ABSTRACT
Recently, there has been a growing interest in the importance of stem cells (SCs) in the development/progression of gastric neoplasms. In this study, we performed a comprehensive analysis of different populations of bone-marrow-derived stem cells (BMSCs) in patients with various types of gastric malignancies, including gastric cancer, gastrointestinal stromal tumors (GISTs), neuroendocrine neoplasms (NENs), and lymphomas. We found significantly lower numbers of circulating Lin-/CD45 +/ CD133 + hematopoietic stem/progenitor cells (HSPCs), and intensified peripheral trafficking of both Lin−/CD45−/CXCR4+/CD34+/CD133+ very small embryonic/epiblast-like stem cells (VSELs) and CD105 + /STRO-1 +/ CD45- mesenchymal SCs (MSCs) in patients with gastric cancer, but not in those with other types of gastric neoplasms. No significant differences in the absolute numbers of circulating CD34 +/ KDR +/ CD31 +/ CD45- endothelial progenitor cells (EPCs) were observed between the groups. This abnormal balance in the peripheral trafficking of BMSCs in patients with gastric cancer was neither associated with clinical stage of the disease nor with systemic levels of stromal-derived factor-1 (SDF-1), as these were comparable to the values observed in control individuals. Interestingly, the absolute numbers of circulating BMSCs correlated with the concentrations of complement cascade-derived anaphylatoxins/molecules (mainly C5b-9/membrane attack complex-MAC) and sphingosine-1-phosphate (S1P). In summary, our translational study revealed that abnormal peripheral trafficking of BMSCs occurs in patients with gastric cancer, but not in those with other types of gastric neoplasms. Further, our findings indicate that highlighted complement cascade-derived molecules and S1P, but not SDF-1, are significant players associated with this phenomenon.
Abbreviations
| BMSCs | = | bone marrow-derived stem cells |
| EPCs | = | endothelial progenitor cells |
| GISTs | = | gastrointestinal stromal tumors |
| HSPCs | = | hematopoietic stem/progenitor cells |
| MAC | = | membrane attack complex |
| MSCs | = | mesenchymal stem cells |
| NENs | = | neuroendocrine neoplasms |
| SCs | = | stem cells |
| SDF-1 | = | stromal-derived factor-1 |
| S1P | = | sphingosine-1-phosphate |
| VSELs | = | very small embryonic-like stem cells. |
Introduction
Gastric neoplasms constitute a diverse group of tumors, the clinical diagnosis and treatment of which represents a major challenge in modern gastroenterology. In the vast majority of cases, it is the gastric cancer that affects humans. This type of gastric malignancy is, unfortunately, also associated with the worst overall prognosis characterized by a very high mortality rate that constantly ranks this disease among the top five of the deadliest neoplasms in humans.Citation1,2 However, within the gastric tissue, other less aggressive types of tumors may also develop. These rare malignancies represent around 5% of all the gastric neoplasms, and most commonly include GISTs, NENs of the stomach, and/or primary gastric lymphomas.Citation3-5 From the molecular standpoint, the pathogenesis of gastric tumors is diverse, and even though several risk factors for stomach cancer have been identified recently, the exact biochemical and cellular mechanisms underlying the formation of gastric tumors remain only partially known.
Recently there has been an increased interest in the potential role of SCs in the development/progression of gastric tumors.Citation6-9 This interest was first generated by the study of Houghton et al.Citation10, who demonstrated that BMSCs migrate to and repopulate the gastric tissue in experimental animals infected by H. pylori, and that these BMSCs may undergo all the steps of molecular and histological trans-differentiation that enables them to participate in carcinogenesis. These preliminary experimental observations were supported by later experimental studies and clinical case reports, in which the presence of bone-marrow-derived cells was detected within the gastric cancer microenvironment in patients who underwent bone marrow transplantation.Citation11,12 Nevertheless, till today, it is not known if gastric tumors in fact arise from SCs originating from the bone marrow. In addition, whether molecular interactions between different types of BMSC populations participate in and support the development/progression of different gastric neoplasms in humans as well as the underlying mechanisms of the same remain unclear.
It is important to highlight that within the bone marrow environment, different populations of stem/progenitor cells including EPCs, HSPCs, MSCs, and VSELs exist. All of these are proved to be actively involved in the regeneration of solid organs or blood compartments.Citation13 Theoretically, their molecular armament may also enable them to actively support and/or even initiate the development of tumors in the gastrointestinal tract. Therefore, in this study, we took the first translational step by analyzing the systemic trafficking of various populations of BMSCs in patients with different types of gastric neoplasms. Furthermore, we aimed to elucidate the potential biochemical/immunological mechanism(s) that might be associated with this phenomenon. In particular, we focused on determining if this process is associated with peripheral levels of the currently well-known chemoattractant, stromal-derived factor-1 (SDF-1), and/or immune-derived molecules such as complement cascade-derived anaphylatoxins (C3a, C5a, C5b-9/membrane attack complex—MAC) or S1P as suggested previously.Citation14-18 In addition, we aimed to examine potential associations between the absolute numbers of circulating BMSCs and the clinical stage of gastric neoplasms. Our hypothesis was that intensified peripheral trafficking of selected populations of BMSCs occurs in patients with gastric cancer, but not in those with other types of gastric neoplasms, which is irrespective of the clinical stage. Moreover, we hypothesized that this egress of the BMSCs is associated with systemic levels of complement cascade-derived molecules, such as C5b-9/MAC, but not with concentrations of the currently known chemoattractant for BMSCs, SDF-1.
