ABSTRACT
TNF has been associated with both inhibition and promotion of tumor growth. We recently described a mechanism by which tumor cells attract TNF producing cells via expression of MHC class II molecules.
Tumor necrosis factor (TNF, formerly TNF-α) was first characterized in the 70s as a cytotoxic molecule for cancer cells. Today, more than 40 y later it is still used for treatment of locally advanced tumors of the extremities in combination with melphalan (reviewed inCitation1). However, only in the last decade we are starting to appreciate its pleiotropic roles in cancer biology. Multiple recent reports have shown that TNF associated inflammation as well as its direct effects on tumor cells may actually be cancer promoting. In melanoma, preclinical models have shown that TNF can induce cell invasion Citation2 and angiotropism,Citation3 thus increasing the likelihood of intravasation and hematogenous dissemination; dedifferentiation, and thereby impaired sensitivity to melanocyte-differentiation antigen (MDA)-directed CD8+ immune responses; Citation4 trigger effector CD8+ T cell death impairing accumulation of CD8+ T cells in the tumor microenvironment.Citation5 Importantly, TNF inhibition prevents lung metastatization in animal models Citation6 and, in contrast to earlier reports, it is now demonstrated that chronic treatment with TNF inhibitors does not increase the risk of developing melanoma in human subjects.Citation7
Our recent studies add additional insights to the puzzling roles of TNF in cancer, pointing out tumor-specific CD4+ T cells as one major source of TNF in the tumor microenvironment of metastatic melanoma in humans driven by tumor MHC class II expression.Citation8
Of note, aberrant expression of MHC class II has repeatedly been reported in melanoma and other tumors. Tumor-antigens (TA) presented in association with MHC class II molecules may trigger activation of TA-specific CD4+ T cells in the tumor microenvironment, and lead to direct recognition of MHC II+ cancer cells. Under physiological conditions and in the absence of a local inflammatory response, expression of MHC class II molecules occurs only in hematopoietic cells and thymus epithelium. This suggests that dysregulated gene-networks leading to abnormal transcription of class II transactivator (CIITA) are responsible for MHC class II expression.
We provide evidence that the accumulation of melanoma-specific CD4+ T cells is promoted by constitutive de novo aberrant expression of MHC class II on tumor cells. In contrast to melanoma-specific CD8+ T cells, the vast majority of these tumor specific CD4+ T cells were mono-functional with TNF as principal function.
During immune attack of melanoma, activation of CD8+ T cells lead to release of large quantities of IFNγ in the tumor microenvironment. Our data indicate that, in tumors enriched with melanoma-specific CD4+ T cells, IFNγ induced upregulation of MHC class II molecules increase tumor-recognition and activation of resident CD4+ T cells, with local release of TNF which in turn impairs CD8+ T cell function ().
Figure 1. Schematic representation of immune escape of “melanoma subset I."Citation8 (A) Aberrant expression of MHC class II induce accumulation of tumor specific CD4+ T cells in the microenvironment. (B) CD8+ T cells produce IFNγ upon recognition of tumor antigens. (C) IFNγ promotes upregulation of MHC class II molecules which increase tumor recognition by tumor specific CD4+ T cells, leading to TNF release. (D) TNF increase expression of immune suppressive molecules in tumor cells, leading to reduced activation of CD8+ T cells in the tumor microenvironment. (E) Tumor cells escape CD8+ T cell responses and proliferate.
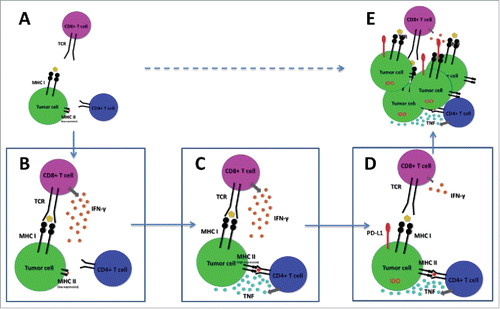
Importantly, downregulation of MHC class I with reduced response to IFNγ has been previously characterized as a major immune escape mechanism.Citation9 Our data indicate that this immune escape pathway is exclusively activated in tumors which are not able to attract a sufficient TNF-producing CD4+ T cell pool.
Thus, our results indicate that a novel mechanism of suppression of CD8+ T cell response may be linked to aberrant expression of MHC class II. However, they also indicate that only a fraction (roughly corresponding to 40%, that we named “melanoma subset I”) of melanoma seems to use this immune-escape pathway efficiently, and suggest that the remaining tumors (or “melanoma subset II”) may instead suppress CD8+ T cell recognition through reduced upregulation of MHC class I in response to IFNγ.
