ABSTRACT
Myeloid-derived suppressor cells (MDSCs) and chronic inflammatory factors induce immunosuppression and disease progression in advanced melanoma patients. They could serve as important markers allowing the identification of patients with high risk of melanoma progression as well as the detection of those patients who may benefit from the therapy with ipilimumab.
Abbreviations
| CTLA-4 | = | cytotoxic T lymphocyte-associated antigen-4 |
| Gr | = | granulocytic |
| HMGB1 | = | high mobility group protein B1 |
| IL | = | interleukin |
| Ipi | = | ipilimumab |
| IFNγ | = | interferon-γ |
| MDSCs | = | myeloid-derived suppressor cells |
| Mo | = | monocytic |
| NO | = | nitric oxide |
| PD-1 | = | program death receptor-1 |
| PD-L1 | = | program death ligand |
| TGF-β | = | transforming growth factor-β |
| TNF-α | = | tumor necrosis factor (TNF)-α |
Skin malignant melanoma is a potentially fatal form of skin cancer characterized by a rapid progression, early spreading in distant organs and poor response to the conventional treatment. Recent application of targeted and immunotherapeutic strategies for metastatic melanoma have brought new optimism into the field. The treatment with antibodies blocking cytotoxic T lymphocyte-associated antigen (CTLA)-4 (ipilimumab, Ipi) or interaction of program death (PD)-1 receptor and PD-1 ligand (PD-L1) (pembrolizumab, nivolumab) has been shown to induce durable responses with long-term survival.Citation1 However, such effect was found only in some patients that could be due to an enrichment of activated MDSCs and chronic inflammatory factors, which stimulate further immunosuppression and tumor progression.Citation2-5
MDSCs represent a heterogeneous population of immature myeloid cells derived from the bone marrow haematopoietic precursors that strongly inhibit T cell antitumor reactivity via different mechanisms.Citation3 MDSCs can be recruited, enriched and activated by various inflammatory mediators including interleukin (IL)-1β, IL-6, transforming growth factor (TGF)-β, tumor necrosis factor (TNF)-α, interferon (IFN)γ, S100A8/A9, high mobility group protein B1 (HMGB1) and chemokines CCL2, CCL3, CCL4, CCL5.Citation3,5 Notably, many of these factors (such as IL-1β, IL-6, TNF-α, IFNγ, CCL2 and CCL5) are known be produced and secreted in the course of acute inflammation, inducing the stimulation of T cell-mediated immune reactions.Citation3 However, a long-term secretion and maintenance of the same mediators creates chronic inflammatory conditions that promote an enrichment and activation of immunosuppressive cells (such as MDSCs) and support tumor progression.Citation3,6 Moreover, being delivered distantly in the soluble form or by tumor-derived microvesicles (exosomes), these factors can alter myelopoiesis and even convert normal peripheral blood monocytes into highly immunosuppressive MDSCs.Citation7
We performed a complex analysis of various inflammatory factors and MDSCs in the peripheral blood of patients suffering from malignant melanoma of different stages.Citation8 In advanced melanoma patients, the levels of serum inflammatory mediators IL-1β, IFNγ and CXCL10 were significantly elevated that was found to be associated with an increased frequency of MDSCs as compared to age- and gender-matched healthy donors. Moreover, an enrichment of circulating monocytic (Mo)-MDSCs significantly correlated with a decreased progression free survival of these patients.Citation8 Importantly, advanced melanoma patients with signs of progression displayed markedly elevated concentrations of IL-1β and CXCL10 as compared to patients with stable disease. These data suggest a complex association between circulating inflammatory mediators, MDSCs and the clinical outcome. The levels of these markers in patients with advanced melanoma could be of important prognostic value allowing the identification of those with high risk of disease progression.
To evaluate predictive immune-related markers of the responsiveness of advanced melanoma patients to the Ipi treatment, we divided them in two groups (responding and non-responding to the treatment) and studied myeloid cells (MDSCs and eosinophils) and related inflammatory factors in patients' peripheral blood.Citation9 A pretreatment frequency of Mo-MDSCs in non-responders was found to be slightly higher than in responders. However, upon the Ipi therapy, we observed a significant increase in the frequency of Mo-MDSCs in non-responders after the first and second Ipi infusion, whereas in responders, the Mo-MDSC level showed a strong reduction upon the therapy as compared to basal values. Furthermore, in responders, the frequencies of Mo-MDSC upon the first and second infusion were significantly higher than in non-responders.Citation9 Next, we measured nitric oxide (NO) production, which was reported to mediate MDSC immunosuppressive function.Citation3,5,6 The production of NO by Mo-MDSC from patients responding to Ipi was decreased as compared to non-responders, suggesting an Ipi-related downregulation of the MDSC activity. Analyzing a correlation between Mo-MDSC frequencies and NO production by these cells measured simultaneously in the same patients, we demonstrated that after the first infusion, higher frequencies of Mo-MDSC in non-responders significantly correlated with an elevated intensity of NO production in these cells.Citation9
In contrast to Mo-MDSC, we observed no reduction of the frequency of granulocytic (Gr)-MDSCs in responders. However, this subset displayed in responders an early significant downregulation of the PD-L1 expression as compared to the baseline and to this parameter in responders. This molecule has been shown to be involved in MDSC-mediated inhibition of T cell reactivity,Citation3,10 suggesting a downregulation of Gr-MDSC functions in patients responding to Ipi therapy.