Results
Analysis of study participants
Our initial comparison of the analyzed groups of recruited individuals revealed significantly higher C-reactive protein (CRP) levels and lower erythrocyte counts in patients with gastric cancer than in the other examined groups (). No other significant differences were found between the analyzed groups, although the comparison of platelet counts between healthy individuals and gastric cancer patients showed a value close to the threshold for statistical significance (p = 0.06).
Table 1. General characteristic of surgical procedure and of individuals enrolled in the study (means ± SD).
Circulating stem cell populations in patients with gastric neoplasms
The mean absolute numbers of various BMSC populations circulating in the peripheral blood in patients with gastric cancer and healthy individuals are shown in . Our analyses showed significantly higher numbers of circulating VSELs and MSCs and lower numbers of HSPCs in patients with gastric cancer than in the healthy individuals (). Interestingly, the absolute numbers of EPCs were statistically comparable between these two analyzed groups. Further, linear regression analyses revealed that the absolute numbers of circulating HSPCs were significantly associated with the cancer stage determined according to the TNM classification (β = −0.57; R2 = 0.32; p < 0.03), whereas the numbers of VSELs and MSCs were not (β = 0.09; R2 = 0.01; p = 0.69 for VSELs, and β = 0.13; R2 = 0.13; p = 0.62 for MSCs). However, when we divided our cancer patients into subgroups of “early” and “advanced” gastric cancer according to the Japanese criteria or into “diffuse” and “intestinal” based on the histological type, no significant differences in the absolute numbers of circulating SC populations were observed between the subgroups (data not shown).
Figure 1. Mean absolute numbers of stem/progenitor cells circulating in the peripheral blood derived from control individuals and patients with different types of gastric neoplasms together with their statistical comparison between the groups. VSEL—very small embryonic-like stem cells, MSC—mesenchymal stem cells, HSPC—hematopoietic stem/progenitor cells, EPC—endothelial progenitor cells, *p < 0.05 (level of significance vs. control individuals).
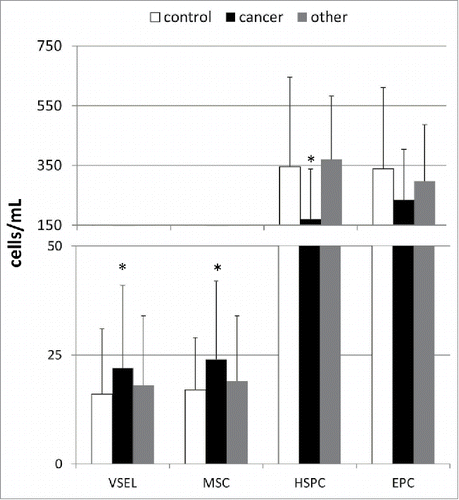
Interestingly, when we compared the numbers of circulating BMSC populations in patients with other types of gastric neoplasms, we could not find any statistically significant differences between those patients and both the gastric cancer patients and healthy controls (). In addition, the comparison of the absolute numbers of circulating BMSCs between patients with GISTs and NENs also did not reveal any significant differences (in all cases at least p > 0.1).
Potential associations of SDF-1, complement cascade-related molecules, and S1P with the intensified trafficking of BMSCs in patients with gastric cancer
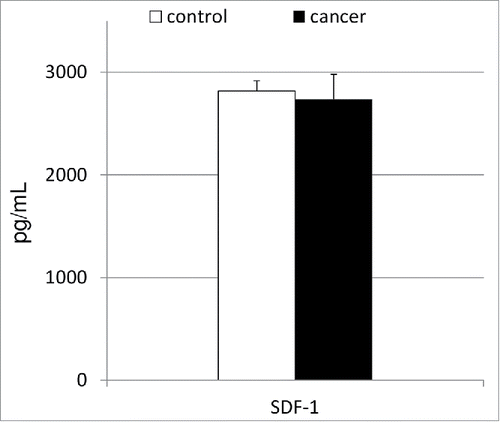
To elucidate the biochemical/molecular mechanisms associated with the observed phenomenon of altered circulation of BMSCs in patients with gastric cancer, we decided to analyze the systemic levels of biochemical molecules that are known to be involved in the orchestration of the peripheral trafficking of SCs—for example, SDF-1 (). Our results showed that patients with gastric cancer have mean SDF-1 levels similar to those in healthy controls, and these values did not significantly correlate with the absolute numbers of VSELs, MSCs, or HSPCs (r = 0.06, r = 0.11 and r = 0.13; in all cases at least p > 0.25, respectively).
Figure 2. Mean concentrations of serum stromal-derived factor-1 in patients with gastric cancer together with their statistical comparison with levels observed in healthy individuals. SDF-1—stromal-derived factor-1.