One previous small clinical trial has investigated the safety and biological activity of infliximab, a TNF inhibitor, in patients with advanced cancers. However, there were no objective responses reported but, at best, disease stabilization in one out of three patients with advanced melanoma.Citation10
Based on these premises and given the pleiotropic roles of TNF, we believe that it is unlikely that strategies blocking TNF may result in marked clinical efficacy if used as monotherapy. However, novel strategies based on selective targeting MHC class II-related immune escape in the tumor microenvironment may instead reach more success.
Importantly, the immune suppressive role of MHC class II may not be limited to the mechanisms described above. Immune inhibitory receptors found on the surface of T cells such as LAG-3 may bind to MHC class II molecules, transmitting immune suppressive signals. Strategies to counteract this mechanism are currently explored in clinical trials (clinicaltrials.gov identifier: NCT01968109 and NCT02061761).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Aggarwal BB, Gupta SC, Kim JH. Review article Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Hematology 2012; 119:651-65; PMID:22053109; http://dx.doi.org/10.1182/blood-2011-04-325225
- Katerinaki E, Evans GS, Lorigan PC, MacNeil S. TNF-alpha increases human melanoma cell invasion and migration in vitro: the role of proteolytic enzymes. Br J Cancer 2003; 89:1123-9. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2376936&tool=pmcentrez&rendertype=abstract; PMID:12966436; http://dx.doi.org/10.1038/sj.bjc.6601257
- Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D, Kohlmeyer J, Riesenberg S, van den Boorn-Konijnenberg D, Hömig-Hölzel C et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 2014; 507:109-13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24572365; PMID:24572365; http://dx.doi.org/10.1038/nature13111
- Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, Fatho M, Lennerz V, Wölfel T, Hölzel M et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature 2012; 490:412-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23051752; PMID:23051752; http://dx.doi.org/10.1038/nature11538
- Bertrand F, Rochotte J, Colacios C, Montfort A, Tilkin-Mariame A-F, Touriol C, Rochaix P, Lajoie-Mazenc I, Andrieu-Abadie N, Levade et al. Blocking Tumor Necrosis Factor enhances CD8 T cell-dependent immunity in experimental melanoma. Cancer Res 2015; 75(13):2619-28; Available from: http://cancerres.aacrjournals.org/cgi/doi/10.1158/0008-5472.CAN-14-2524; PMID: 25977337; http://dx.doi.org/10.1158/0008-5472.CAN-14-2524
- Waterston AM, Salway F, Andreakos E, Butler DM, Feldmann M, Coombes RC. TNF autovaccination induces selfanti-TNF antibodies and inhibits metastasis in a murine melanoma model. BrJ Cancer 2004; 90:1279-84. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2409655&tool=pmcentrez&rendertype=abstract; PMID:15026813; http://dx.doi.org/10.1038/sj.bjc.6601670
- Mercer L, Askling J, Raaschou P, Dixon W, Dreyer L, Hetland ML, Mellemkjær L, Strangfeld A, Zink A, Iannone F et al. No Increased Risk of Developing a First Invasive Melanoma in Rheumatoid Arthritis Patients Treated with Biologics: Results of a Collaborative Project of 11 European Biologics Registers. In: American College of Rheumatology Annual Meeting. 2014. page 1838.
- Donia M, Andersen R, Kjeldsen JW, Fagone P, Munir S, Nicoletti F, Andersen MH, thor Straten P, Svane IM. Aberrant expression of MHC Class II in melanoma attracts inflammatory tumor specific CD4+ T cells which dampen CD8+ T cell antitumor reactivity. Cancer Res 2015; 75(18):3747-59; Available from: http://cancerres.aacrjournals.org/cgi/doi/10.1158/0008-5472.CAN-14-2956; PMID:26183926; http://dx.doi.org/10.1158/0008-5472.CAN-14-2956
- Garrido F, Romero I, Aptsiauri N, Garcia-Lora AM. Generation of MHC class I diversity in primary tumors and selection of the malignant phenotype. Int J Cancer 2014; 2:n/a-n/a. Available from: http://doi.wiley.com/10.1002/ijc.29375; PMID:25471439; http://dx.doi.org/10.1002/ijc.29375
- Brown ER, Charles KA, Hoare SA, Rye RL, Jodrell DI, Aird RE, Vora R, Prabhakar U, Nakada M, Corringham RE et al. A clinical study assessing the tolerability and biological effects of infliximab, a TNF- inhibitor, in patients with advanced cancer. Ann Oncol 2008; 19:1340-6. Available from: http://annonc.oxfordjournals.org/cgi/doi/10.1093/annonc/mdn054; PMID:18325912; http://dx.doi.org/10.1093/annonc/mdn054
