ABSTRACT
Human papilliomavirus (HPV) oncogene E7, essential for the transformation and maintenance of the malignancy of cervical cancer cells, represents an ideal tumor-specific antigen for vaccine development. However, due to the poor immunogenicity of E7 protein, an effective therapeutic E7 vaccine is still lacking. Dendritic cells (DCs) are probably the most potent antigen presenting cells for the induction of cytotoxic T lymphocyte (CTL) response, which is crucial for tumor control. In this study, we tested whether targeting the E7 antigen to DCs in vivo would elicit therapeutic antitumor CTL response. We generated the DEC205-specific single-chain variable fragment (scFv) and E7 long peptide fusion protein [scFv(DEC205)-E7] based on the novel method of protein assembly we recently developed. This fusion protein vaccine demonstrated highly efficient DC-targeting in vivo and elicited much stronger protective CTL response than non-DC-targeting control vaccine in naive mice. Furthermore, the scFv(DEC205)-E7 vaccine showed significant therapeutic antitumor response in TC-1 tumor bearing mice. Importantly, PD-L1 blockade further improved the therapeutic effect of the scFv(DEC205)-E7 vaccine. Thus, the current study suggests an efficient strategy for cervical cancer immunotherapy by combining the DC(DEC205)-targeting E7 vaccine and PD-L1 blockade.
Introduction
High-risk HPVs are the major causative agents for cervical cancers and many other HPV associated tumors. Nearly all (99.7%) cervical cancers are HPV DNA positive.Citation1 Among them, HPV16 and 18 account for about 70% of all cervical cancers.Citation2 The viral oncoproteins E6 and E7 have been well recognized for their crucial roles in cell transformation and malignance maintenance in various mechanisms.Citation3 Therefore, the tumor specific E6/E7 represents an attractive target for cervical cancer treatment.
The importance of immune response, especially CTL response, in tumor control has been increasingly acknowledged. T cell checkpoint blockade has been shown to promote highly effective antitumor T cell response in several types of tumor models in both tumor bearing mice and human patients.Citation4 For cervical cancer treatment, however, anti-PD-L1 treatment alone did not demonstrate therapeutic effects.Citation5 This may be due to poor or suppressed endogenous T cell response in the tolerized tumor bearing host.Citation6,7 Thus, for immunotherapy of cervical cancers, to actively overcome the tolerance and generate effective CTL response in the tolerized tumor bearing host is a key step.
DCs are probably the most potent antigen presenting cells for CTL induction. DC based or targeted vaccines have attracted extensive research for both prophylactic and therapeutic vaccines against various infections, cancers and other diseases. In the treatment of cervical cancers, a few studies have tested DC based HPV E6/E7 vaccines.Citation8-11 Such vaccines are usually produced with in vitro cultured autologous DCs pulsed with E6/E7 polypeptides. The therapeutic efficacies of these vaccines are rather limited, which may be due to the poor survival and trafficking of DC vaccines to draining lymph nodes (DLNs) upon in vivo administration.Citation12 In addition, the production of vaccines is time consuming and costly.
In vivo DC targeting provides an easier and more effective way for vaccine development. Numerous surface molecules on DCs have been chosen for DC targeting in vivo, including DEC205, DNGR1, XCR1, CD11b, CD11c, FcγR, CD40, MHC-II, etc.Citation13,14 FcγR, CD40 and MHC-II are the first to be targeted for antigen delivery.Citation14 Integrins, such as CD11b and CD11c, have also been used for DC targeting. Adenylate cyclase (CyaA) of Bordetella pertussis, which binds specifically to CD11b, was shown to be able to target CD11b+ DCs and induce potent anti-viral and antitumor antibody or T cell responses.Citation15-18 However, most of these target molecules are not specifically expressed on DCs and their exact mechanisms remains elusive. During the last decade, some molecules preferentially expressed on DCs or subsets of DCs have got special attention. Among them, DEC205 is probably the most widely used DC target. Vaccines based on DEC205 have shown potent therapeutic effect against viral infections and tumors not only in mice, but also in non-human primates and humans.Citation19-25 However, an efficient DEC205-targeting HPV E6/E7 vaccine has not been reported. More importantly, whether it could generate effective antitumor CTL response in tumor bearing host and provide therapeutic effect remains unclear.
In this study, we have generated a synthetic vaccine composing scFv(DEC205) and HPV16 E7 long peptide employing the SpyTag/SpyCatcher based synthetic vaccine technology we developed recently.Citation26 Such a vaccine induced a potent antigen-specific antitumor CTL response in vivo and showed significantly better therapeutic effect than non-DC-targeting vaccine in a TC-1 tumor bearing mouse model. Furthermore, combinational therapy with PD-L1 blockade produced synergistic antitumor effect. Thus, our results provide an efficient strategy for cervical cancer immunotherapy.