We next aimed to determine whether, as in our previous study on patients with pancreatic cancer,Citation18 in this study too, the activation of the complement cascade occurs in patients with gastric cancer, which is characterized by increased systemic levels of its anaphylatoxins/molecules (C3a, C5a, and C5b-9/MAC). We also aimed to determine whether this phenomenon might be associated with the observed alterations in the peripheral profile of circulating BMSCs in patients with gastric cancer. In our study, the gastric cancer patients had significantly increased systemic levels of C5b-9/MAC, while C3a and C5a concentrations were similar to those observed in healthy individuals (). More importantly, the levels of the examined complement fractions significantly correlated with the absolute numbers of circulating VSELs and MSCs in patients with gastric cancer (), while such tendencies were not detected in the control group (data not shown).
Figure 3. Mean concentrations of examined complement anaphylatoxins in patients with gastric cancer, and their statistical comparison with levels observed in healthy individuals. MAC—membrane attack complex, *p <0.01 (level of significance vs. control individuals).
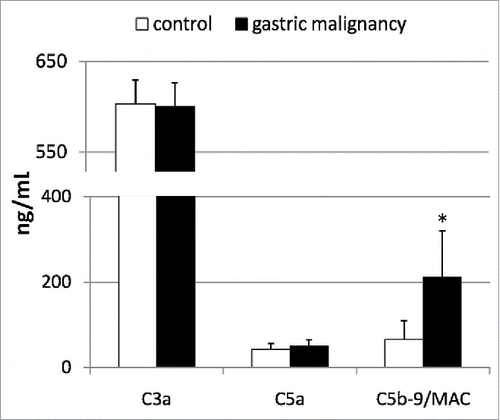
Table 2. Coefficients of correlations between absolute numbers of circulating stem cells' populations and systemic levels of complement anaphylatoxins measured in patients with gastric cancer.
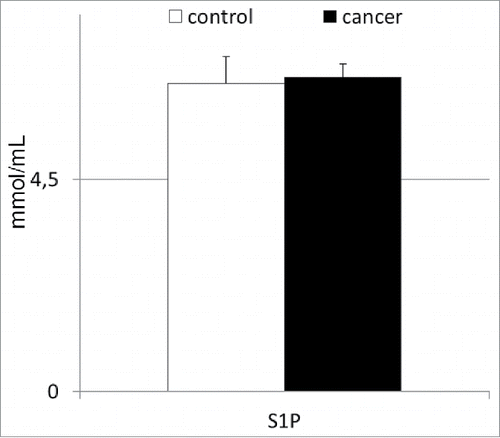
Finally, we decided to analyze the plasma S1P levels in patients with gastric cancer, as this molecule has recently been shown to potentially influence the peripheral trafficking of BMSCs in experimental animals.Citation14-16 In our study, patients with gastric cancer had mean systemic S1P levels similar to those of the healthy individuals (). Interestingly, these values significantly correlated with the absolute numbers of circulating HSPCs (r = 0.32; p < 0.05), but not with those of other populations of BMSCs (in all cases at least p > 0.3) enumerated in the peripheral blood derived from patients with gastric cancer.
Discussion
The fact that BMSCs may support and participate in gastric carcinogenesis in an experimental setting has been revealed recently.Citation10-12 While this preliminary discovery generated a lot of interest in the potential role of BMSCs in cancer in general, very little is known about BMSC homeostasis in patients with different types of gastric neoplasms. Therefore, in this study, we made a translational effort to comprehensively examine and compare the peripheral trafficking of different populations of BMSCs in these patients, as well as to (at least preliminarily) elucidate the biochemical/immune mechanisms that might be associated with this phenomenon in individuals affected by various gastric tumors.
First, we found abnormal peripheral trafficking of BMSCs in patients with gastric cancer, but not in those with other types of gastric neoplasms. This is characterized by an intensified egress of these SC populations that mainly participate in the regeneration of solid organs. In addition, we also noticed that this phenomenon occurs irrespective of the tumor stage. From the molecular standpoint, this observation seems perfectly compatible with the results of several previous experimental studies that showed intensified accumulation of Oct-4+ cells within gastric cancer tissues, which was not associated with TNM stage, depth of invasion, or the presence of metastasis in the lymph nodes; these studies also described the significant contribution of MSCs in the different stages of gastric carcinogenesis.Citation19-22 However, in our study, other populations of BMSCs that are usually not so “directly” involved in the regeneration of solid organs (such as EPCs and/or HSPCs) were not mobilized from the BM microenvironment. Similar results were also reported by Kim et al., who did not observe a significant peripheral egress of EPCs in patients with gastric cancer.Citation23 Moreover, a similar profile of circulating BMSCs was also observed in another study in patients with pancreatic adenocarcinoma.Citation18 Therefore, it seems justified to conclude that cancers developing in the gastrointestinal tract are associated with modulated peripheral trafficking of various populations of BMSCs in humans, and that this process seems to occur irrespective of the clinical stage of the malignancy.
