ABSTRACT
Purpose: Therapy targeting CTLA-4 immune checkpoint provides increased survival in patients with advanced melanoma. However, immunotherapy is frequently associated with delayed and heterogeneous clinical responses and it is important to identify prognostic immunological correlates of clinical endpoints. Experimental design: 77 patients with stage III/IV melanoma were treated with ipilimumab alone every 3 weeks, during 9 weeks. Blood samples were collected at the baseline and before each dose for in depth immune monitoring. Results: The median follow-up was 28 mo with a median survival of 7 mo. Survival and clinical benefit were significantly improved when absolute lymphocyte count at the baseline was above 1 × 109/L. Notably, ipilimumab had a global effect on memory T cells, with an early increase of central and effector subsets in patients with disease control. By contrast, percentages of stem cell memory T cells (TSCM) gradually decreased despite stable absolute counts and sustained proliferation, suggesting a process of differentiation. Higher proportions of eomes+ and Ki-67+ T cells were observed, with enhanced skin homing potential and induction of cytotoxic markers. Conclusion: These results suggest that CTLA-4 blockade is able to reshape the memory subset with the potential involvement of Eomes and memory subsets including TSCM.
Abbreviations
| ALC | = | absolute lymphocyte count |
| CLA | = | cutaneous addressin |
| CTLA-4 | = | cytotoxic T-lymphocyte-associated antigen 4 |
| DC | = | disease control |
| eomes | = | eomesodermin |
| HD | = | healthy donor |
| HR | = | Hazard Ratio |
| IrAEs | = | immune-related adverse events |
| NR | = | non-responder |
| OS | = | overall survival |
| PBL | = | peripheral blood lymphocyte |
| TCM | = | central memory T cell |
| TEM | = | effector memory T cell |
| TEMRA | = | terminal effector memory T cell |
| TIL | = | tumor-infiltrating lymphocyte |
| TN | = | naive T cell |
| TSCM | = | stem cell memory T cell |
| TTM | = | transitional memory T cell |
Introduction
Melanoma is one of the most rapidly spreading cancers in terms of worldwide incidence. Until recently, survival outcomes for stage IV melanoma patients treated with standard therapies such as dacarbazine have been poor, with a median survival less than 8 mo and a 5-y survival rate of approximately 10%.Citation1,2 Melanoma is a long-lasting cancer known for its ability to induce specific immunological responses, providing the first evidences of successful immunotherapy by activating the immune system with high doses of IFN-α or IL-2.Citation3 The use of autologous tumor-infiltrating lymphocytes (TIL) in metastatic melanoma by Rosenberg and colleaguesCitation4 Three decades ago emphasized the potential role of tumor-specific T cellsCitation5 and paved the way for the development of melanoma specific vaccines. However, patients with detectable tumor Ag-specific T cells can still develop progressive metastatic melanoma. Results from many clinical trials indicate that the common denominator of therapy benefit in melanoma is the augmentation of host immunity. Recent advances in the understanding of the complex cellular mechanisms regulating cancer immunity have led to the development of new strategies aimed at targeting specific immune-checkpoints.Citation6 The early stages of T cell activation are regulated by the interaction of B7-1 and B7-2, whose expression is restricted to professional antigen presenting cells, with their counter receptors CD28 and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4).Citation7 The costimulatory signal through CD28 lowers the threshold for full activation, allowing lower affinity T cell clones to become activated. Subsequently, CD28 primes the system for regulation by increasing CTLA-4 on the T cell surface (for a review, see ref.Citation8). CTLA-4 is a key element in immune tolerance and a main negative regulator of T-cell-mediated antitumor immune responses. It out competes the interaction of CD28-B7 molecules and antagonizes early T cell activation, IL-2 production, cell cycle progression and modulation of TCR signaling.Citation9 Therefore, blockade of CTLA-4 aims to prevent the attenuation of T cell activation, potentiating tumor-specific T cells.Citation10,11 Ipilimumab, previously known as MDX-010, is a fully humanized IgG1 monoclonal antibody against the extracellular domain of CTLA-4. Since the initial human studies in previously vaccinated melanoma patients,Citation12,13 several trials have assessed the efficacy of anti-CTLA-4 therapy alone or in association with targeted therapy, chemotherapy, peptide vaccine or other immunotherapies (for a review, see ref.Citation14). A survival benefit with ipilimumab was demonstrated in two pivotal phase III trials in a subset of patients with advanced melanoma, leading to its approval by the US Food and Drug Administration in March 2011.Citation15,16 Data from subsequent clinical trials also suggested that a proportion of patients treated with ipilimumab can achieve survival of at least 5 y.Citation17 A recent pooled analysis of 12 studies reported a remarkable effect in terms of durability illustrated by a 3-y survival rate of 22%.Citation18
However, this therapy appears to benefit only a subset of patients. Consequently, identifying prognostic immunologic correlates of clinical endpoints is a challenging issue. Although durable objective clinical responses are found to be associated with the induction of immune-related adverse events (IrAEs),Citation19 autoimmune disorders do not predict improved antitumor response.Citation20 Immunological monitoring has always constituted a relevant aspect of completed and ongoing clinical trials. Several immunologic endpoints with a strong biologic rationale are proposed to correlate with clinical activity. Increased absolute lymphocyte count (ALC) during the therapy is found to be associated with clinical benefit and overall survival (OS) in uncontrolled studies.Citation21,22,23,24 In a clinical trial using tremelimumab, another humanized anti-CTLA-4, increased levels of memory and activated T cells were reported in 3 out of 12 patients achieving disease control (DC).Citation25 Several reports demonstrate immunological changes under ipilimumab therapy, shedding light on in vivo mechanisms of anti-CTLA-4, but their pertinence to predict clinical responses and OS remains to be clarified.Citation26-29 In this report, we present results from the longitudinal immunological monitoring of a cohort of 77 ipilimumab-treated patients. The extended characterization of peripheral lymphocyte subsets allowed us to define early markers of survival and/or clinical response, such as ALC at the baseline. We report major changes within the memory T cell subsets, which are associated with response to the treatment, and a potential implication of T memory stem cells (TSCM).