Results
Construction of a synthetic DC targeting vaccine against HPV associated cervical cancer
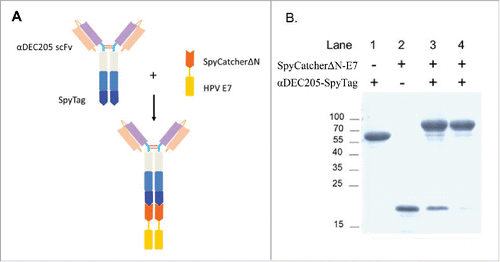
HPV16 E7 is an ideal antigen for cervical cancer immunotherapy. To achieve the goal of in vivo DC targeted antigen delivery, the HPV16 E7 long peptide (amino acid 43-62) containing the dominant T cell epitopes was fused to the single-chain fragment variable (scFv) specific for DEC-205 (αDEC205) using a protein assembly strategy based on the SpyCatcher-SpyTag system as described previously.Citation26 The SpyTag was genetically fused to the C-terminus of αDEC205 to obtain αDEC205-SpyTag and the E7 long peptide was added to the C-terminus of SpyCatcherΔN to obtain Sc-E7, respectively (). Upon mixture of these two proteins, SpyTag and SpyCatcherΔN mediate rapid and efficient covalent conjugation to form a fully functional vaccine. αDEC205-SpyTag (, Lane 1) and Sc-E7 (, Lane 2) fusion proteins were expressed, purified and combined as described previously. SDS-PAGE result showed that more than 90% of the input αDEC205-SpyTag was conjugated (, Lane 3) and the purity of assembled DC targeting tumor vaccine (αDEC205-Sc-E7) was above 90% (, Lane 4).
Figure 1. Design and generation of DC targeting vaccine against HPV associated tumor. (A) Schematic diagram of the DC targeting tumor vaccine design and assembly. The αDEC205-SpyTag was prepared previously, and the HPV16 E743-62 polypeptide was fused to the C-terminus of SpyCatcherΔN. Once mixed, they conjugated to form an intact vaccine. (B) Vaccine production and purification. Purified αDEC205-SpyTag was mixed with SpyCatcherΔN-E7 at a molar ratio of 1:1.5 at 4°C for 2 h. The assembled αDEC205-Sc-E7 adduct was purified by Protein A chromatography. The purified adduct was analyzed by SDS-PAGE.
The αDEC205-Sc-E7 vaccine efficiently targets DC in vivo
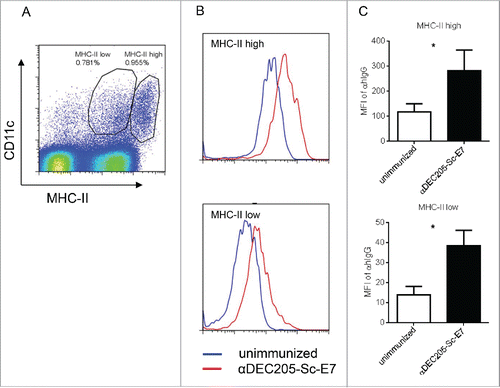
To test the in vivo DC targeting capacity of the vaccine, naive C57BL/6 mice were injected with the αDEC205 fusion proteins subcutaneously at the tail base, 20 h later, the inguinal DLNs were isolated and digested into single-cell suspensions. Given that αDEC205 targeting mediates quick endocytosis of conjugated antigens,Citation27,28 DC-targeted delivery of vaccine was detected by intracellular staining of the hIgG portion of the vaccine. As shown in , both MHC-IIhigh and MHC-IIlow DCs were targeted by αDEC205-Sc-E7 vaccine upon immunization as compared with those from unimmunized mice. Interestingly, MHC-IIhigh DCs were more targeted than MHC-IIlow DCs as shown by the higher mean fluorescence intensity (MFI) (). Considering MHC-IIhigh DCs are activated DCs, targeting this DC population may endow αDEC205-Sc-E7 good capability for CTL induction.
Figure 2. DC targeting vaccine efficiently targets DCs in vivo. C57BL/6 mice were injected with 200 pmol αDEC205-Sc-E7 protein, along with 30 μg CpG1826 and 30 μg Poly I:C as an adjuvant. Twenty hours later, cells were isolated from DLNs and stained. (A) Anti-MHC II and anti-CD11c antibodies were used to gate the DC populations. (B) and (C) Vaccine uptaken by DCs was measured by intracellular staining of the hIgG portion of the vaccine. Representative FACS plots (B) and the statistical analysis of the isotype-subtracted geometric MFI are shown (C). Data represent two independent experiments.
DC targeting tumor vaccine generates significantly enhanced cytotoxic T cell response
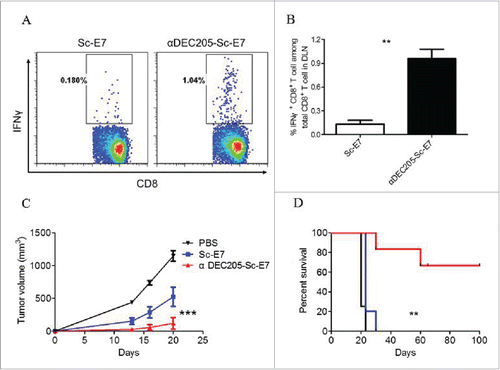
To study whether the αDEC205-Sc-E7 vaccine can induce efficient antigen-specific CTL response, naive C57BL/6 mice were immunized with the αDEC205-Sc-E7 or the Sc-E7 fusion protein in combination with adjuvant CpG1826/PolyI:C as before.Citation26 7 d later, the DLNs from the immunized mice were isolated and digested into single-cell suspensions, and then stimulated with 5 μg/mL E749-57 peptide in a U-bottom 96-well plate for 6 h. The IFNγ producing CD8+ T cells were then determined and analyzed by intracellular staining and flow cytometry. As shown in , αDEC205-Sc-E7 generated a significantly higher percentage of IFNγ producing CD8+ T cells compared with Sc-E7. To further test the antitumor effect of the E7-specific CTL response, immunized mice were challenged with TC-1 tumor cells 14 d after vaccination. As shown in , single vaccination significantly delayed the tumor onset. Furthermore, while control vaccine immunized group all develop tumors around 30 d after tumor challenge, more than 60% of αDEC205-Sc-E7 vaccine group remained tumor free till 100 d ().
