs | = | tumor associated antigens |
| TLRs | = | toll-like receptors |
| TNFL | = | tumor necrosis family ligand |
Background
TAA-based therapeutic vaccines are particularly attractive because of their ease of production, scale-up, storage, and administration to a broad patient population. The efficacy of TAA-based subunit vaccines, however, is contingent upon the inclusion of an immune adjuvant in the formulation. Indeed, accumulating evidences in cancer vaccinology indicate the requirement for combinatorial use of adjuvants for better therapeutic efficacy. The choice of adjuvants is also a key consideration and dictates the outcome of vaccination. Adjuvants that not only generate potent immune responses against TAAs with long-term immunologic memory, but also overcome various immune evasion mechanisms will likely have better success in the clinic. Toward this goal, we recently published an article entitled “SA-4-1BBL and monophosphoryl lipid A constitute an efficacious combination adjuvant for cancer vaccines”Citation1 that demonstrated the utility of combinatorial administration of 2 immune agonists, SA-4-1BBL and MPL, with synergistic mechanisms of action as an adjuvant system. As a component of TAA-based subunit vaccine formulations, this adjuvant combination generated robust antitumor immune responses, displaying therapeutic efficacy in various preclinical models with an excellent safety profile.
Choice of adjuvants
Adjuvants have been shown to enhance the magnitude, breadth, quality, and longevity of the immune response to a given antigen. As such, identification of novel adjuvants remains an active and important area of investigation for both academia and industry. Several classes of adjuvants have been tested as part of cancer vaccine formulations, including alum-salts, bacterial products such as lipopolysaccharides, liposomes, and cytokines. Unfortunately, the majority of these adjuvants have shown very modest efficacy coupled with toxicity concerns that raise significant regulatory hurdles. In fact, alum-salt-based adjuvants were the only ones used clinically in the U.S. until 2010, when monophosphoryl lipid A (MPL), in combination with aluminum hydroxide, was approved by the FDA as an adjuvant component of Cervarix, a preventive vaccine against human papillomavirus (HPV). The limited choice of adjuvants is mainly due to a lack of a comprehensive understanding of mechanistic insight and precise knowledge of the constituents of many adjuvants. The substantial progress made in recent years in the elucidation of immune responses in general, and signals that are required for the generation of effective innate and adaptive immune responses in particular, has led to the rational design and/or discovery of agents that have the potential to generate robust and long-lasting cellular and humoral immune responses with acceptable safety profiles. A more complete understanding of the mechanistic basis of these agents may also yield opportunities to combine adjuvants that target distinct immune pathways with potential additive or synergistic effects for better immune efficacy.
Adjuvants to guide innate, adaptive and regulatory immunity for therapeutic response against cancer
The use of immunological adjuvants in cancer vaccines requires an inherent ability to primarily improve the quality and quantity of effector and long-term cellular immune response by targeting both innate and adaptive immunity. However, the majority of adjuvants achieve their activity by acting as pathogen-associated molecular patterns that work on evolutionary conserved innate immune receptors to mimic natural infections. In fact, almost all clinically approved adjuvants as well as most adjuvants under development primarily trigger innate immune responses via the recruitment, activation and maturation of antigen presenting cells (APCs) that serve as a bridge between innate and adaptive immunity. The majority of adjuvants from this class are ligands of pattern recognition receptors (PRRs) with toll-like receptors (TLRs) being the main family. Despite some promising results, the choice of such adjuvants for cancer vaccines has been very limited, mostly due to a lack of efficacy for generating robust and long-lasting cellular immune responses but also partly due to toxicity concerns.