Results
Patient clinical characteristics, response to treatment and immune-related adverse events
The majority of patients included in this study were stage IV (90%) (). The median follow-up was 28 mo with a median survival of 7 mo in the cohort of patients treated with ipilimumab alone (95% IC 6–10). DC group was defined as patients achieving complete response (CR), or partial response (PR) or stable disease (SD) at week 16, whereas NR group included patients with progressive disease (PR) or death before week 16. DC was reported in 30% of cases. 52 patients received the total course of four cures ipilimumab and presented a better clinical response at week 16, with 35% of these patients achieving DC, compared to 24% in the group of patients receiving less than four doses of ipilimumab (p = 0.01). This was expected since the number of doses of anti-CTLA-4, reflecting the continuation of the therapy, depends on a good tolerability of the treatment by the patient, potentiating a better response. The overall survival was however not affected by the dose number of anti-CTLA-4 (data not shown). Patients receiving less than four doses were the ones with a higher frequency of grade 3 irAEs (p = 0.007), resulting in treatment discontinuation.
Table 1. Patients characteristics. The whole cohort is described as well as the two groups of patients treated or not with the full course of four cures of ipilimumab. Adverse events were graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (version 4.0).
IrAEs occurred 49 d in median after the beginning of treatment with values ranging from 7 to 186 d. The most clinically significant IrAE was enterocolitis (grade III/IV in 14% of cases) followed by rash/pruritus or hepatitis (5%). These IrAEs were in most cases treatable with vigilance and early intervention with corticosteroids. Of note, we did not find any correlation between patients who develop IrAEs and those who achieved clinical benefit (data not shown).
ALC at the baseline is a predictive marker of survival and clinical response
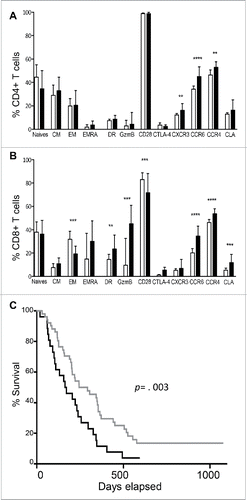
The success of therapies aimed at immune checkpoints relies on the ability of the immune system to mount specific and sustained antitumor responses. Therefore, the immune status at the baseline may be especially relevant. Our results showed that ALC before Ipilimumab initiation was lower in patients when compared to healthy donors (HD) (median = 1.18 × 109/L versus median = 1.58 × 109/L respectively, p = 0.00008). This was mainly due to a defect in both CD4+ (p = 0.005) and CD8+ T cells (p = 0.006), with a more pronounced defect in effector memory CD8+ T cells (p < 10−6). B and NK-cell counts were similar in patients and HD (data not shown). Characteristics of patients T cells at the baseline are represented in and are consistent with an activated phenotype, as evidenced by increased percentages of HLA-DR+ and granzyme B+ CD8+ T cells and a lower expression of CD28 on CD8+ T cells. Although not significant, a shift toward a higher CTLA-4 expression was detected on memory CD8+ T cells of patients when compared to healthy donors (HD) (p = 0.057).
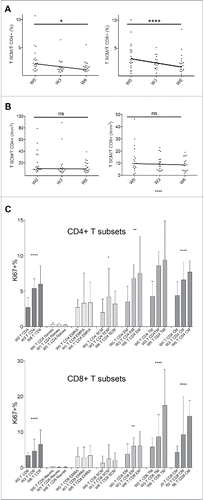
Figure 1. Patients immunological status at the baseline. Percentages of CD4+ (A) and CD8+ (B) T cell subsets (CM for central memory, EM for effector memory, EMRA for terminally effector memory, GzmB for Granzyme B). Results from HD and patients are shown as open bars and black bars respectively. *for p < 0.05, ** for p < 0.01, *** for p < 0.001 and **** for p < 0.0001. (C) Kaplan–Meier survival curves from patients with ALC ≥ 1 × 109/L (gray line, n = 43) or < 1 × 109/L (black line, n = 26) at the baseline.
In addition, the cutaneous addressin CLA, as well as CCR4 and CXCR3 were expressed at higher levels in patients.
We then delineated a threshold value of ALC = 1 × 109/L below which only 2.5% of HD was found. A median survival of 11 mo (95%CI 7–14) was observed in the group with a baseline ALC ≥ 1 × 109/L, vs. 5 mo (95%CI 3–10) in the group with ALC < 1 × 109/L (p = 0.003) (). The prognostic impact of baseline ALC was still significant when adjusting for baseline LDH (Hazard Ratio HR = 0.49, 95%CI = 0.28-0.86) or for the use of glucocorticoids (HR = 0.5 95%CI 0.29–0.88). Moreover, the proportion of patients achieving disease control was higher in the group with an ALC ≥ 1 × 109/L at the baseline (44% vs. 19% if ALC < 1 × 109/L, p = 0.04).
