ABSTRACT
The purpose of combining targeted agents and immunotherapy is to achieve a chance of long-term tumor control in highly advanced patients. Between April 2012 and December 2013, 10 patients with metastatic melanoma were treated with a combination treatment of vemurafenib and ipilimumab as an individual treatment decision after detailed information and giving written informed consent. All the patients had advanced symptomatic disease, seven with elevated serum lactate dehydrogenase (LDH) levels and six with brain metastases on MRI. After clinical improvement under vemurafenib monotherapy (median 11.5 weeks), four cycles of ipilimumab were administered additionally. Combination treatment was tolerated well, with only two patients developing ≥ grade 3 elevation of transaminases; this was asymptomatic and resolved on cessation of BRAF inhibitor treatment. Staging 12 weeks after initiation of ipilimumab revealed partial response for five patients, stable disease for two, and disease progression for three. Of the seven patients with disease control, we stopped vemurafenib for five, to determine whether ipilimumab treatment led to disease control. Two revealed progressive disease 2 mo later, and received vemurafenib again, but for three the disease was controlled for at least a year, and two are still in partial remission without any further treatment. Progression-free survival was a median of 8.0 mo (95% CI 4.8–11.2), overall survival (OS) was 13.0 mo (95% CI 5.0–21.0), and four patients are still alive. In conclusion, the combination of vemurafenib and ipilimumab was well tolerated and clinical outcome was promising. The combination of targeted and immunotherapies is currently addressed in clinical trials.
Abbreviations
| BRAF | = | B-Raf kinase (Raf: Rapidly Accelerated Fibrosarcoma) |
| BRIM2/3 | = | Phase 2/3 trial with the BRAF inhibitor Vemurafenib in melanoma |
| CD | = | cluster of differentiation |
| CR | = | Complete response |
| CTC-AE | = | Common toxicity criteria for adverse events |
| LDH | = | lactate dehydrogenase |
| MEK | = | MAPK ERK Kinase |
| MRI | = | Magnetic resonance imaging |
| OS | = | Overall survival |
| PD | = | Progressive disease |
| PFS | = | Progression free survival |
| PR | = | Partial response |
| SD | = | Stable disease |
Introduction
The combination of targeted therapy with immunotherapy is of much interest for treatment of metastasized melanoma, especially for patients with a large tumor burden. Targeted treatments with the selective BRAF inhibitors vemurafenib or dabrafenib have shown high response rates even in patients with a high tumor burden but tend to loose efficacy by the development of melanoma resistance about 7 mo after initiation of treatment.Citation1,2 The addition of another targeted drug namely a MEK inhibitor can delay the timepoint of resistance but the rate for a long-term tumor control seems to be low.Citation3,4 Concerning immunotherapy with the immune checkpoint blocker ipilimumab at least 20% of patients seem to be able to achieve this long-term tumor control.Citation5 Here, the serum LDH level was shown to correlate with survival upon ipilimumab treatment.Citation6 Ribas et al.Citation7 conducted a phase I trial in which the BRAF inhibitor vemurafenib was combined with the immune checkpoint blocker ipilimumab; the trial was stopped early because of significant liver toxicity.Citation7 Independently, we offered selected patients this combination treatment in a similar regimen in Germany as an individual treatment decision.
Results
We report results from a cohort of 10 patients who received vemurafenib and ipilimumab combination therapy between April 2012 and December 2013 at the Dermato–oncology clinic at the National Center for Tumor diseases of the University Hospital Heidelberg, Germany (patient characteristics are listed in ). All patients (median age 44.5 y, range 25–66; five males, five females) had very advanced disease (AJCC stage IV M1c). Elevated LDH levels were measured for seven patients (median LDH 1.3 times ULN; range up to 2.5 times ULN). 9 of the 10 patients had symptomatic disease; six had brain metastases (two patients with solitary brain metastases and four with several brain metastases). Five of the six patients with brain metastases had received previous treatment of the brain before the onset of vemurafenib treatment. Solitary brain metastases were treated by use of either neurosurgery or stereotactic radiotherapy. Multiple metastases were treated by use of whole-brain radiotherapy. One patient received vemurafenib immediately, because of symptomatic metastases outside the brain namely in hepatic and bone metastases.
Table 1. Patient characteristics.