To decipher the mechanisms of MDSC alterations under the treatment with Ipi, we measured serum levels of inflammatory factors S100A8/A9 and HMGB1 that were reported to be involved in MDSC recruitment and stimulation.Citation3,6 In non-responders, we detected an accumulation of these mediators suggesting their involvement in the observed regulation of MDSC frequency and immunosuppressive phenotype.Citation9
In addition to MDSCs, changes in eosinophils in the peripheral blood also correlated with the responsiveness to Ipi. Already after first infusion, we observed a strong increase in eosinophil counts in responding patients as compared to the baseline level.Citation9 Moreover, in non-responding patients, we detected a significant reduction in the concentration of eotaxin-1 (CCL11) as compared to baseline levels, indicating poor conditions for eosinophil accumulation in patients resistant to Ipi therapy. The mechanisms of eosinophil implication in the response to the therapy with negative checkpoint inhibitors are currently under investigation.
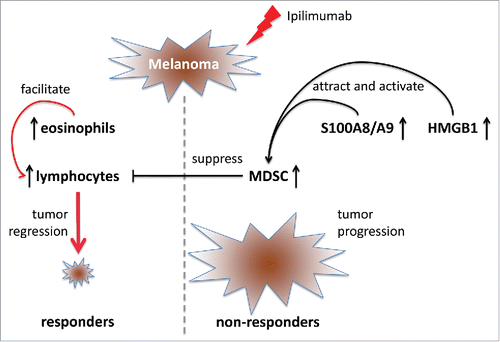
Taken together, we suggest that MDSCs and eosinophils as well as related chronic inflammatory factors S100A8/A9 and HMGB1 could be involved in the therapeutic effect of ipilimumab and could serve as new biomarkers detecting the group of advanced melanoma patients who may benefit from Ipi therapy ().
Figure 1. The treatment of advanced melanoma patients with ipilimumab induces clinical response in some patients (responders), whereas others showed no clinical response. Non-responders are characterized by an increase in the frequency of circulating myeloid-derived suppressor cells (MDSCs) and their immunosuppressive features leading to T lymphocyte inhibition and tumor progression. MDSC accumulation and stimulation is supported by elevated serum levels of inflammatory factors S100A8/A9 and HMGB1. Responders displayed increased counts of circulating eosinophils that may facilitate T lymphocyte recruitment. Therefore, MDSCs and eosinophils as well as S100A8/A9 and HMGB1 could serve as new biomarkers detecting the group of advanced melanoma patients who may benefit from ipilimumab therapy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Stadler S, Weina K, Gebhardt C, Utikal J. New therapeutic options for advanced non-resectable malignant melanoma. Adv Med Sci 2015; 60:83-8; PMID:25596540; http://dx.doi.org/10.1016/j.advms.2014.12.002
- Umansky V, Sevko A, Gebhardt C, Utikal J. Myeloid-derived suppressor cells in malignant melanoma. J Dtsch Dermatol Ges 2014; 12:1021-7; PMID:25263083; http://dx.doi.org/10.1111/ddg.12411
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253-68; PMID:22437938; http://dx.doi.org/10.1038/nri3175
- Kanterman J, Sade-Feldman M, Baniyash M. New insights into chronic inflammation-induced immunosuppression. Semin Cancer Biol 2012; 22:307-18; PMID:22387003; http://dx.doi.org/10.1016/j.semcancer.2012.02.008
- Umansky V, Sevko A. Melanoma-induced immunosuppression and its neutralization. Semin Cancer Biol 2012; 22:319-26; PMID:22349515; http://dx.doi.org/10.1016/j.semcancer.2012.02.003
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 2009; 182:4499-506; PMID:19342621; http://dx.doi.org/10.4049/jimmunol.0802740
- Altevogt P, Bretz NP, Ridinger J, Utikal J, Umansky V. Novel insights into exosome-induced, tumor-associated inflammation and immunomodulation. Semin Cancer Biol 2014; 28:51-7; PMID:24769223; http://dx.doi.org/10.1016/j.semcancer.2014.04.008
- Jiang H, Gebhardt C, Umansky L, Beckhove P, Schulze TJ, Utikal J, Umansky V. Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int J Cancer 2015; 136:2352-60; PMID:25353097; http://dx.doi.org/10.1002/ijc.29297
- Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, Holland-Letz T, Umansky L, Beckhove P, Sucker A et al. Myeloid Cells and Related Chronic Inflammatory Factors as Novel Predictive Markers in Melanoma Treatment with Ipilimumab. Clin Cancer Res 2015; 21:5453-9; PMID:26289067; http://dx.doi.org/10.1158/1078-0432.CCR-15-0676
- Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014; 211:781-90; PMID:24778419; http://dx.doi.org/10.1084/jem.20131916