Ipilimumab induces early immunological changes of T cell subsets
A significant increase in ALC, CD4+ and CD8+ T cells absolute counts was observed 3 weeks after the first dose of ipilimumab (p = 0.0006, p = 0.0002 and p = 0.008, respectively) with values remaining stable from week 3 to week 12 (data not shown). A marked increase in HLA-DR activated T cells was also reported, lasting at least over 12 weeks for CD8+ T cells (). An early and transient upregulation of CD25 on CD4+ and CD8+ T cells was observed at week 3 (data not shown).
Figure 2. Ipilimumab induces activated and memory T cells. The two slope model is visualized by the black line. CD4+ and CD8+ T cell subsets are presented in the left and the right panels respectively. (A) Percentage of HLA-DR+ T cells, absolute counts per mm3 of (B) CM, (C) EM and (D) EMRA. The same results are observed in terms of percentages of parental subsets (data not shown). *for p < 0.05, ** for p < 0.01, *** for p < 0.001 and **** for p < 0.0001.
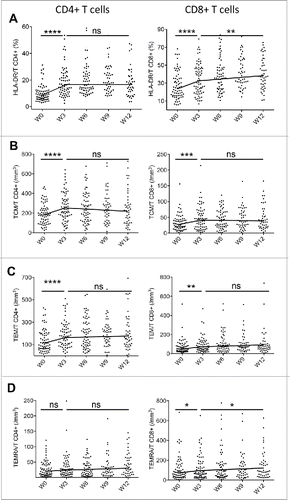
Naive and memory T cell subsets were identified based on CD45RA and CCR7 expression. Naive cells (TN) were characterized as CD45RA+ CCR7+, central memory T cells (TCM) had the CD45RA-CCR7+ phenotype, effector memory T cells (TEM) were defined by the lack of expression of these markers and terminal effector memory (TEMRA) exhibited CD45RA+CCR7- phenotype (). An early effect of ipilimumab was detected on CD4+ and CD8+ TCM and TEM subsets with an increase in both percentages and absolute counts (), whereas the increase of CD8+ TEMRA subset was delayed (). These results suggest that the major dynamic changes between naïve and memory subsets operate within 3 weeks after the first injection of ipilimumab.
ICOS (CD278) is a receptor belonging to the CD28/CTLA-4 family expressed upon T-cell activation.Citation30 It has been shown that ICOS critically controls the pool size of both effector memory and regulatory T cells.Citation31 In our study, comprising the global cohort of 77 patients, no significant changes in the frequency of ICOS+ CD4+ T and CD8+ T cells from baseline to week 6 were observed ().
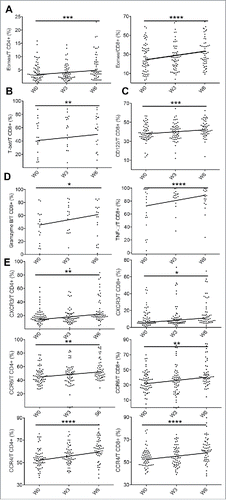
Eomes and T-bet are the two T-box transcription factors which are involved in the generation and function of effector and memory CD8+ T cells. Our results showed that ipilimumab treatment was associated with the expansion of lymphocytes expressing eomes (CD4+ and CD8+ T cells) and T-bet (CD8+ T cells) (). These transcription factors cooperate to maintain memory CD8+ T cells homeostasis through the expression of IL-2Rβ (CD122), favoring IL-15 mediated signaling and proliferation of memory T cells.Citation32 The increased expression of CD122 () on CD8+ T cells was consistent with the enhanced expression of T-bet and eomes. In addition, cytotoxic molecules such as TNF-α and granzyme B were induced on CD8+ T cells but not in CD4+ T cells during the course of treatment, whereas no changes in IFNγ expression were observed in T cells ( and data not shown). Finally, CTLA-4 blockade had an effect on chemokine receptors with an expansion of CXCR3, CCR6 and CCR4 positive T cells from week 0 to week 6 ().
Figure 3. Pharmacodynamic changes during ipilimumab therapy. Expression of eomes on CD4+ and CD8+ T cells (A), T-bet (B) and CD122 (C) on. Cytotoxic markers expression (GzmB and TNF-a) on CD8+ T cells (D). Evolution of chemokine receptor expression in T cells (E). Note: T-bet and GzmB were evaluated on 20 patients as mentioned in material and method section. *for p < 0.05, ** for p < 0.01, *** for p < 0.001 and **** for p < 0.0001.
Occurrence of IrAEs is not associated to any of the biomarkers tested in this study
Although targeted immunotherapies such as ipilimumab are well related to the induction of IrAEs, we did not find any association between the occurrence of such events and the activation status of lymphocytes, assessed by CD25, HLA-DR and ICOS expression on T cells, at baseline and during the course of immunomonitoring. Similarly, none of the biomarkers used in our experimental design was found to be associated with the development of IrAEs (data not shown).
Clinical response is associated to the generation of memory T cells with a potential role of T memory stem cells (TSCM)
An increase in ALC between week 3 and week 12 was significantly associated with DC (p = 0.013). Importantly, although no association between the evolution of total count of CD4+ T cells and clinical response was observed, the early raise of CD4+ TCM and TEM (from week 0 to week 3) was significantly higher for patients achieving DC than for non-responders (NR) (). This early augmentation in CD4+ TEM was followed by an increase between week 3 and week 12 in patients achieving DC, and a decrease in patients with no response (median slope = −2.1 and +5.9 in NR and DC respectively, p = 0.025).