At this stage of our research, it is very difficult to explain the molecular basis underlying this aforementioned phenomenon. Hence, we also focused on the analysis of potential biochemical and/or immunological substances that might be involved in the orchestration of systemic BMSC trafficking in humans. One of the most important molecules believed to be involved in the orchestration of the systemic egress and anchoring of BMSCs in both physiological and/or pathological conditions is SDF-1.Citation24,25 However, in our study, we found that the systemic levels of this well-known chemoattractant for BMSCs were not significantly associated with the absolute numbers of circulating BMSCs in the gastric patients; these levels were also comparable to those in healthy individuals. Thus, our results indicate that abnormal peripheral trafficking of BMSCs observed in patients with gastric cancer seems to occur in an SDF-1-independent manner. Interestingly, some experimental reports have suggested that SDF-1, together with other biochemical molecules and/or growth factors, may play a significant role in the promotion of gastric carcinogenesis.Citation26-28 Paradoxically, the results of our clinical study do not depreciate the potential significance of this chemoattractant in gastric carcinogenesis in humans. While the systemic changes in the peripheral trafficking of BMSCs in our study were not associated with SDF-1 concentrations, the activity of this molecule may still successfully contribute to the promotion of cancerogenesis, for example via its local/tissue influence on the process of anchoring of cancer (stem) cells to the microenvironment of a developing tumor.Citation29 Such mechanisms have already been proposed by various authors, who also found increase in the local expression of SDF-1 genes in human gastric cancer samples.Citation26,27
However, our findings additionally highlighted the importance of complement cascade-derived anaphylatoxins and molecules as potential important “systemic” players in the molecular dialog between the bone marrow microenvironment and the developing gastric cancer in humans. Based on the analyses of different types of malignancies in humans, several investigators have recently proved that complement derivatives may exert some pro-metastatic and chemotactic influence on various cancer (stem) cells, as well as, on normal mobilized BMSCs.Citation30,31 Interestingly, in our previous study based on the analysis of patients with pancreatic cancer, we observed a similar network of associations between the complement cascade-derived anaphylatoxins/molecules, BMSCs, and the developing cancer.Citation18 So far, very little is known about the clinical impact of these molecular interactions, but our results suggest that these may play an important role in development/progression of different types of gastrointestinal cancers. We believe that the modulation of these interactions using various inhibitors of the complement cascade may offer some potential therapeutic benefits for gastric cancer in the future. Undoubtedly, this aspect should be verified in further clinical studies.
It seems to be important to highlight the fact that although our study reports a very interesting phenomenon, a lot of challenging concerns remain. Currently, one cannot clearly state the exact function of BMSCs that egress from the bone marrow in patients with gastric cancer. Further, whether these cells are indeed migrating to and/or anchoring within the gastric tissue in humans needs to be fully elucidated; it is also possible that their contribution to the developing/progressing cancer is rather more “indirect”. In addition, the potential of these SCs as eventual biomarkers should be verified, and it remains to be determined if the measurement and/or modulation of the homeostasis of BMSCs may offer some diagnostic, therapeutic and/or prognostic benefits for gastric cancer patients, as hoped by some researchersCitation32-34 It will also be important to verify if, among the various populations of BMSCs, selected SCs populations such as Oct-4+ VSELs do not possess the properties of the tumor-initiating cells. In terms of human gastric cancer, the molecular signatures of these cells have not yet been clearly defined and remain a matter of a debate.Citation35-39
Despite the above-mentioned challenges, our study showed that abnormal peripheral trafficking of BMSCs occurs in patients with gastric cancer, but not in those with other types of gastric neoplasms. Moreover, our findings highlighted the importance of the complement cascade-derived molecules and S1P, but not SDF-1, as significant players associated with this phenomenon.
Materials and methods
Patients and blood samples
A total of 53 individuals with generally good and stable health were included in the study. Among the recruited individuals, 23 patients had recently diagnosed gastric cancer and 15 were diagnosed with other types of gastric neoplasms (GISTs [n = 9], NENs [n = 5] and/or gastric lymphomas [n = 1]). In addition, 15 healthy individuals were included in the study as controls. All patients were recruited from the Department of Gastroenterology of the Pomeranian Medical University in Szczecin. At the time of inclusion in the study, none of the patients was on chemotherapy, had received any cytotoxic agents/drugs within the last 12 mo before the study, nor presented signs of an active infectious disease.
Final diagnosis of the gastric neoplasm was based on analysis of the biopsy specimen. In order to establish staging of the disease, all patients underwent ultrasonography, computed tomography, and/or endoscopic ultrasonography, as well as chest X-ray examinations. In the “cancer” group, 11 patients were diagnosed with early and 12 with advanced gastric cancer according to the Japanese criteria. According to the Tumor Node Metastasis (TNM) classification, 11 patients had stage I gastric cancer, 2 had stage II, and 8 patients presented with metastasis (stage IV). Two patients died before the exact stage of the malignancy could be determined by further diagnostic/clinical assessment. Histological analysis revealed “intestinal” type gastric cancer in 13 patients, “diffuse” type in 9, and a “mixed” type in 1 patient. Among the patients with GISTs, six stage I patients showed low-grade tumors, and three stage II and III patients showed moderate/high grade of malignant potential. In patients with NEN lesions, all of the diagnosed tumors were non-functional and had low/moderate grade malignancy (NEN G1/G2). None of the NEN patients presented any signs of metastasis, neither to lymph nodes nor to distant solid organs. One of the recruited patients had primary gastric diffuse large B-cell lymphoma. The general characteristics of the individuals enrolled in the study, together with a statistical comparison of these features between the examined groups, are presented in .