Figure 3. DC targeting tumor vaccine generates increased cytotoxic T cell response. C57BL/6 mice were subcutaneously immunized with 120 pmol αDEC205-Sc-E7 or Sc-E7, with 30 μg CpG1826 and 30 μg Poly I:C as adjuvant. (A) Seven days later, cells were isolated from the DLNs and restimulated with HPV16 E749-57 peptide (5 μg/mL) for 6 h in the presence of brefeldin A. (B) The frequency of IFNγ+ cells among CD8+ T cells was analyzed. Mean ± SD (n = 5). **p < 0.01. (C) and (D) Fourteen days later, 5 × 105 TC-1 cells were injected subcutaneously, then tumor volumes were monitored. The growth curve is shown in panel C and the survival curve is shown in panel D. (n = 5–6). **p < 0.01, ***p < 0.001.
To further test whether αDEC205-Sc-E7 vaccine induced T cell responses would provide protection in the genital area, vaccinated mice were challenged intravaginally with 1.5 × 106 TC-1 cells. Two weeks later, mice were sacrificed and the genital tumor was measured. The data showed that αDEC205-Sc-E7 vaccine significantly inhibited tumor growth (Fig. S2). Therefore, DC targeting αDEC205-Sc-E7 vaccine induces efficient antitumor CTL responses not only to ectopic but also to orthotopic TC-1 tumor development.
Therapeutic vaccination with DC targeting tumor vaccine significantly inhibits tumor growth
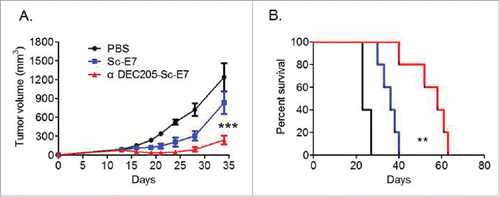
In order to assess whether the DC targeting tumor vaccine can generate therapeutic antitumor effects, TC-1 tumor cells were injected subcutaneously on the right flank of naive C57BL/6 mice for better later treatment and monitoring. After the tumor was established, the tumor bearing mice were immunized with the αDEC205-Sc-E7 or the Sc-E7 fusion protein at day 9 after tumor cell inoculation and boosted on day 14. The tumor growth was monitored twice a week. As shown in , vaccination with αDEC205-Sc-E7 more efficiently delayed tumor growth compared to the Sc-E7 vaccine group or PBS control group. In addition, the survival of tumor bearing mice was significantly improved by the αDEC205-Sc-E7 vaccination (). Thus, these data suggest that the DC targeting tumor vaccine could be a more potent therapeutic vaccine compared with the conventional non-DC targeting protein vaccines.
Figure 4. DC targeting tumor vaccine can efficiently inhibit tumor growth. (A) and (B) C57BL/6 mice were inoculated with 5 × 104 TC-1 cells subcutaneously at right flank. Nine and fourteen days later, mice were injected with 120 pmol Sc-E7 or αDEC205-Sc-E7, respectively. CpG & Poly I:C were used as adjuvant as before. The tumor size was monitored. The growth curve is shown in panel A and the survival curve is shown in panel B. (n = 5–6). **p < 0.01, ***p < 0.001.
Vaccination increases PD-L1 and blockade of which can synergize with DC targeting tumor vaccine to produce significantly enhanced antitumor effect
Although the increased CTL response generated by DC targeting tumor vaccine contributes to better tumor control, relapses still occur. PD-L1 expression on tumor and immune cells has been associated with poor prognosis following chemo and radiotherapy in both pre-clinical and clinical studies. We wondered whether PD-L1 expression may be upregulated by vaccination, thus limiting the antitumor CTL response. TC-1 tumor bearing mice were vaccinated as before. Three days later, DLNs and tumors were digested into single cell suspension for flow cytometric analysis. Indeed, significant PD-L1 upregulation was observed upon vaccination on total CD45+ cells in the DLNs and on both tumor cells (CD45−) and hematopoietic CD45+ cells in tumor microenvironment ().
Figure 5. Vaccination increases PD-L1 and anti-PD-L1 blockade can synergize with DC targeting tumor vaccine to control tumor growth. C57BL/6 mice were inoculated with 5 × 104 TC-1 cells subcutaneously at right flank. (A) Nine days later, mice were immunized with αDEC205-Sc-E7 protein, together with 30 μg CpG and 30 μg Poly I:C as adjuvant. Three days later, PD-L1 expression on cells from DLNs and tumors (A) was analyzed. (B) For combinational therapy, 12 and 18 d after tumor inoculation, mice were injected with 120 pmol αDEC205-Sc-E7. Meanwhile, anti-PD-L1 was administered intratumorally on days 14, 18, 21 and 25; Rat-IgG was used as an isotype control. The tumor size was monitored. (C) and (D) The growth curve is shown in panel C and the survival curve is shown in panel D. (n = 5–6). **p < 0.01, ***p < 0.001.