Figure 4. CTLA-4 blockade and markers associated with clinical response. Patients were subdivided in two groups, DC (gray line and dots) and NR (black line and dots). Clinical benefit is associated with a more marked increase of memory subsets (A and B) and of eomes expressing CD8+ T cells (C). *for p < 0.05, ** for p < 0.01
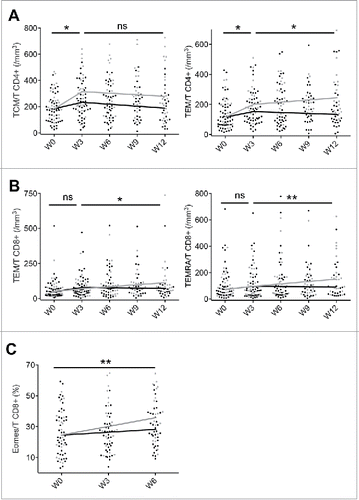
In addition, patients with DC had a slightly delayed, but sustained increase of effector and terminal effector memory CD8+ T cells () with a higher proportion of CD8+ T cells expressing eomes (median slope = 0.7 and 2.1 in NR and DC respectively, p = 0.008) (). Of note, the two response groups had similar immunological profiles in terms of ICOS+ T cell evolution (data not shown).
Further phenotypic characterization of memory T cells was performed for 20 patients (10 DC and 10 NR, randomly chosen), allowing the study of transitional memory T cells TTM (CD45RA-CCR7-CD27+)Citation33 and the recently identified TSCM (defined as CD45RA+CCR7+CD27+CD95+) ().Citation34 Upon ipilimumab therapy, percentages of both CD4+ and CD8+ TSCM significantly decreased whereas absolute counts remained unchanged (). In order to analyze the dynamics amidst memory T cell subsets, we followed the expression of the Ki67 protein, a cellular marker for proliferation and cell cycling.Citation35 Ipilimumab treatment resulted in a significant increase of CD4+ and CD8+ T cell proliferation (median slope = +0.6, p < 10−15 and +0.8, p < 10−5, respectively), involving TCM, TTM and TEM subsets, and remaining barely detectable in naive T cells (). Notably, both CD4+ and CD8+ TSCM had significant proliferative activity and CD4+ TSCM exhibited an enhanced proliferative capacity from week 0 to week 6 (median slope = +0.4, p = 0.034).
Discussion
Anti-CTLA-4 is the first therapeutic agent aimed at overcoming cancer immune tolerance. It relies on the ability of T cells to enhance antigen recognition by shifting their activation threshold.
Prior to ipilimumab therapy, patients are characterized by a lymphopenia, which may reflect either the tumor burden, through tumor-derived factors,Citation36 or previous conventional therapies such as dacarbazine.Citation37 In the present study, we reported that ALC above 1 × 109/L before therapy was significantly associated with an extended survival and disease control. Moreover, only patients mounting a sustained expansion of total lymphocytes, as well as CD4+ and CD8+ T cells during the course of immunotherapy were associated with a higher rate of disease control at week 16. Our data reinforce previous results from clinical trials involving either ipilimumab at a higher dose or another CTLA-4 abrogating antibody, tremelimumab.Citation21,22,23,38 Prior to treatment, patients' circulating T cells displayed a phenotype compatible with a chronic activation by the tumor, with a lower expression of the costimulatory molecule CD28 and increased percentage of HLA-DR and granzyme B positive CD8+ T cells, when compared to HD. In addition, although not significant, a trend toward higher percentages of TCD8+ T cells expressing CTLA-4 by was also observed, suggesting a post-activation status of T cells. An early expansion of CD4+ and CD8+ T cells was reported at the induction of immunotherapy, with CD8+ T cells gradually expressing higher levels of granzyme B and TNF-α.
A correlation between CTLA-4 blockade and ICOS induction has been reported in several studies.Citation39 An increase of ICOShi CD4+ T cells producing IFNγ was first observed in six bladder cancer patients, in the absence of any association with clinical outcome.Citation40 In a subsequent study, Carthon et al. showed that increases in ICOShi CD4+ T cells were more pronounced with 10 mg/kg ipilimumab treatment than with 3 mg/kg. In addition, these authors correlated the increased and sustained frequency of ICOShi CD4+ T cell to improved overall survival in an independent cohort of 14 melanoma patients treated with 10 mg/kg ipilimumab.Citation41 More recently, in a study analyzing 36 patients treated with ipilimumab, an increased frequency of ICOS+ CD4+ T cells was proposed as a pharmacodynamic biomarker of CTLA-4 blockade activity at the dose of 3 mg/kg.Citation26 We were not able to confirm these observations in our cohort of 77 patients. Further investigations are therefore needed in order to confirm these data and to better characterize the phenotype of ICOS+ CD4+ T cells in terms of memory subsets.