Given the importance of T-cell responses in cancer immunotherapy and the inability of agonists of PRRs to directly act on these cells, it is intuitive that adjuvants directly targeting and generating optimal CD4+ and CD8+ T-cell responses may have better efficacy in therapeutic cancer settings. In this context, agonistic ligands to the costimulatory tumor necrosis family receptors (TNFRs) may potentially be used as adjuvants of choice for TAA-based subunit vaccines, largely due to their pleiotropic effects on cells of innate, adaptive, and regulatory immunity. Spearheading this perspective, our group has targeted the 4-1BB receptor of the TNFR costimulatory family and developed a novel agonistic ligand, SA-4-1BBL.Citation2-5 In extensive studies, SA-4-1BBL was shown to play a critical role in the generation and maintenance of CD8+ T-cell responses, while having a negative impact on the frequency and suppressor function of CD4+CD25+FoxP3+ regulatory T cells that are important culprits of tumor immune evasion.Citation2,6-8 Importantly, as an adjuvant component of TAA-based subunit vaccines, SA-4-1BBL showed robust therapeutic efficacy in various preclinical tumor models,Citation1,3,4,9,10 establishing this molecule as an important new class of adjuvant.
Combinatorial adjuvants as the next logical approach to cancer immunotherapy
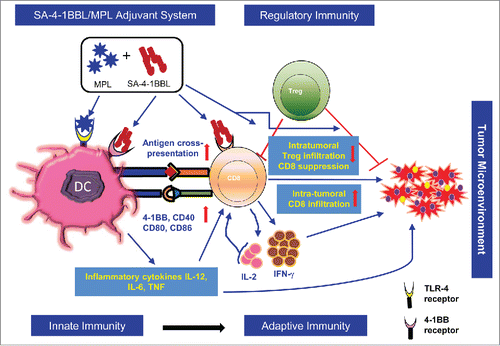
Although the preclinical antitumor impact of SA-4-1BBL is impressive, we aimed to further improve and expand the scope of its utilization in clinical settings. Thus, we next sought to use this adjuvant in combination with TLR agonists to directly target both innate and adaptive immunity for a robust anticancer response. This strategy is especially warranted to counteract complex immune evasion mechanisms employed by tumors during disease progression and to overcome immune evasion as well as to generate robust effector responses, which will require a combination of multiple adjuvants directed at diverse targets to maximize therapeutic outcome. We focused on TLR4 agonist MPL for combinatorial use with SA-4-1BBL because of its safety profile and FDA approved status as component of a preventive vaccine against cervical cancer. Combinatorial use of these 2 adjuvants was also warranted due to their distinct immune cell targets and signaling pathways, as well as their demonstrated significant roles as individual agents for the activation of innate immunity and the generation and maintenance of cellular immune responses. Our results (Fig. 1) demonstrated that the MPL component of the adjuvant system targeted TLR expressed on DCs for activation and upregulation of various costimulatory receptors/ligands and production of pro-inflammatory cytokines that altogether primed T cells for activation. The SA-4-1BBL component of the vaccine, on the other hand, directly targeted primed CD8+ T cells expressing 4-1BB for expansion, survival, and acquisition of effector function, as well as potentiating long-term memory. These combined effects resulted in eradication of tumors in various preclinical models without detectable toxicity. Most importantly, therapeutic efficacy of the combined adjuvants was associated with significant infiltration of CD8+ T cells into the tumor and marked reduction of CD4+CD25+FoxP3+ regulatory T cells with a favorable intra-tumoral T effector to regulatory T cell ratio, a hallmark of successful clinical prognosis.
Concluding remarks
The positive developments in cancer immunotherapy in the past decade combined with the recent FDA approval of several immune-check point blockers have set the stage for cancer immunotherapy. The impressive clinical results garnered so far along with a better comprehension of the complexities of the immune system will further accelerate the development of new immune strategies to combat cancer in general and engineer cancer vaccines in particular. However, establishing successful cancer vaccine strategies will require careful design of adjuvant systems tailored to a given cancer type and formulated to generate the desired immune responses for therapeutic efficacy without significant toxicity. Our findings with SA-4-1BBL/MPL, although preclinical, have revealed the attributes of a successful therapeutic approach and display promise as a potential strategy that should be evaluated in the clinic.