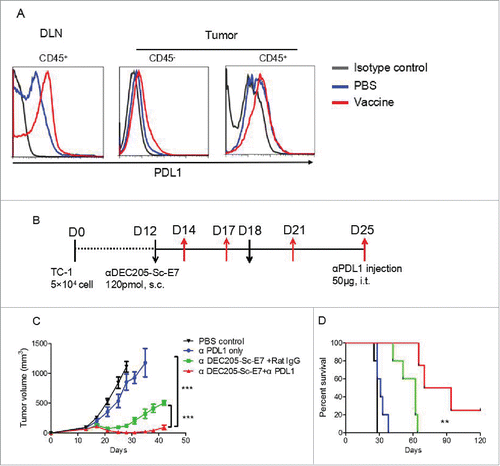
We next tested whether blocking PD-L1/PD-1 signaling pathway may significantly enhance the antitumor effect elicited by DC targeting tumor vaccine. TC-1 tumor bearing mice were established as before. Twelve days later, the tumor bearing mice were treated with anti-PD-L1 alone, αDEC205-Sc-E7 vaccine plus Rat-IgG or αDEC205-Sc-E7 vaccine plus anti-PD-L1, respectively (). The tumor growth was monitored twice a week. Anti-PD-L1 alone had a minor impact on tumor growth, whereas αDEC205-Sc-E7 vaccine plus Rat-IgG slowed the tumor progression significantly (). Strikingly, the combination of αDEC205-Sc-E7 vaccine and anti-PD-L1 treatment dramatically suppressed the tumor growth (), and 20% of treated mice survived at least 120 d (). Together, these data suggest that the combination of DC targeting tumor vaccine and PD-L1 blockade could be a potent therapeutic strategy against cervical cancers.
Discussion
While prophylactic HPV vaccines have already gained great success for human health, a commercial therapeutic vaccine is still lacking. Various types of therapeutic vaccines against HPV-associated cervical cancer have been actively explored in murine models and humans.Citation8-11,29 These mainly include nucleic acid vaccines, bacterial or viral vector-based vaccines, protein/peptide vaccines and DC-based vaccines. Even so, a safe, effective and easy vaccine still remains a difficult task. Recently, an encouraging clinical effect of a plasmid therapeutic vaccine VGX-3100 against HPV16/18 associated cervical intraepithelial neoplasia (CIN) 2/3 was reported.Citation30 This study further encourages the development of HPV therapeutic vaccines. In current study, we have constructed a new DC targeting synthetic protein vaccine based on recently developed protein assembly system, and tested its therapeutic effect in TC-1 tumor bearing mouse model. Our study provides a novel and easier strategy for generation of protein based DC-targeting tumor vaccine. This may be especially useful when multiple neoantigens need to be targeted.Citation31,32
In the current vaccine design, the N-terminus truncated SpyCatcher is an integral part of the vaccine. This short foreign polypeptide does not have major negative impact on the vaccination. In fact, the immunogenicity of this polypeptide may actually positively influence the vaccine efficacy, since we found that fusion of SpyCatcherΔN to an antigen containing the model epitope OVA257-264 significantly improved the CTL response against it (Fig. S1A). One possibility is that SpyCatcherΔN contains CD4+ Th epitopes that helps CTL generation. Supporting this, significant SpyCatcherΔN specific Th1 cells were induced upon vaccine immunization (Fig. S1B). In addition, it may be also contributed by the PEST-like region present in SpyCatcherΔN (Fig. S1C). PEST regions have been found to target proteins for rapid degradation and presentation.Citation33-35 Therefore, inclusion of SpyCatcherΔN may offer an addition benefit for inducing CTL responses.
Immune suppression is a common obstacle for cancer immunotherapy. The PD-L1-PD-1 axis plays important roles in limiting CTL responses and antitumor control. Blockade of this signaling pathway has recently shown impressive effects in cancer immunotherapy.Citation36-41 However, whether and how PD-L1 expression is regulated remains incompletely understood. Previous study found that vaccination with TEGVAX induced IFNγ dependent PD-L1 upregulation on tumor cells,Citation42 which may limiting vaccine efficacy at the effector phase. Here, we found that vaccination also induced significantly higher PD-L1 expression on the antigen presenting cells in DLNs, which may add an additional level of immune suppression at the priming phase during therapeutic vaccine treatment. Since the upregulation of PD-L1 is quickly induced after vaccination when large amount of IFNγ is possibly lacking, we hypothesized that this is more likely due to the type I IFN induced by the adjuvant TLR ligands (polyI:C and CpG in current study). In fact, type I IFN has been shown to directly upregulate PD-L1 expression on antigen presenting cells.Citation43 Given the important beneficial roles of type I IFN during cancer immunotherapy,Citation44-47 an ideal solution for improving the efficacy of a therapeutic vaccine is probably via combinational PD-L1 blockade while maintaining type I IFN signaling. Indeed, combinational therapy of αDEC205-Sc-E7 vaccine plus anti-PD-L1 blockade showed significantly enhanced therapeutic effect than vaccine alone.