As in our previous studiesCitation18,40 peripheral blood (PB) samples (8–10 mL) were collected from all included individuals. The absolute numbers of leukocytes and lymphocytes in PB were determined at the time of sample collection using an automatic cell counter (SYSMEX XT-2000i). Blood samples were centrifuged to obtain whole cell pellet and plasma fractions. Subsequently, plasma samples were frozen and stored at –80°C until further assessment of selected biochemical and immunomodulatory molecules. The population of PB-derived leukocytes was obtained from collected cell pellets after lysis of red blood cells with ammonium chloride-based lysing solution (BD Pharm Lyse Buffer; BD Biosciences PharMingen, San Diego, CA). Purified whole leukocyte fractions were further used for staining and flow cytometric analysis for stem/progenitor cell identification as described below.
Flow cytometry analysis of circulating populations of BMSCs
Flow cytometry analyses were performed according to the procedures previously described.Citation18,40 Briefly, circulating VSELs (FSClow/SSClow/CD45–/Lin–/CD133+ and FSClow/SSClow/CD45–/Lin–/CD34+ cells) and HSPCs (CD45+/Lin–/CD133+ and CD45+/Lin–/CD34+ cells) were identified following immunostaining of the whole PB-derived nucleated cell fraction against hematopoietic lineage markers (Lin), CD45 antigen, CD133, or CD34. Antibodies for Lin markers included fluorescein isothiocyanate (FITC)-conjugated murine anti-human antibodies against the following antigens: CD2, CD3, CD14, CD66b, CD24, CD56, CD16, CD19, and CD235a. EPCs (CD45−/CD31+/CD133+ and CD45−/CD31+/CD34+/KDR+ cells) were stained with fluorescent-labeled antibodies against CD45, CD31, CD133, CD34, and KDR (also known as VEGFR2), while MSCs were labeled using antibodies against CD45, CD105, and Stro-1. Appropriate sets of isotype control antibodies were used for each staining and negative control samples were used to set up the gating strategy for identification of all indicated SC populations.
Additionally, a single-cell suspension was stained for lineage markers (CD56, CD235a, CD3, CD66b, CD24, CD19, CD14, CD16, and CD2) conjugated with fluorescein isothiocyanate, CD45 conjugated with PE, and CXCR4 conjugated with APC. Samples were incubated with antibodies in PBS containing 2% fetal bovine serum (FBS; Invitrogen) for 30 min on ice and then were washed and fixed with 4% paraformaldehyde solution for 20 min. Fixed cells were subsequently stained with Hoechst 33342 (2 μg/mL, Sigma-Aldrich) to visualize nucleated objects and exclude debris from subsequent analysis with an LSR II flow cytometer (Becton Dickinson). The absolute numbers of circulating stem/progenitor cells per microliter of PB were computed based on (1) the percentage content of each subpopulation within the whole leukocyte fraction and (2) the white blood cell (WBC) count. The absolute numbers of circulating SCs were then re-calculated per milliliter of PB.
Analysis of systemic levels of complement cascade protein cleavage fragments, SDF-1 and S1P
The concentrations of SDF-1 and complement cascade molecules (C3a, C5a, C5b-9/MAC) were measured using commercially available, high-sensitivity ELISA kits (R&D Systems, Minneapolis, MN, USA and BD Bioscience OptEIA ELISA Kits, MD, USA) according to the manufacturer's instructions. However, S1P concentrations were measured using reverse-phase high performance liquid chromatography (RP-HPLC) according to previously published protocols.Citation41,42 Briefly, specified volume of internal standard (S1P C17), 1M NaCL and methanol was added to plasma, mixed, chloroform was added and mixture was again centrifuged. Lower organic phase was transferred to another tube. To non-organic phase, chloroform was added and mixture was centrifuged. Two organic phases were combined, and dried in vacuum centrifuge. Dried substance was diluted in methanol, reactive mixture was added (o-phthaldialdehyde, methanol, mercaptoethanol, boric acid pH 10.5), incubated and finally centrifuged. Clear supernatant was transferred to a fresh tube and analyzed using high-performance liquid chromatography. Separation was performed using isocractic method and following separation conditions/tools were applied: Cosmosil 5 µm C18-ARII (150 × 4.6) column; cartridge 5 µm C18-ARII (10 × 4.6); moving phase:10 mM K2HPO4:methanol:1 M TBAP (78:21:5:0.5), temperature 25°C, flow 1.0 mL/min; fluorescence detector (excitation: 340 nm; emission: 455 nm).