Treatment was started with vemurafenib (960 mg orally, twice daily). After clinical improvement of symptoms had been achieved, ipilimumab was also administered. Treatment with ipilimumab was initiated 3–30 weeks (median 11.5 weeks) after starting vemurafenib. For two patients, the dose of vemurafenib had to be reduced to 720 mg twice daily because of skin toxicity. 9 of the 10 patients received four doses of ipilimumab; for one patient, treatment was stopped after three cycles because of rapid progression of the disease.
Overall, the combination therapy was well tolerated. Although treatment-related side effects were frequently observed, most were mild ()—rashes, diarrhea, arthralgia, or elevation of liver transaminases. The last of these was more frequent than expected during combination treatment for ipilimumab monotherapy, as reported elsewhere.Citation7 Elevation of transaminases had to be scored ≥ grade 3 (CTC-AE) for two patients only, however, and (although asymptomatic) led to interruption of vemurafenib treatment. Because, for these patients, autoimmune hepatitis could not be excluded, high-dose systemic steroid treatment was given for a week () then tapered down for one patient and discontinued completely for the other after having no effect on transaminases and no further signs of autoimmune hepatitis. For both patients, pausing of vemurafenib resulted in significant reduction of liver transaminases, which reached grade 1 within 3 weeks.
The best responses to the combination treatment 12 weeks after initiation of ipilimumab were: PR for five patients, SD for two patients, progression of disease (PD) for three patients, according to RECIST. Thus, disease control was achieved for seven patients. After detailed discussion with the patients, we stopped vemurafenib treatment for five of these to determine whether ipilimumab treatment led to disease control. In subsequent staging after 2 mo without further treatment, progression of the disease was observed for two patients, who were again given vemurafenib. The other three were without further PD for at least a year, and two are still in partial remission, for 16 and 17 mo, respectively. One of these patients is demonstrated in . Statistically, median progression-free survival (PFS) was 8.0 mo (95% CI 4.8–11.2), and hence better than expected for monotherapy with one of the drugs, especially so because the treated patients had very advanced disease. OS was 13.0 mo (95% CI 5.0–21.0), and four patients are still alive.
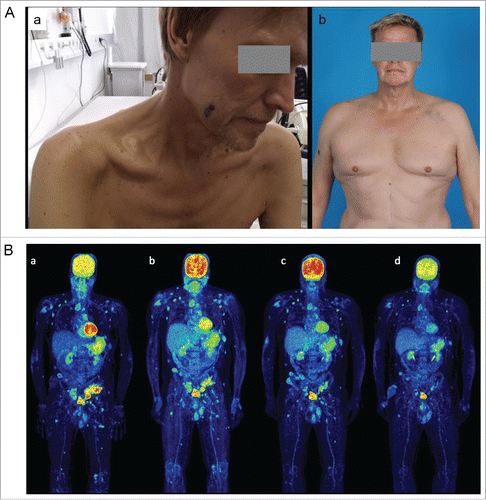
Figure 1. (A). Cachectic 56 y old patient with extensive stage IV disease before start of vemurafenib (a) and after 6 mo of combination treatment with vemurafenib and ipilimumab (b). He presented with an ECOG 4 after enlarged hemicolectomy with ileostoma because of metastases induced small and great bowel ileus. Under the combination treatment the patient gained 30 kg of weight and is back to normal day life. (B). PET-CT scans before start of ipilimumab (a; after 4 mo of vemurafenib treatment, LDH decreased to normal serum levels), after four doses of ipilimumab (b; after 7 mo of vemurafenib treatment), at discontinuation of vemurafenib (c; one year after treatment start) and at last staging (d; 2.5 y after start of treatment and 1.5 y without any treatment).