In most current single PD-L1/PD-1 blockade therapies, the major effective site has been proposed to be the tumor tissue during T cell effector phase.Citation4,48 Therefore, we have chosen intratumoral injection of anti-PD-L1 and demonstrated its synergistic effect when in combination with vaccines, indicating PD-L1 inside tumor indeed plays an essential role in limiting CTL there. However, it should be noted that the PD-L1/PD-1 pathway is also upregulated early during T cell activation in DLNs upon vaccination. In fact, PD-1 was upregulated on antigen-specific IFNγ producing CD8+ T cells upon vaccine immunization, which is consistent with the previous study.Citation5 In addition, PD-L1 was also significantly upregulated on CD45+ cell in the DLNs (). Therefore, inhibition of this pathway during early vaccination may enhance CTL generation, which has been actually shown in previous studies.Citation49,50 Thus, early treatment with anti-PD-L1 targeting T cell priming in the DLNs may provide additional benefit. DLN or tumor specific targeting delivery of anti-PD-L1 and the timing related to vaccination need to be comprehensively explored in future to further dissect the role of anti-PD-L1 on CTL generation in DLNs and CTL effector function in tumor tissues during combinational cancer therapy.
In summary, our study constructed in a novel way a new DC-targeting HPV vaccine. This vaccine targets HPV16 E7 antigen to DCs in vivo and generates potent antitumor CTL response in TC-1 tumor bearing mice, which allows PD-L1 blockade to further activate the vaccine induced antitumor efficacy. Our study provides preclinical evidence that HPV associated cervical cancers can be treated by the combinational therapy of active vaccination and PD-L1 blockade.
Materials and methods
Mice and cell lines
Six to eight week-old female C57BL/6 mice were obtained from Vital River Laboratory Animal Technology Co. (Beijing, China). All mice were housed under specific pathogen-free conditions in the animal care facilities at the Institute of Biophysics, Chinese Academy of Sciences. All animal experiments were performed in accordance with the guidelines of the Institute of Biophysics, Chinese Academy of Sciences, using protocols approved by the Institutional Laboratory Animal Care and Use Committee.
TC-1, a tumor cell line transformed from C57BL/6 primary mouse lung epithelial cells and expressing HPV16 E6 and E7 oncoproteins, was kindly provided by Dr. Xuemei Xu (Institute of Basic Medical Sciences Chinese Academy of Medical Sciences & School of Basic Medicine, Peking Union Medical College, Beijing, China). FreeStyle™ 293-F cells, a cell line derived from 293 cell line and used for large-scale transfection and protein production, were purchased from Invitrogen (Carlsbad, CA).
Protein production and purification
Generation of αDEC205 fusion protein-based DC targeting vaccine with SpyCatcher/SpyTag system was described previously.Citation26 Briefly, the αDEC205-SpyTag fusion protein was expressed by transient transfection in FreeStyleTM 293-F cells and purified on protein A column (GE Healthcare Life Sciences, Pittsburgh, PA, USA). For SpyCatcher-E7 production, the cDNA coding sequence of HPV16 E743-62 was subcloned into the C-terminus of SpyCatcherΔN (SpyCatcher with deletion at N-terminus as described previously Citation26) to construct the pDEST14-SpyCatcherΔN-E7, then the expression plasmid was transformed into E. coli and the protein was purified by a Ni-NTA agarose column (ComWin Biotech, Beijing, China). For DC targeting vaccine generation, αDEC205-SpyTag (10 μM) and SpyCatcherΔN-E7 (or Sc-E7) (15 μM) were mixed in PBS at a molar ratio of 1:1.5 at 4°C for 2 h. Subsequently, the conjugated αDEC205-Sc-E7 adduct was then purified by Protein A column. All these protein production and purification steps were analyzed by SDS-PAGE.
Flow cytometry analysis
Lymph nodes, spleens and tumors from treated or naive C57BL/6 mice were processed into single cell suspensions, and 2 × 106 cells each sample were used for flow cytometry assays. For PD-L1 expression detection, the cells from DLNs and tumors were incubated with an anti-FcγR mAb (2.4G2) to block non-specific binding. Then, cells were stained with fluorescence-conjugated antibodies: anti-CD45 (30-F11) and PD-L1 (MIH5). Finally, data were collected on FACSCalibur or LSRFortessa (BD Biosciences, San Jose, CA, USA) and analyzed with FlowJo software (Tree Star Inc., Ashland, OR, USA). All antibodies used in this study were obtained from eBioscience (San Diego, CA, USA). Fixable viability dye (L-34967, ThermoFisher) was used to exclude dead cells.
Intracellular staining
Naive C57BL/6 mice were subcutaneously immunized with 120 pmol αDEC205-Sc-E7 or Sc-E7 at tail base, along with 30 μg CpG1826 (Invitrogen Life Technologies, Beijing, China) and 30 μg PolyI:C (InvivoGen, San Diego, CA, USA) as an adjuvant. Seven days later, DLNs of the immunized mice were harvested and processed into single-cell suspensions. Cells (1 × 106 cells/well) were restimulated in U-bottom 96-well plates with 5 μg/mL E749-57 peptide (RAHYNIVTF) (GL Biochem, Shanghai, China) for 6 h and 5 μg/mL Brefeldin A (eBioscience) was added to accumulate intracellular cytokines. After restimulation, the cells were first incubated with anti-mouse CD8α antibody (53-6.7) for surface staining. Subsequently, fixation/permeabilization and intracellular staining for IFNγ were performed with anti-IFNγ (XMG1.2) or isotype control (RTK2071). Fixable viability dye (L-34967) was used to exclude dead cells. Data were collected and analyzed as above.