Statistical methods
Analogically as in our previous studiesCitation43-46 in order to determine the distribution of the continuous variables analyzed, the Shapiro–Wilk's test was used. For comparison of mean parameter values between examined groups, Student's t-test was used (for normally distributed variables). For variables that were not normally distributed, the variable values were log transformed. If a normal distribution was then achieved, these transformed variables were also compared using Student's t-test. However, if the transformation did not create a normal distribution, the Mann–Whitney U-test was performed. Correlations between various analyzed parameters were calculated using Pearson's test or Spearman's rank test, according to the normality of the distribution. To evaluate the effects of continuous variables on numbers of circulating BMSCs in patients with gastric cancer, multivariate regression analyses were performed using a stepwise selection method. Variables excluded from the initial model were re-entered individually to exclude residual confounding. During development of multivariate regression models, the number of inserted independent variables did not exceed 10% of the total number of analyzed patients. Constructed models were verified using the Akaike information criterion (AIC), and wrongly constructed matrices resulted in rejection of the model. Statistical analysis was performed using SPSS statistical analysis software, and significance was defined as p < 0.05.
This study was performed in accordance with appropriate regulations and guidelines highlighted in the “World Medical Association Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects”. The study protocol was approved by the Institutional Bioethical Committee of the Pomeranian Medical University in Szczecin, and all patients provided written informed consent prior to inclusion in the study.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was financed the European Union Structural Funds grant for Innovative Economy Operational Program (POIG 01.01.02–00–109/09). The grant founders had no role in the study design, data acquisition and analysis, or in the decision to submit the article for publication. WB receives the “START” stipend awarded by the Foundation for Polish Science.
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics. CA Cancer J Clin 2015; 65:87-108; PMID:25651787; http://dx.doi.org/10.3322/caac.21262
- Park JY, von Karsa L, Herrero R. Prevention strategies for gastric cancer: a global perspective. Clin Endosc 2014; 47:478-89; PMID:25505712; http://dx.doi.org/10.5946/ce.2014.47.6.478
- Barrios CH, Blackstein ME, Blay JY, Casali PG, Chacon M, Gu J, Kang YK, Nishida T, Purkayastha D, Woodman RC et al. The GOLD ReGISTry: a Global, Prospective, Observational Registry Collecting Longitudinal Data on Patients with Advanced and Localised Gastrointestinal Stromal Tumours. Eur J Cancer 2015; 51:2423–33; PMID:26248685; http://dx.doi.org/10.1016/j.ejca.2015.07.010
- Scherubl H, Streller B, Stabenow R, Herbst H, Hopfner M, Schwertner C, Steinberg J, Eick J, Ring W, Tiwari K et al. Clinically detected gastroenteropancreatic neuroendocrine tumors are on the rise: epidemiological changes in Germany. World J Gastroenterol 2013; 19:9012-9; PMID:24379626; http://dx.doi.org/10.3748/wjg.v19.i47.9012
- Belaid I, Chabchoub I, Mejri N, Zaghouani H, Chafai R, Ezairi F, Hochlaf M, Gharbi O, Fatma LB, Ahmed SB. Clinicopathological study of primary gastric lymphomas in the central region of Tunisia, with survival analysis. Eur J Gastroenterol Hepatol 2013; 25:1060-7; PMID:23778310; http://dx.doi.org/10.1097/MEG.0b013e3283636233
- Saikawa Y, Fukuda K, Takahashi T, Nakamura R, Takeuchi H, Kitagawa Y. Gastric carcinogenesis and the cancer stem cell hypothesis. Gastric Cancer 2010; 13:11-24; PMID:20373071; http://dx.doi.org/10.1007/s10120-009-0537-4
- Zhao Y, Feng F, Zhou YN. Stem cells in gastric cancer. World J Gastroenterol 2015; 21:112-23; PMID:25574084; http://dx.doi.org/10.3748/wjg.v21.i1.112
- Li K, Dan Z, Nie YQ. Gastric cancer stem cells in gastric carcinogenesis, progression, prevention and treatment. World J Gastroenterol 2014; 20:5420-6; PMID:24833872; http://dx.doi.org/10.3748/wjg.v20.i18.5420
- Wu XZ, Chen D, Xie GR. Bone marrow-derived cells: roles in solid tumor. Neoplasma 2007; 54:1-6; PMID:17203886
- Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science 2004; 306:1568-71; PMID:15567866; http://dx.doi.org/10.1126/science.1099513