Discussion
Sequencing of targeted drugs and immune checkpoint inhibitors for treatment of stage IV melanoma is still a matter of debate. Ackermann et al.Citation8 showed retrospectively for 40 patients that none responded to ipilimumab after progression on a BRAF inhibitor. Fifty-nine percent of patients had elevated LDH at the time ipilimumab treatment was started. Kelderman et al.Citation6 found that for patients treated with ipilimumab tumor load, as measured on the basis of LDH levels, was highly predictive of OS in a multivariate model. Hence, reduction of tumor load by targeted therapy might enable immune therapy to work more efficiently. However, even the clinical outcome of BRAF inhibitor treatment in melanoma patients seems to correlate with tumor load. Pooled data analysis of the BRIM2/3 trials with vemurafenib revealed that patients who responded to treatment longer than a year had significantly lower LDH levels at treatment start.Citation4,9 Hence, clinical outcome in our patient cohort with highly advanced disease was better than expectable by monotherapy. Preclinical data also indicate that the combination might have greater efficacy. In melanoma biopsies from patients before and under BRAF inhibitor treatment, expression of melanoma antigens and infiltration with CD8+ T-cells increased.Citation10,11 The side effects of combination treatments must be monitored carefully, however. As reported by Ribas et al.,Citation7 elevation of transaminases was frequent during the combination treatment but was never associated with symptoms and always resolved after cessation of the BRAF inhibitor. In our work, the frequency of this side effect was lower, possibly as a result of different patient characteristics or treatment schedule, because we treated only patients with very advanced disease and added the ipilimumab after the clinical condition of the patients improved as a result of vemurafenib treatment. Results from a clinical trial of the combination of the BRAF inhibitor dabrafenib with or without the MEK inhibitors trametinib and ipilimumab were recently reported.Citation12 In this trial, eight patients received dabrafenib and ipilimumab and seven received dabrafenib, trametinib, and ipilimumab. The double treatment was very well tolerated with no dose-limiting toxicity; one of eight patients developed grade 3 elevation of transaminases. This, however, is in the same range as we observed for our combination of vemurafenib and ipilimumab. The triple combination was much more toxic and had to be stopped because of two cases of perforating colitis. Efficacy data for this trial are still limited because of the short follow-up. In addition, the combination of dabrafenib/trametinib with PD1/PDL1 directed immune checkpoint blockers is investigated currently in phase 1 trials (NCT02130466, NCT02027962). Combination of BRAF/MEK inhibition might be superior as it leads to increased melanosomal antigen and major histocompatibility complex (MHC) expression in a mouse model.Citation13 Combination with an anti-PD1 or anti-PDL1 antibody instead of ipilimumab seems to have less severe side-effects.Citation14
Especially because of the increased risk for side-effects in the combination treatment a sequential treatment with an ‘early switch’ of the targeted substance to the immune checkpoint blocker might be a solution. One phase 2 trial that has been completed recently enrolled 46 patients with metastasized V600 mutated melanoma (NCT01673854). Patients were treated with vemurafenib for 6 weeks and then switched to ipilimumab 10 mg/kg body weight; results on the trial are pending. However, in such a short induction phase with a targeted substance you risk a regrowth of the metastases after switching to the immune checkpoint blocker in fast growing tumors.
Strength and limitations of the study
We are aware, that this is a case series and not a clinical trial. However, all treated patients are reported. In addition, patients treated had a much more advanced disease than patients usually included in clinical trials with generally a high tumor load, symptomatic disease and a high frequency of brain metastases. Good clinical outcomes in such patients are rare and hence, the results of this case series are remarkably good.
In summary, combination treatment with targeted therapy and immunotherapy has the potential to lead to long-term responses, even for patients with a high tumor load. Although side effects must be monitored carefully, we should continue to investigate these combinations among larger patient cohorts.
Patients and methods
All patients received detailed information about chances and risks of vemurafenib and ipilimumab monotherapy (e.g., the high response rates of the targeted treatment with the risk of development of resistancy and the chance of long-term response to ipilimumab in patients with a low tumor load) and the possible advantage and risks especially concerning new side-effects when combining these substances. After presentation of the phase 1 trial investigating this combination,Citation7 the patients were informed about termination of the study because of the occurred liver toxicity and that our experience in the first eight treated patients was not that severe. All patients gave written consent to this individual and experimental treatment decision; of course guidelines from the declaration of Helsinki were followed. Patient selection was based on the clinical need for rapid tumor reduction, because of symptomatic metastases, or a high tumor load treatable by use of BRAF inhibitors, for example vemurafenib. After the start of vemurafenib treatment, and improvement of melanoma-induced symptoms, combination with ipilimumab treatment was offered.
Tumor assessments were done before start of vemurafenib and before start of ipilimumab combination treatment (baseline), and then after 12 weeks after start of ipilimumab and then every 2–3 mo as done in clinical routine. For scientific analysis clinical data were extracted from patients' clinical record and analyzed using suitable statistical methods like frequency tables and Kaplan Meier analysis (SPSS Statistics version 21).