To determine the DC targeting delivery of αDEC205-Sc-E7, intracellular staining of captured vaccines were performed. Briefly, 1 d after vaccine immunization, DLNs of the immunized were harvested and processed into single-cell suspensions. DLN cells from non-immunized mice were used as control. The cells were first stained with anti-mouse IA/I-E (M5/114.15.2, Biolegend) and anti-mouse CD11c antibody (N418, Biolegend). Subsequently, fixation/permeabilization and were performed as described above and intracellular hIgG was stained with polyclonal antibody (12-4998, eBioscience) or isotype control (12-4321, eBioscience). Geometric MFI of isotype staining was subtracted for each sample for further analysis. Fixable viability dye was used to exclude dead cells.
Tumor model and vaccination
To test the functionality of CTLs in vivo, C57BL/6 mice were immunized subcutaneously with 120 pmol αDEC205-Sc-E7 or Sc-E7 together with 30 μg CpG1826 and 30 μg PolyI:C, 14 d later, mice were inoculated with 5 × 105 TC-1 cells in the right flank. Then, tumor size was measured twice a week by using a caliper and calculated according to the formula: length × (width)2 × 0.5. All mice were executed once the tumor size researched or exceeded 1000 mm3. For orthotopic tumor model, mice were immunized twice with the same dose as above with 1 week interval. One week after the last vaccination, 1.5 × 106 TC-1 cells were inoculated intravaginally as described.Citation51,52 Mice were sacrificed 2 weeks later for genital tumor dissection and measurement.
For therapeutic experiments, C57BL/6 mice were subcutaneously inoculated with 5 × 104 TC-1 cells in the right flank. Nine and fourteen days later, tumor bearing mice were immunized with 120 pmol αDEC205-Sc-E7 or Sc-E7, along with 30 μg CpG1826 and 30 μg PolyI:C as adjuvant. Then, the tumor size was monitored as described above.
Therapeutic vaccination combined with anti-PD-L1 blockade
5 × 104 TC-1 tumor cells were inoculated subcutaneously into C57BL/6 mice. Twelve days later, tumor bearing mice were immunized with 120 pmol αDEC205-Sc-E7 vaccine for twice at 6 d interval. Meanwhile, 50 μg anti-PD-L1(10F.9G2, BioXCell, West Lebanon, NH, USA) were administered intratumorally on days 14, 18, 21 and 25, Rat-IgG was used as an isotype control. Tumor growth and mice survival were monitored twice a week.
Statistical analysis
All analysis was performed using GraphPad Prism statistical software (GraphPad Software Inc., San Diego, CA, USA). Two way ANOVA and log-rank (Mantel-Cox) tests were used to analyze the tumor growth and mice survival data, respectively. All the other data were analyzed using unpaired two-tailed t tests. A value of p < 0.05 was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_A_1147641_s02.docx
Download MS Word (581.8 KB)Funding
This work was supported by grants from National Natural Science Foundation of China (81261130022 to M.Z.) and Chinese Academy of Sciences (Hundred Talents Program to M.Z.).
References
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12-9; PMID:10451482; http://dx.doi.org/10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F
- Steben M, Duarte-Franco E. Human papillomavirus infection: epidemiology and pathophysiology. Gynecologic Oncol 2007; 107:S2-5; PMID:17938014; http://dx.doi.org/10.1016/j.ygyno.2007.07.067
- Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer 2010; 10:550-60; PMID:20592731; http://dx.doi.org/10.1038/nrc2886
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science (New York, NY) 2015; 348:56-61; PMID:25838373; http://dx.doi.org/10.1126/science.aaa8172
- Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res 2013; 73:128-38; PMID:23135914; http://dx.doi.org/10.1158/0008-5472.CAN-12-2606
- Piersma SJ. Immunosuppressive tumor microenvironment in cervical cancer patients. Cancer Micro Environment 2011; 4:361-75; PMID:21626415; http://dx.doi.org/10.1007/s12307-011-0066-7
- Patel S, Chiplunkar S. Host immune responses to cervical cancer. Curr Opin Obstetrics Gynecol 2009; 21:54-9; PMID:19125004; http://dx.doi.org/10.1097/GCO.0b013e32831a9890
- Wang HL, Xu H, Lu WH, Zhu L, Yu YH, Hong FZ. In vitro and in vivo evaluations of human papillomavirus type 16 (HPV16)-derived peptide-loaded dendritic cells (DCs) with a CpG oligodeoxynucleotide (CpG-ODN) adjuvant as tumor vaccines for immunotherapy of cervical cancer. Arch Gynecol Obstetrics 2014; 289:155-62; PMID:23912529; http://dx.doi.org/10.1007/s00404-013-2938-1
- Santin AD, Bellone S, Palmieri M, Zanolini A, Ravaggi A, Siegel ER, Roman JJ, Pecorelli S, Cannon MJ. Human papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination of stage IB or IIA cervical cancer patients: a phase I escalating-dose trial. J Virol 2008; 82:1968-79; PMID:18057249; http://dx.doi.org/10.1128/JVI.02343-07