- Varon C, Dubus P, Mazurier F, Asencio C, Chambonnier L, Ferrand J, Giese A, Senant-Dugot N, Carlotti M, Megraud F. Helicobacter pylori infection recruits bone marrow-derived cells that participate in gastric preneoplasia in mice. Gastroenterology 2012; 142:281-91; PMID:22062361; http://dx.doi.org/10.1053/j.gastro.2011.10.036
- Worthley DL, Ruszkiewicz A, Davies R, Moore S, Nivison-Smith I, Bik To L, Browett P, Western R, Durrant S, So J et al. Human gastrointestinal neoplasia-associated myofibroblasts can develop from bone marrow-derived cells following allogeneic stem cell transplantation. Stem Cells 2009; 27:1463-8; PMID:19492298; http://dx.doi.org/10.1002/stem.63
- Ratajczak MZ, Liu R, Marlicz W, Blogowski W, Starzynska T, Wojakowski W, Zuba-Surma E. Identification of very small embryonic/epiblast-like stem cells (VSELs) circulating in peripheral blood during organ/tissue injuries. Methods Cell Biol 2011; 103:31-54; PMID:21722799; http://dx.doi.org/10.1016/B978-0-12-385493-3.00003-6
- Ratajczak MZ, Kim C. Bioactive sphingolipids and complement cascade as new emerging regulators of stem cell mobilization and homing. J Stem Cell Res Ther 2011; 1:102; PMID:24380038; http://dx.doi.org/10.4172/2157-7633.1000e102
- Golan K, Vagima Y, Ludin A, Itkin T, Cohen-Gur S, Kalinkovich A, Kollet O, Kim C, Schajnovitz A, Ovadya Y et al. S1P promotes murine progenitor cell egress and mobilization via S1P1 mediated ROS signaling and SDF-1 release. Blood 2012; 119:2478-88; PMID:22279055; http://dx.doi.org/10.1182/blood-2011-06-358614
- Marquez-Curtis LA, Turner AR, Sridharan S, Ratajczak MZ, Janowska-Wieczorek A. The ins and outs of hematopoietic stem cells: studies to improve transplantation outcomes. Stem Cell Rev 2011; 7:590-607; PMID:21140298; http://dx.doi.org/10.1007/s12015-010-9212-8
- Kong Y, Wang H, Lin T, Wang S. Sphingosine-1-phosphate/S1P receptors signaling modulates cell migration in human bone marrow-derived mesenchymal stem cells. Mediators Inflamm 2014; 2014:565369; PMID:25147438; http://dx.doi.org/10.1155/2014/565369
- Starzynska T, Dabkowski K, Blogowski W, Zuba-Surma E, Budkowska M, Sałata D, Dołęgowska B, Marlicz W, Lubikowski J, Ratajczak MZ. An intensified systemic trafficking of bone marrow-derived stem/progenitor cells in patients with pancreatic cancer. J Cell Mol Med 2013; 17:792-9; PMID:23672538; http://dx.doi.org/10.1111/jcmm.12065
- Asadi MH, Mowla SJ, Fathi F, Aleyasin A, Asadzadeh J, Atlasi Y. OCT4B1, a novel spliced variant of OCT4, is highly expressed in gastric cancer and acts as an antiapoptotic factor. Int J Cancer 2011; 128:2645-52; PMID:20824712; http://dx.doi.org/10.1002/ijc.25643
- Chen Z, Xu WR, Qian H, Zhu W, Bu XF, Wang S, Yan YM, Mao F, Gu HB, Cao HL et al. Oct4, a novel marker for human gastric cancer. J Surg Oncol 2009; 99:414-9; PMID:19347886; http://dx.doi.org/10.1002/jso.21270
- Okumura T, Wang SS, Takaishi S, Tu SP, Ng V, Ericksen RE, Rustgi AK, Wang TC. Identification of a bone marrow-derived mesenchymal progenitor cell subset that can contribute to the gastric epithelium. Lab Invest 2009; 89:1410-22; PMID:19841619; http://dx.doi.org/10.1038/labinvest.2009.88
- Lin R, Ma H, Ding Z, Shi W, Qian W, Song J, Hou X. Bone marrow-derived mesenchymal stem cells favor the immunosuppressive T cells skewing in a Helicobacter pylori model of gastric cancer. Stem Cells Dev 2013; 22:2836-48; PMID:23777268; http://dx.doi.org/10.1089/scd.2013.0166
- Kim HK, Song KS, Kim HO, Chung JH, Lee KR, Lee YJ, Lee DH, Lee ES, Kim HK, Ryu KW et al. Circulating numbers of endothelial progenitor cells in patients with gastric and breast cancer. Cancer Lett 2003; 198:83-8; PMID:12893434; http://dx.doi.org/10.1016/S0304-3835(03)00268-4
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 2004; 10:858-64; PMID:15235597; http://dx.doi.org/10.1038/nm1075
- Ceradini DJ, Gurtner GC. Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc Med 2005; 15:57-63; PMID:15885571; http://dx.doi.org/10.1016/j.tcm.2005.02.002
- Iwasa S, Yanagawa T, Fan J, Katoh R. Expression of CXCR4 and its ligand SDF-1 in intestinal-type gastric cancer is associated with lymph node and liver metastasis. Anticancer Res 2009; 29:4751-8; PMID:20032431
- Zhao BC, Wang ZJ, Mao WZ, Ma HC, Han JG, Zhao B, Xu HM. CXCR4/SDF-1 axis is involved in lymph node metastasis of gastric carcinoma. World J Gastroenterol 2011; 17:2389-96; PMID:21633638; http://dx.doi.org/10.3748/wjg.v17.i19.2389
- Wang X, Zhang H, He H, Shen Z, Tang Z, Xu J, Sun Y. Prognostic value of stromal cell-derived factor 1 expression in patients with gastric cancer after surgical resection. Cancer Sci 2014; 105:1447-56; PMID:25220301; http://dx.doi.org/10.1111/cas.12531