Figure 1. Pleiotropic effects of the SA-4-1BBL/MPL adjuvant system on cells of innate, adaptive, and regulatory immunity. Monophosphoryl lipid A (MPL) interaction with Toll-like receptor 4 (TLR4) expressed on dendritic cells (DCs) results in DC activation, including the up-regulation of costimulatory receptors/ligands and production of various pro-inflammatory cytokines, such as interleukin (IL)-12, IL-6, and tumor necrosis factor (TNF), that together prime adoptive cellular immune responses. SA-4-1BBL further improves the MPL effect by interaction with 4-1BB receptor constitutively expressed on DCs for their activation/maturation and overcomes regulatory T cell (Treg) mediated immune suppression. Most importantly, SA-4-1BBL drive robust cellular immune responses by interacting with 4-1BB receptor upregulated on antigen-primed CD8+ T cells, which allows their survival, acquisition of effector function, and establishment of long-term memory. The combined adjuvant further contributes to a favorable intratumoral T effector to Treg ratio by facilitating the infiltration of CD8+ T cells and reducing the frequency of Tregs.
Disclosure of potential conflicts of interest
Haval Shirwan is an inventor on a patent filed by the University of Louisville Research Foundation for the technology described herein.
References
- Srivastava AK, Dinc G, Sharma RK, Yolcu ES, Zhao H, Shirwan H. SA-4-1BBL and monophosphoryl lipid A constitute an efficacious combination adjuvant for cancer vaccines. Cancer Res 2014; 74:6441-51; PMID:25252915; http://dx.doi.org/10.1158/0008-5472.CAN-14-1768-A
- Madireddi S, Schabowsky RH, Srivastava AK, Sharma RK, Yolcu ES, Shirwan H. SA-4-1BBL costimulation inhibits conversion of conventional CD4+ T cells into CD4+ FoxP3+ T regulatory cells by production of IFN-gamma. PLoS One 2012; 7:e42459; PMID:22870329; http://dx.doi.org/10.1371/journal.pone.0042459
- Sharma RK, Schabowsky RH, Srivastava A, Elpek KG, Madireddi S, Zhao H, Zhong Z, Miller RW, Macleod KJ, Yolcu ES, et al. 4-1BB ligand as an effective multifunctional immunomodulator and antigen delivery vehicle for the development of therapeutic cancer vaccines. Cancer Res 2010; 70:3945–54; PMID:20406989; http://dx.doi.org/10.1158/0008-5472.CAN-09-4480
- Sharma RK, Elpek KG, Yolcu ES, Schabowsky RH, Zhao H, Bandura-Morgan L, Shirwan H. Costimulation as a platform for the development of vaccines: a peptide-based vaccine containing a novel from of 4-1BBL eradicates established tumors. Cancer Res 2009; 69:4319–26; PMID:19435920; http://dx.doi.org/10.1158/0008-5472.CAN-08-3141
- Elpek KG, Yolcu ES, Franke DD, Lacelle C, Schabowsky RH, Shirwan H. Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4-1BB signaling. J Immunol 2007; 179:7295–304; PMID:18025172; http://dx.doi.org/10.4049/jimmunol.179.11.7295
- Sharma RK, Yolcu ES, Shirwan H. SA-4-1BBL as a novel adjuvant for the development of therapeutic cancer vaccines. Expert Rev Vaccines 2014; 13:387–98; PMID:24521311; http://dx.doi.org/10.1586/14760584.2014.880340
- Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest 2005; 115:3623–33; PMID:16308572; http://dx.doi.org/10.1172/JCI25947
- Schabowsky RH, Madireddi S, Sharma R, Yolcu ES, Shirwan H. Targeting CD4+CD25+FoxP3+ regulatory T-cells for the augmentation of cancer immunotherapy. Curr Opin Investig Drugs 2007; 8:1002–8; PMID:18058571
- Sharma RK, Yolcu ES, Srivastava AK, Shirwan H. CD4(+) T cells play a critical role in the generation of primary and memory antitumor immune responses elicited by SA-4-1BBL and TAA-based vaccines in mouse tumor models. PLoS One 2013; 8:e73145; PMID:24066030; http://dx.doi.org/10.1371/journal.pone.0073145
- Srivastava AK, Sharma RK, Yolcu ES, Ulker V, MacLeod K, Dinc G, Shirwan H. Prime-boost vaccination with SA-4-1BBL costimulatory molecule and survivin eradicates lung carcinoma in CD8+ T and NK cell dependent manner. PLoS One 2012; 7:e48463; PMID:23144888; http://dx.doi.org/10.1371/journal.pone.0048463