- Bellone S, Pecorelli S, Cannon MJ, Santin AD. Advances in dendritic-cell-based therapeutic vaccines for cervical cancer. Expert Rev Anti Cancer Therapy 2007; 7:1473-86; PMID:17944571; http://dx.doi.org/10.1586/14737140.7.10.1473
- Santin AD, Bellone S, Palmieri M, Ravaggi A, Romani C, Tassi R, Roman JJ, Burnett A, Pecorelli S, Cannon MJ. HPV16/18 E7-pulsed dendritic cell vaccination in cervical cancer patients with recurrent disease refractory to standard treatment modalities. Gynecologic Oncol 2006; 100:469-78; PMID:16249018; http://dx.doi.org/10.1016/j.ygyno.2005.09.040
- Adema GJ, de Vries IJ, Punt CJ, Figdor CG. Migration of dendritic cell based cancer vaccines: in vivo veritas? Curr Opin Immunol 2005; 17:170-4; PMID:15766677; http://dx.doi.org/10.1016/j.coi.2005.01.004
- Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013; 39:38-48; PMID:23890062; http://dx.doi.org/10.1016/j.immuni.2013.07.004
- Kastenmuller W, Kastenmuller K, Kurts C, Seder RA. Dendritic cell-targeted vaccines-hope or hype? Nat Rev Immunol 2014; 14:705-11; PMID:25190285; http://dx.doi.org/10.1038/nri3727
- Guermonprez P, Khelef N, Blouin E, Rieu P, Ricciardi-Castagnoli P, Guiso N, Ladant D, Leclerc C. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the α(M)β(2) integrin (CD11b/CD18). J Exp Med 2001; 193:1035-44; PMID:11342588; http://dx.doi.org/10.1084/jem.193.9.1035
- Moron G, Dadaglio G, Leclerc C. New tools for antigen delivery to the MHC class I pathway. Trends Immunol 2004; 25:92-7; PMID:15102368; http://dx.doi.org/10.1016/j.it.2003.11.008
- Berraondo P, Nouze C, Preville X, Ladant D, Leclerc C. Eradication of large tumors in mice by a tritherapy targeting the innate, adaptive, and regulatory components of the immune system. Cancer Res 2007; 67:8847-55; PMID:17875726; http://dx.doi.org/10.1158/0008-5472.CAN-07-0321
- Preville X, Ladant D, Timmerman B, Leclerc C. Eradication of established tumors by vaccination with recombinant Bordetella pertussis adenylate cyclase carrying the human papillomavirus 16 E7 oncoprotein. Cancer Res 2005; 65:641-9; PMID:15695409
- Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML, Chuang E, Sanborn RE, Lutzky J, Powderly J et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Translational Med 2014; 6:232ra51; PMID:24739759; http://dx.doi.org/10.1126/scitranslmed.3008068
- Swee LK, Guimaraes CP, Sehrawat S, Spooner E, Barrasa MI, Ploegh HL. Sortase-mediated modification of alphaDEC205 affords optimization of antigen presentation and immunization against a set of viral epitopes. Proc Natl Acad Sci USA 2013; 110:1428-33; PMID:23297227; http://dx.doi.org/10.1073/pnas.1214994110
- Flynn BJ, Kastenmuller K, Wille-Reece U, Tomaras GD, Alam M, Lindsay RW, Salazar AM, Perdiguero B, Gomez CE, Wagner R et al. Immunization with HIV Gag targeted to dendritic cells followed by recombinant New York vaccinia virus induces robust T-cell immunity in nonhuman primates. Proc Natl Acad Sci U S A 2011; 108:7131-6; PMID:21467219; http://dx.doi.org/10.1073/pnas.1103869108
- Cheong C, Choi JH, Vitale L, He LZ, Trumpfheller C, Bozzacco L, Do Y, Nchinda G, Park SH, Dandamudi DB et al. Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti-human DEC205 monoclonal antibody. Blood 2010; 116:3828-38; PMID:20668230; http://dx.doi.org/10.1182/blood-2010-06-288068
- Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med 2004; 199:815-24; PMID:15024047; http://dx.doi.org/10.1084/jem.20032220
- Nchinda G, Kuroiwa J, Oks M, Trumpfheller C, Park CG, Huang Y, Hannaman D, Schlesinger SJ, Mizenina O, Nussenzweig MC et al. The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells. J Clin Invest 2008; 118:1427-36; PMID:18324335; http://dx.doi.org/10.1172/JCI34224
- Johnson TS, Mahnke K, Storn V, Schonfeld K, Ring S, Nettelbeck DM, Haisma HJ, Le Gall F, Kontermann RE, Enk AH. Inhibition of melanoma growth by targeting of antigen to dendritic cells via an anti-DEC-205 single-chain fragment variable molecule. Clin Cancer Res 2008; 14:8169-77; PMID:19088032; http://dx.doi.org/10.1158/1078-0432.CCR-08-1474
- Liu Z, Zhou H, Wang W, Tan W, Fu YX, Zhu M. A novel method for synthetic vaccine construction based on protein assembly. Scientific Reports 2014; 4:7266; PMID:25434527; http://dx.doi.org/10.1038/srep07266
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med 2002; 196:1627-38; PMID:12486105; http://dx.doi.org/10.1084/jem.20021598
- Kamphorst AO, Guermonprez P, Dudziak D, Nussenzweig MC. Route of antigen uptake differentially impacts presentation by dendritic cells and activated monocytes. J Immunol (Baltimore, Md: 1950) 2010; 185:3426-35; PMID:20729332; http://dx.doi.org/10.4049/jimmunol.1001205
- Hung CF, Ma B, Monie A, Tsen SW, Wu TC. Therapeutic human papillomavirus vaccines: current clinical trials and future directions. Exp Opin Biol Therapy 2008; 8:421-39; PMID:18352847; http://dx.doi.org/10.1517/14712598.8.4.421