Chemokines are critical mediators of routine immune surveillance, providing directional signals for cell trafficking.Citation42 Some of these molecules orchestrate the migration of T cells to the skin. CCR4 is involved during the first step of T cell skin homing, followed by the extravasation to the skin due to CLA interaction with its ligand, E-selectin. In addition to other T cell types, CCR6 is expressed on a T cell subset with skin homing properties.Citation43 CXCR3 is a chemokine receptor that favors migration of Th1 cells and effector CD8+ T cells into inflamed tissuesCitation44 and tumors.Citation45 Before CTLA-4 blockade, patients with advanced melanoma had a higher proportion of CD4+ and CD8+ T cells expressing CXCR3, CCR6, CCR4 and/or CLA, suggesting an increased capacity of T cells to relocate to the skin. This phenotype was observed in all patients at the baseline, regardless of clinical responses. Furthermore, ipilimumab therapy potentiated the expression of these chemokine receptors on both CD4+ and CD8+ T cells. A previous study, where patient-derived melanoma-specific T cell lines were analyzed, reported a positive association between survival and the expression of CXCR3 and CCR4 by T-cells, highlighting the importance of these molecules in the maintenance of effective in situ antitumor immunity.Citation46 Our data further emphasize the potential of CTLA-blockade in the induction of tumor infiltrating cells, already demonstrated in previous reports.Citation47,48
Upon therapy, we observed an expansion of memory subsets, with an increase of central and effector memory T cells, followed by a gradual raise in TEMRA cell counts. More importantly, our data showed for the first time a significant association between clinical benefit at week 16 and an early increase of CD4+ TCM and TEM followed by a delayed increase of TEMRA (), consistent with the stepwise differentiation pattern of memory subsets from TCM to TEMRA.Citation49 There was an increased proliferative state of memory T cells, and more specifically of TTM, in accordance with previous studies.Citation50 In contrast, the late expansion of TEMRA seemed to rather depend upon differentiation than proliferation, as suggested by the stable Ki-67 expression. Therefore, the immunological changes induced by ipilimumab may not be solely explained by enhanced proliferation. The functional development of memory T cell subsets is directed by regulatory transcription factors such as eomes and T-bet. They sustain memory CD8+ T cell homeostasis through the expression of CD122 and are involved in the differentiation of memory precursor and effector CD8+ T cells respectively.Citation51 In addition, they coordinately promote T cell migration to inflamed tissues via chemokine receptors and cooperate to generate cytotoxic T cells.Citation52-54 In mice, CTLA-4 reduces the expression of eomes at the transcriptional level resulting in less IFNγ and granzyme B producing CD8+ T cells.Citation55 In accordance with this observation, our results showed that CTLA-4 blockade induced a higher expression of eomes in CD8+ T cells from patients with DC, suggesting a pivotal role of this transcriptional factor during ipilimumab therapy.
Recently, TSCM have been identified.Citation34 These cells, which are the least differentiated memory subset, are long lived, with an enhanced self-renewal capacity and multipotency to differentiate into all memory T cells. In addition, emerging evidence indicate that TSCM are involved in antitumor immunity, thus representing valuable candidates for adoptive T cell therapies. They are the most potent cells among memory subsets to trigger tumor regression in humanized mice. Moreover, some patients with metastatic melanoma have a significant fraction of MART-1-specific CD8+ TSCM.Citation34 Interestingly, experiments conducted in the setting of adoptive immunotherapy revealed that T cells invalidated for both T-bet and eomes were unable to trigger an antitumor response and bored characteristics consistent with TSCM. Therefore, the antitumor potential of TSCM seems to rely more on their further differentiation into effector memory cells than in their intrinsic activity.Citation56 After ipilimumab initiation, CD4+ TSCM percentages decreased despite enhanced proliferative activity suggesting a differentiation process into classical memory cells. Whether this phenomenon was driven or not by T-bet and eomes remains to be elucidated.
Factors that determine the development of IrAEs under CTLA-4 blockade are a field of intensive research. Although it was hoped in early anti-CTLA-4 trials that the induction of IrAEs,Citation19 that are well correlated to immunotherapies, were associated with durable objective clinical responses, it is now more established that autoimmune disorders are not good predictors of improvement in anti-CTLA-4 therapy.Citation20 We were not able to confirm the overlap between patients who developed IrAEs and those who achieved durable objective clinical response (for review and references thereinCitation39). In addition, we did not find any correlation between the occurrence of IrAEs and the biomarkers investigated in this study. This lack of correlation may however be related to the observation of IrAEs over a wide range of time points (7 to 186 d) which increases the complexity of the statistical analysis.
In conclusion, this study identified pharmacodynamic markers and biomarkers associated with survival and/or clinical benefit. Our data show that CTLA-4 blockade targets the early steps of memory T cell generation, inducing both differentiation and proliferation. These results suggest that ipilimumab is able to reshape the memory subset and provide new insights into the mechanisms of memory T cell subset expansion with the potential involvement of TSCM and eomes (). In response to ipilimumab, T cells gradually acquire skin homing and cytotoxic capacities, which may increase in situ antitumor responses. Identifying immune biomarkers as reliable intermediate endpoint may therefore facilitate the management of patients, providing insight into the selection of most effective therapeutic strategies.
Patients and methods
Patient characteristics and study design
Patients with unresectable stage III or IV melanoma were eligible for ipilimumab treatment if they had previously failed to one or more systemic therapy. Between June 2010 and September 2013, 77 patients were prospectively included (59 at Saint-Louis hospital and 18 at Bichat hospital, Paris, France). They received intravenous ipilimumab 3 mg/kg every 3 weeks, for an expected total course of four doses. Dose omission or discontinuation, mainly due to the occurrence of IrAEs, was discussed during multidisciplinary meetings. Blood samples were collected before each injection of ipilimumab at week 0, 3, 6, 9, and at week 12. All patients gave their informed consent for this study, which was approved by the Institutional Review Board of Saint-Louis Hospital, in accordance with the Declaration of Helsinki. Thirty samples from healthy donors were collected from the blood donor center (Etablissement Français du Sang, Hôpital Saint-Louis).