Disclosure of potential conflicts of interest
JCH received honoraria from BMS, Roche, GSK and MSD; FM from GSK, Roche, Amgen, BMS; ADS from GSK; AE from MSD and BMS—all outside the submitted work. All remaining authors have declared no conflicts of interest.
Acknowledgments
We thank Ian Davis for English revision of the manuscript.
Funding
JCH was supported by the Olympia Morata Grant of the University of Heidelberg.
References
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364:2507-16; PMID:21639808; http://dx.doi.org/10.1056/NEJMoa1103782
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH Jr, Kaempgen E et al. Dabrafenib in BRAF-mutated metastatic melanoma. A multicenter, open-label, phase 3 randomised controlled trial. Lancet 2012; 380:358-65; PMID:22735384; http://dx.doi.org/10.1016/S0140-6736(12)60868-X
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015; 372:30-9; PMID:25399551; http://dx.doi.org/10.1056/NEJMoa1412690
- Menzies AM, Wilmott JS, Drummond M, Lo S, Lyle M, Chan MM, Thompson JF, Guminski A, Carlino MS, Scolyer RA et al. Clinicopathologic features associated with efficacy and long-term survival in metastatic melanoma patients treated with BRAF or combined BRAF and MEK inhibitors. Cancer 2015; 121:3826-35; PMID:26218930; http://dx.doi.org/10.1002/cncr.29586
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 2015; 33:1889-94; PMID:25667295; http://dx.doi.org/10.1200/JCO.2014.56.2736
- Kelderman S, Heemskerk B, van Tinteren H, van den Brom RR, Hospers GA, van den Eertwegh AJ, Kapiteijn EW, de Groot JW, Soetekouw P, Jansen RL et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother 2014; 63:449-58; PMID:24609989; http://dx.doi.org/10.1007/s00262-014-1528-9
- Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med 2013; 368:1365-6; PMID:23550685; http://dx.doi.org/10.1056/NEJMc-1302338
- Ackerman A, Klein O, McDermott DF, Wang W, Ibrahim N, Lawrence DP, Gunturi A, Flaherty KT, Hodi FS, Kefford R et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer 2014; 120:1695-701; PMID:24577748; http://dx.doi.org/10.1002/cncr.28620
- Ribas A, Chapman P, McArthur G, Robert C, Sosman JA, Hauschild A, Donica M, Antic V, Makrutzki M, Flaherty KT. Long-term response in patients with BRAFV600 mutation-positive metastatic melanoma who received vemurafenib: pooled data analyses from BRIM2 and BRIM3. Pigment Cell Melanom Res 2014; 27:1179; http://dx.doi.org/10.1111/pcmr.12317
- Frederick D, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, Mitra D, Boni A, Newton LP, Liu C et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favourable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res 2013; 19:1225-31; PMID:23307859; http://dx.doi.org/10.1158/1078-0432.CCR-12-1630
- Cooper ZA, Juneja VR, Sage PT, Frederick DT, Piris A, Mitra D, Lo JA, Hodi FS, Freeman GJ, Bosenberg MW et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockage. Cancer Immunology Res 2014; 2:643-54; http://dx.doi.org/10.1158/2326-6066.CIR-13-0215
- Minor DR, Puzanov I, Callahan MK, Hug BA, Hoos A. Severe gastrointestinal toxicity with administration of trametinib in combination with dabrafenib and ipilimumab. Pigment Cell Melanoma Res 2015; 28:611-2; PMID:25996827; http://dx.doi.org/10.1111/pcmr.12383
- Hu-Lieskovan S, Mok S, Homet Moreno B, Tsoi J, Robert L, Goedert L, Pinheiro EM, Koya RC, Graeber TG, Comin-Anduix B et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci Transl Med 2015; 7:279ra41; PMID:25787767; http://dx.doi.org/10.1126/scitranslmed.aaa4691
- Ribas A, Butler M, Lutzky J, Lawrence DP, Robert C, Miller W, Linette GP, Ascierto PA, Kuzel T, Algazi AP et al. J Clin Oncol 2015; 33:1865-6, suppl; abstr 3003.Tbl.1: Patient characteristics; PMID:25667273; http://dx.doi.org/10.1200/JCO.2014.59.5041