- Trimble CL, Morrow MP, Kraynyak KA, Shen X, Dallas M, Yan J, Edwards L, Parker RL, Denny L, Giffear M et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet (London, England) 2015; 386:2078-88; PMID:26386540; http://dx.doi.org/10.1016/S0140-6736(15)00239-1
- Delamarre L, Mellman I, Yadav M. Cancer immunotherapy. Neo approaches to cancer vaccines. Science (New York, NY) 2015; 348:760-1; PMID:25977539; http://dx.doi.org/10.1126/science.aab3465
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science (New York, NY) 2015; 348:69-74; PMID:25838375; http://dx.doi.org/10.1126/science.aaa4971
- Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science (New York, NY) 1986; 234:364-8; PMID:2876518; http://dx.doi.org/10.1126/science.2876518
- Decatur AL, Portnoy DA. A PEST-like sequence in listeriolysin O essential for Listeria monocytogenes pathogenicity. Science (New York, NY) 2000; 290:992-5; PMID:11062133; http://dx.doi.org/10.1126/science.290.5493.992
- Sewell DA, Shahabi V, Gunn GR, 3rd, Pan ZK, Dominiecki ME, Paterson Y. Recombinant Listeria vaccines containing PEST sequences are potent immune adjuvants for the tumor-associated antigen human papillomavirus-16 E7. Cancer Res 2004; 64:8821-5; PMID:15604239; http://dx.doi.org/10.1158/0008-5472.CAN-04-1958
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science (New York, NY) 2013; 342:1432-3; PMID:24357284; http://dx.doi.org/10.1126/science.342.6165.1432
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Eng J Med 2015; 372:311-9; PMID:25482239; http://dx.doi.org/10.1056/NEJMoa1411087
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455-65; PMID:22658128; http://dx.doi.org/10.1056/NEJMoa1200694
- Hamid O, Carvajal RD. Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy. Exp Opin Biol Therapy 2013; 13:847-61; PMID:23421934; http://dx.doi.org/10.1517/14712598.2013.770836
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012; 24:207-12; PMID:22236695; http://dx.doi.org/10.1016/j.coi.2011.12.009
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K et al. Nivolumab plus ipilimumab in advanced melanoma. N Eng J Med 2013; 369:122-33; PMID:23724867; http://dx.doi.org/10.1056/NEJMoa1302369
- Fu J, Malm IJ, Kadayakkara DK, Levitsky H, Pardoll D, Kim YJ. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Cancer Res 2014; 74:4042-52; PMID:24812273; http://dx.doi.org/10.1158/0008-5472.CAN-13-2685
- Schreiner B, Mitsdoerffer M, Kieseier BC, Chen L, Hartung HP, Weller M, Wiendl H. Interferon-β enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J Neuro Immunol 2004; 155:172-82; PMID:15342209; http://dx.doi.org/10.1016/j.jneuroim.2004.06.013
- Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{α}+ dendritic cells. J Exp Med 2011; 208:2005-16; PMID:21930765; http://dx.doi.org/10.1084/jem.20101159
- Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med 2011; 208:1989-2003; PMID:21930769; http://dx.doi.org/10.1084/jem.20101158
- Yang X, Zhang X, Fu ML, Weichselbaum RR, Gajewski TF, Guo Y, Fu YX. Targeting the tumor microenvironment with interferon-β bridges innate and adaptive immune responses. Cancer Cell 2014; 25:37-48; PMID:24434209; http://dx.doi.org/10.1016/j.ccr.2013.12.004
- Fuertes MB, Woo SR, Burnett B, Fu YX, Gajewski TF. Type I interferon response and innate immune sensing of cancer. Trends Immunol 2013; 34:67-73; PMID:23122052; http://dx.doi.org/10.1016/j.it.2012.10.004
- Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012; 12:237-51; PMID:22437869; http://dx.doi.org/10.1038/nrc3237
- Mkrtichyan M, Chong N, Abu Eid R, Wallecha A, Singh R, Rothman J, Khleif SN. Anti-PD-1 antibody significantly increases therapeutic efficacy of Listeria monocytogenes (Lm)-LLO immunotherapy. J Immunother Cancer 2013; 1:15; PMID:24829751 http://dx.doi.org/10.1186/2051-1426-1-15
- Dai B, Xiao L, Bryson PD, Fang J, Wang P. PD-1/PD-L1 Blockade Can Enhance HIV-1 Gag-specific T Cell Immunity Elicited by Dendritic Cell-Directed Lentiviral Vaccines. Mol Ther 2012; 20:1800-9; PMID:22588271; http://dx.doi.org/10.1038/mt.2012.98
- Soong RS, Song L, Trieu J, Knoff J, He L, Tsai YC, Huh W, Chang YN, Cheng WF, Roden RB et al. Toll-like receptor agonist imiquimod facilitates antigen-specific CD8+ T-cell accumulation in the genital tract leading to tumor control through IFNgamma. Clin Cancer Res 2014; 20:5456-67; PMID:24893628; http://dx.doi.org/10.1158/1078-0432.CCR-14-0344
- Zeng Q, Peng S, Monie A, Yang M, Pang X, Hung CF, Wu TC. Control of cervicovaginal HPV-16 E7-expressing tumors by the combination of therapeutic HPV vaccination and vascular disrupting agents. Human Gene Therapy 2011; 22:809-19; PMID:21128743; http://dx.doi.org/10.1089/hum.2010.071