Flow cytometry and monoclonal antibodies
Lymphocyte immunophenotyping was performed on freshly collected EDTA whole blood samples, using a FACS Canto II flow cytometer and FACS DIVA software (BD Biosciences), in a laboratory that operates under GLP principles. Absolute counts were determined using the TruCount system (BD Biosciences) with anti-CD3 FITC, -CD8 PE, -CD45 PerCP, and -CD4 APC mAbs (BD Multitest, BD Biosciences). Eight colors labeling was performed with the following mAbs (all from BD unless specified): anti-CD45 FITC and PercP, CD3 V450, -CD4 V500, -CD8 PerCP, -CD16 APCH7, -CD56 PECy7, -CD45RO PE, -CD45RA APC, -CCR7 PECy7 and BV421, -CD27 PE and APCH7, -CD28 PE, -CD25 PECy7, -HLA-DR APCH7, -CD57 FITC, -CD122 PE, -CLA FITC, -CCR4 PE, -CCR6 PECy7, -CXCR3 APC, -CD278 PE and eomesodermin (eomes) PE (e-Bioscience). Additional immunostainings were done for 20 patients from stored frozen peripheral blood lymphocytes (PBL), using anti-CD95 FITC and APC, -CTLA-4 PE, -T-bet PE, -Ki67 PECy7, -IFNγ Vio770, -TNF-α PE and granzyme B FITC (last three mAbs from Miltenyi). Intracellular staining was performed using either Foxp3 staining buffer (e-Biosciences) or inside stain buffer (Miltenyi), according to the manufacturer's instructions. These studies were performed using standard operating protocols. Acquisition was performed using a FACSCanto II™ flow cytometer. Results were expressed according to the « fluorescence minus one » (FMO) control, allowing the definition of the background signal and data were analyzed using FACS Diva™ (BD Biosciences).
Definition of tumor assessment and main clinical outcomes
Tumor assessment using clinical examination, total bodyscan, cerebral MRI and ultrasound lymph node scan if necessary was performed at baseline, week 12 and week 16, and then every 2 mo. Because of the atypical pattern of response to immunotherapy, week 16 evaluation was systematically maintained even if the patient exhibited tumor progression at week 12. The overall response was assessed according to the immune-response-related criteria (irRCs) derived from time-point response assessments (based on tumor burden).
Statistical analyses
Data are described using means and standard deviation for quantitative variables and count and percentages for qualitative variables. Comparisons between patients and HD immunological status at baseline were performed using Wilcoxon rank sum test. p values where adjusted for multiple comparisons using the Hochberg correction.
Overall survival was defined as time between inclusion and death from any cause and was estimated using the Kaplan-Meier method. Effect of ALC at baseline on survival was assessed using Cox proportional hazard model.
Linear mixed-effect models were fitted to model the evolution of T cells between baseline and week 12. A two-slope model was used to account for a potential early and late effect of ipilimumab. The early effect was defined as the initial rise from baseline to week 3, and the late effect was modeled as a linear increase from week 3 to week 12. Random effects were added for the intercept and the two slopes. Immunological changes of T cell during the follow-up were modeled using linear mixed-effect models. To test the effect of response at week 16 on the evolution of T cells, two interaction terms between response and the two slopes were added to the model. Regarding several subsets of T cells, quantification was only performed at week 0, 3, and 6. In these cases, linear mixed-effect models were fitted with a single slope.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_A_1136045_s02-s04.zip
Download Zip (538.8 KB)Acknowledgments
J.F. is a Paris Diderot University junior fellowship. We thank all the technicians from the Immunocellular laboratory, Hôpital Saint-Louis.
Funding
This work was supported by INSERM, Société Française de Dermatologie and Institut National du Cancer, PAIR melanoma program.