- Stoicov C, Li H, Liu JH, Houghton J. Mesenchymal stem cells utilize CXCR4-SDF-1 signaling for acute, but not chronic, trafficking to gastric mucosal inflammation. Dig Dis Sci 2013; 58:2466-77; PMID:23873382; http://dx.doi.org/10.1007/s10620-013-2782-y
- Orr FW, Delikatny EJ, Mokashi S, Krepart GV, Stiver HG. Detection of a complement-derived chemotactic factor for tumor cells in human inflammatory and neoplastic effusions. Am J Pathol 1983; 110:41-7; PMID:6185003
- Schraufstatter IU, Discipio RG, Zhao M, Khaldoyanidi SK. C3a and C5a are chemotactic factors for human mesenchymal stem cells, which cause prolonged ERK1/2 phosphorylation. J Immunol 2009; 182:3827-36; PMID:19265162; http://dx.doi.org/10.4049/jimmunol.0803055
- Shin SJ, Jeung HC, Ahn JB, Rha SY, Yoo NC, Roh JK, Noh SH, Chung HC. Mobilized CD34+ cells as a biomarker candidate for the efficacy of combined maximal tolerance dose and continuous infusional chemotherapy and G-CSF surge in gastric cancer. Cancer Lett 2008; 270:269-76; PMID:18555590; http://dx.doi.org/10.1016/j.canlet.2008.05.011
- Pantel K, Alix-Panabieres C. Bone marrow as a reservoir for disseminated tumor cells: a special source for liquid biopsy in cancer patients. Bonekey Rep 2014; 3:584; PMID:25419458; http://dx.doi.org/10.1038/bonekey.2014.79
- Stojnev S, Krstic M, Ristic-Petrovic A, Stefanovic V, Hattori T. Gastric cancer stem cells: therapeutic targets. Gastric Cancer 2014; 17:13-25; PMID:23563919; http://dx.doi.org/10.1007/s10120-013-0254-x
- Takaishi S, Okumura T, Wang TC. Gastric cancer stem cells. J Clin Oncol 2008; 26:2876-82; PMID:18539967; http://dx.doi.org/10.1200/JCO.2007.15.2603
- Chen T, Yang K, Yu J, Meng W, Yuan D, Bi F, Liu F, Liu J, Dai B, Chen X et al. Identification and expansion of cancer stem cells in tumor tissues and peripheral blood derived from gastric adenocarcinoma patients. Cell Res 2012; 22:248-58; PMID:21727908; http://dx.doi.org/10.1038/cr.2011.109
- Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 2009; 27:1006-20; PMID:19415765; http://dx.doi.org/10.1002/stem.30
- Xu GF, Zhang WJ, Sun Q, Xu X, Zou X, Guan W. Combined epithelial-mesenchymal transition with cancer stem cell-like marker as predictors of recurrence after radical resection for gastric cancer. World J Surg Oncol 2014; 12:368; PMID:25441488; http://dx.doi.org/10.1186/1477-7819-12-368
- Rocco A, Liquori E, Pirozzi G, Tirino V, Compare D, Franco R, Tatangelo F, Palaia R, D'Armiento FP, Pollastrone G et al. CD133 and CD44 cell surface markers do not identify cancer stem cells in primary human gastric tumors. J Cell Physiol 2012; 227:2686-93; PMID:21898409; http://dx.doi.org/10.1002/jcp.23013
- Blogowski W, Serwin K, Salata D, Budkowska M, Dolegowska B, Lokaj M, Prowans P, Starzynska T. Plasma and adipose tissue levels of selected growth/inhibitory factors, proteolytic enzymes and sphingosine-1-phosphate in humans. Eur J Inflamm 2012; 3:279-88.
- Marlicz W, Zuba-Surma E, Kucia M, Blogowski W, Starzynska T, Ratajczak MZ. Various types of stem cells, including a population of very small embryonic-like stem cells, are mobilized into peripheral blood in patients with Crohn's disease. Inflamm Bowel Dis 2012; 18:1711-22; PMID:22238186; http://dx.doi.org/10.1002/ibd.22875
- Blogowski W, Dolegowska B, Budkowska M, Salata D, Domanski L, Starzynska T. Perioperative release of pro-regenerative biochemical signals from human renal allografts subjected to ischemia-reperfusion injury. Innate Immun 2014; 20:126-32; PMID:23608824; http://dx.doi.org/10.1177/1753425913482018
- Blogowski W, Serwin K, Budkowska M, Salata D, Dolegowska B, Lokaj M, Prowans P, Starzynska T. Clinical analysis of systemic and adipose tissue levels of selected hormones/adipokines and stromal-derived factor-1. J Biol Regul Homeostat Agents 2012; 26:607-15; PMID:23241111
- Blogowski W, Dolegowska B, Salata D, Budkowska M, Domanski L, Starzynska T. Clinical analysis of perioperative complement activity during ischemia/reperfusion injury following renal transplantation. Clin J Am Soc Nephrol 2012; 7:1843-51; PMID:22904122; http://dx.doi.org/10.2215/CJN.02200312
- Dolegowska B, Blogowski W, Domanski L. Association between the perioperative antioxidative ability of platelets and early post-transplant function of kidney allografts: a pilot study. PLoS One 2012; 7:e29779; PMID:22279544; http://dx.doi.org/10.1371/journal.pone.0029779
- Deskur A, Salata D, Budkowska M, Dolegowska B, Starzynska T, Blogowski W. Selected hemostatic parameters in patients with pancreatic tumors. Am J Transl Res 2014; 6:768-76; PMID:25628787