References
- Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist 2011; 16:5-24; PMID:21212434; http://dx.doi.org/10.1634/theoncologist.2010-0190
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol: Off J Am Soc Clin Oncol 2009; 27:6199-206; PMID:19917835; http://dx.doi.org/10.1200/JCO.2009.23.4799
- Lotze MT, Rosenberg SA. The immunologic treatment of cancer. CA: Cancer J Clin 1988; 38:68-94; PMID:2450624; http://dx.doi.org/10.3322/canjclin.38.2.68
- Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. New Engl J Med 1988; 319:1676-80; PMID:3264384; http://dx.doi.org/10.1056/NEJM198812223192527
- Lee S, Margolin K. Tumor-infiltrating lymphocytes in melanoma. Curr Oncol Rep 2012; 14:468-74; PMID:22878966; http://dx.doi.org/10.1007/s11912-012-0257-5
- Haanen JB. Immunotherapy of melanoma. EJC Suppl 2013; 11:97-105; PMID:26217118; http://dx.doi.org/10.1016/j.ejcsup.2013.07.013
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol 2005; 23:515-48; PMID:15771580; http://dx.doi.org/10.1146/annurev.immunol.23.021704.115611
- Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol 2001; 19:225-52; PMID:11244036; http://dx.doi.org/10.1146/annurev.immunol.19.1.225
- Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1994; 1:405-13; PMID:7882171; http://dx.doi.org/10.1016/1074-7613(94)90071-X
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science (New York, NY) 1996; 271:1734-6; PMID:8596936; http://dx.doi.org/10.1126/science.271.5256.1734
- Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity 1997; 7:445-50; PMID:9354465; http://dx.doi.org/10.1016/S1074-7613(00)80366-0
- Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proce Natl Acad Sci U S A 2003; 100:8372-7; PMID:12826605; http://dx.doi.org/10.1073/pnas.1533209100
- Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A 2003; 100:4712-7; PMID:12682289; http://dx.doi.org/10.1073/pnas.0830997100
- Lesokhin AM, Callahan MK, Postow MA, Wolchok JD. On being less tolerant: Enhanced cancer immunosurveillance enabled by targeting checkpoints and agonists of T cell activation. Sci Transl Med 2015; 7:280sr1; PMID:25810313; http://dx.doi.org/10.1126/scitranslmed.3010274
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al. Improved survival with ipilimumab in patients with metastatic melanoma. New Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Robert C, Thomas L, Bondarenko I, O'Day S, M DJ, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. New Engl J Med 2011; 364:2517-26; PMID:21639810; http://dx.doi.org/10.1056/NEJMoa1104621
- McDermott D, Lebbe C, Hodi FS, Maio M, Weber JS, Wolchok JD, Thompson JA, Balch CM. Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treatment Rev 2014; 40:1056-64; PMID:25060490; http://dx.doi.org/10.1016/j.ctrv.2014.06.012
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol: Off J Am Soc Clin Oncol 2015; 33:1889-94; PMID: 25667295; http://dx.doi.org/10.1200/JCO.2014.56.2736
- Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res: Off J Am Assoc Cancer Res 2007; 13:6681-8; PMID:17982122; http://dx.doi.org/10.1158/1078-0432.CCR-07-0187
- Kong YC, Wei WZ, Tomer Y. Opportunistic autoimmune disorders: from immunotherapy to immune dysregulation. Ann New York Acad Sci 2010; 1183:222-36; PMID:20146718; http://dx.doi.org/10.1111/j.1749-6632.2009.05138.x
- Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, Chapman PB, Schwartz GK, Allison JP, Wolchok JD. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer 2010; 116:1767-75; PMID:20143434; http://dx.doi.org/10.1002/cncr.24951
- Di Giacomo AM, Danielli R, Calabro L, Bertocci E, Nannicini C, Giannarelli D, Balestrazzi A, Vigni F, Riversi V, Miracco C et al. Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the University Hospital of Siena (Italy). Cancer Immunol, Immunother: CII 2011; 60:467-77; PMID:21170646; http://dx.doi.org/10.1007/s00262-010-0958-2
- Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N, Roy S, Eggermont AM, Routier E, Robert C. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol: Off J Euro Soc Med Oncol / ESMO 2013; 24:1697-703; PMID:23439861; http://dx.doi.org/10.1093/annonc/mdt027
- Chasset F, Pages C, Biard L, Roux J, Sidina I, Madelaine I, Basset-Seguin N, Viguier M, Madjlessi-EzrA N, Schneider P et al. Single-center study under a French Temporary Authorization for Use (TAU) protocol for ipilimumab in metastatic melanoma: negative impact of baseline corticosteroids. Eur J Dermatol 2015; 25:36-44; PMID:25500362; http://dx.doi.org/10.1684/ejd.2014.2471
- Comin-Anduix B, Lee Y, Jalil J, Algazi A, de la Rocha P, Camacho LH, Bozon VA, Bulanhagui CA, Seja E, Villanueva A et al. Detailed analysis of immunologic effects of the cytotoxic T lymphocyte-associated antigen 4-blocking monoclonal antibody tremelimumab in peripheral blood of patients with melanoma. J Trans Med 2008; 6:22; PMID:18452610; http://dx.doi.org/10.1186/1479-5876-6-22
- Ng Tang D, Shen Y, Sun J, Wen S, Wolchok JD, Yuan J, Allison JP, Sharma P. Increased frequency of ICOS+ CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer Immunol Res 2013; 1:229-34; PMID:24777852; http://dx.doi.org/10.1158/2326-6066.CIR-13-0020
- Luke JJ, Ott PA. PD-1 pathway inhibitors: the next generation of immunotherapy for advanced melanoma. Oncotarget 2015; 6:3479-92; PMID:25682878; http://dx.doi.org/10.18632/oncotarget.2980
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. New Engl J Med 2015; 372:2006-17; PMID:25891304; http://dx.doi.org/10.1056/NEJMoa1414428
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. New Engl J Med 2015; 373:23-34; PMID:26027431; http://dx.doi.org/10.1056/NEJMoa1504030
- Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 1999; 397:263-6; PMID:9930702; http://dx.doi.org/10.1038/16717
- Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam KP, Coyle AJ, Kroczek RA, Hutloff A. ICOS controls the pool size of effector-memory and regulatory T cells. J Immunol 2008; 180:774-82; PMID:18178815; http://dx.doi.org/10.4049/jimmunol.180.2.774
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol 2005; 6:1236-44; PMID:16273099; http://dx.doi.org/10.1038/ni1268
- Fritsch RD, Shen X, Sims GP, Hathcock KS, Hodes RJ, Lipsky PE. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J Immunol 2005; 175:6489-97; PMID:16272303; http://dx.doi.org/10.4049/jimmunol.175.10.6489
- Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C et al. A human memory T cell subset with stem cell-like properties. Nat Med 2011; 17:1290-7; PMID:21926977; http://dx.doi.org/10.1038/nm.2446
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000; 182:311-22; PMID:10653597; http://dx.doi.org/10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9
- Whiteside TL. Immune responses to cancer: are they potential biomarkers of prognosis? Front Oncol 2013; 3:107; PMID:23730621; http://dx.doi.org/10.3389/fonc.2013.00107
- Mignot G, Hervieu A, Vabres P, Dalac S, Jeudy G, Bel B, Apetoh L, Ghiringhelli F. Prospective study of the evolution of blood lymphoid immune parameters during dacarbazine chemotherapy in metastatic and locally advanced melanoma patients. PloS One 2014; 9:e105907; PMID:25170840; http://dx.doi.org/10.1371/journal.pone.0105907
- Simeone E, Gentilcore G, Giannarelli D, Grimaldi AM, Caraco C, Curvietto M, Esposito A, Paone M, Palla M, Cavalcanti E et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol, Immunother: CII 2014; 63:675-83; PMID:24695951; http://dx.doi.org/10.1007/s00262-014-1545-8
- Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol 2010; 37:473-84; PMID:21074063; http://dx.doi.org/10.1053/j.seminoncol.2010.09.001
- Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, Logothetis C, Sharma P. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A 2008; 105:14987-92; PMID:18818309; http://dx.doi.org/10.1073/pnas.0806075105
- Carthon BC, Wolchok JD, Yuan J, Kamat A, Ng Tang DS, Sun J, Ku G, Troncoso P, Logothetis CJ, Allison JP et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res: Off J Am Assoc Cancer Res 2010; 16:2861-71; PMID:20460488; http://dx.doi.org/10.1158/1078-0432.CCR-10-0569
- Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity 2012; 36:705-16; PMID:22633458; http://dx.doi.org/10.1016/j.immuni.2012.05.008
- Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol 2009; 10:857-63; PMID:19578369; http://dx.doi.org/10.1038/ni.1767
- Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res 2011; 317:620-31; PMID:21376175; http://dx.doi.org/10.1016/j.yexcr.2010.12.017
- Gorbachev AV, Kobayashi H, Kudo D, Tannenbaum CS, Finke JH, Shu S, Farber JM, Fairchild RL. CXC chemokine ligand 9/monokine induced by IFN-gamma production by tumor cells is critical for T cell-mediated suppression of cutaneous tumors. J Immunol 2007; 178:2278-86; PMID:17277133; http://dx.doi.org/10.4049/jimmunol.178.4.2278
- Mullins IM, Slingluff CL, Lee JK, Garbee CF, Shu J, Anderson SG, Mayer ME, Knaus WA, Mullins DW. CXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III disease. Cancer Res 2004; 64:7697-701; PMID:15520172; http://dx.doi.org/10.1158/0008-5472.CAN-04-2059
- Ribas A, Comin-Anduix B, Economou JS, Donahue TR, de la Rocha P, Morris LF, Jalil J, Dissette VB, Shintaku IP, Glaspy JA et al. Intratumoral immune cell infiltrates, FoxP3, and indoleamine 2,3-dioxygenase in patients with melanoma undergoing CTLA4 blockade. Clin Cancer Res: Off J Am Assoc Cancer Res 2009; 15:390-9; PMID:19118070; http://dx.doi.org/10.1158/1078-0432.CCR-08-0783
- Huang RR, Jalil J, Economou JS, Chmielowski B, Koya RC, Mok S, Sazegar H, Seja E, Villanueva A, Gomez-Navarro J et al. CTLA4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans. Clin Cancer Res: Off J Am Assoc Cancer Res 2011; 17:4101-9; PMID:21558401; http://dx.doi.org/10.1158/1078-0432.CCR-11-0407
- Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who's who of T-cell differentiation: human memory T-cell subsets. Euro J Immunol 2013; 43:2797-809; PMID:24258910; http://dx.doi.org/10.1002/eji.201343751
- Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H, Callahan M, Wolchok JD, Halaban R, Dhodapkar MV et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol 2015; 194:950-9; PMID:25539810; http://dx.doi.org/10.4049/jimmunol.1401686
- Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 2012; 12:749-61; PMID:23080391; http://dx.doi.org/10.1038/nri3307
- Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science (New York, NY) 2003; 302:1041-3; PMID:14605368; http://dx.doi.org/10.1126/science.1090148
- Lord GM, Rao RM, Choe H, Sullivan BM, Lichtman AH, Luscinskas FW, Glimcher LH. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood 2005; 106:3432-9; PMID:16014561; http://dx.doi.org/10.1182/blood-2005-04-1393
- Zhu Y, Ju S, Chen E, Dai S, Li C, Morel P, Liu L, Zhang X, Lu B. T-bet and eomesodermin are required for T cell-mediated antitumor immune responses. J Immunol 2010; 185:3174-83; PMID:20713880; http://dx.doi.org/10.4049/jimmunol.1000749
- Hegel JK, Knieke K, Kolar P, Reiner SL, Brunner-Weinzierl MC. CD152 (CTLA-4) regulates effector functions of CD8+ T lymphocytes by repressing Eomesodermin. Euro J Immunol 2009; 39:883-93; PMID:19224637; http://dx.doi.org/10.1002/eji.200838770
- Li G, Yang Q, Zhu Y, Wang HR, Chen X, Zhang X, Lu B. T-Bet and eomes regulate the balance between the effector/central memory T cells versus memory stem like T cells. PloS One 2013; 8:e67401; PMID:23826287; http://dx.doi.org/10.1371/journal.pone.0067401