ABSTRACT
The functional status of CD4+ T cells is a critical determinant of antitumor immunity. Polyfunctional CD4+ T cells possess the ability to concomitantly produce multiple Th1-type cytokines, exhibiting a functional attribute desirable for cancer immunotherapy. However, the mechanisms by which these cells are induced are neither defined nor it is clear if these cells can be used therapeutically to treat cancer. Here, we report that CD4+ T cells exposed to exogenous IL-7 during antigenic stimulation can acquire a polyfunctional phenotype, characterized by their ability to simultaneously express IFNγ, IL-2, TNFα and granzyme B. This IL-7-driven polyfunctional phenotype was associated with increased histone acetylation in the promoters of the effector genes, indicative of increased chromatin accessibility. Moreover, forced expression of a constitutively active (CA) form of STAT5 recapitulated IL-7 in inducing CD4+ T-cell polyfunctionality. Conversely, the expression of a dominant negative (DN) form of STAT5 abolished the ability of IL-7 to induce polyfunctional CD4+ T cells. These in-vitro-generated polyfunctional CD4+ T cells can traffic to tumor and expand intratumorally in response to immunization. Importantly, adoptive transfer of polyfunctional CD4+ T cells following lymphodepletive chemotherapy was able to eradicate large established tumors. This beneficial outcome was associated with the occurrence of antigen epitope spreading, activation of the endogenous CD8+ T cells and persistence of donor CD4+ T cells exhibiting memory stem cell attributes. These findings indicate that IL-7 signaling can impart polyfunctionality and stemness potential to CD4+ T cells, revealing a previously unknown property of IL-7 that can be exploited in adoptive T-cell immunotherapy.
Introduction
The critical role of CD4+ T cells in orchestrating antitumor immunity has been well-established.Citation1 CD4+ T helper (Th) cells, by providing CD40L and releasing inflammatory cytokines such as IFNγ, IL-2 and TNFα, can activate a variety of tumor-reactive immune cells including cytotoxic CD8+ T lymphocytes (CTLs),Citation2-5 NK cells,Citation6 and macrophages.Citation7 In addition, cytolytic CD4+ T cells can mediate direct tumor destruction through perforin and granzyme B.Citation8-10 More importantly, the therapeutic efficacy of antitumor CD4+ T cells has been manifested in a number of clinical studies.Citation11,12
There is mounting evidence that CD4+ T cells can be polyfunctional, as characterized by their ability to concomitantly express two or more effector molecules, including CD40L, IFNγ, IL-2, TNFα, and granzyme B. For years, polyfunctional T cells (both CD4+ and CD8+) have gleaned much attention in the field of microbiology because these cells correlate with more effective control of viral or bacterial infections.Citation13-16 Polyfunctional T cells have also been identified in tumor settings. In preclinical studies, we and others have demonstrated that tumor-specific CD4+ T cells can acquire polyfunctionality upon transferring into tumor-bearing hosts preconditioned by chemotherapy or total body irradiation (TBI).Citation8,10,17-19 Polyfunctional T cells can be induced in cancer patients after vaccinations.Citation20-22 However, mechanistically how these cells are induced is not understood. Moreover, it is unclear whether these cells are associated with better prognosis, or can be used therapeutically to treat cancer.
IL-7 is a hematopoietic growth factor involved in regulating multiple aspects of T-cell biology, including survival, homeostasis, memory formation and metabolism.Citation23,24 The initial evidence that IL-7 plays a role in enhancing antitumor immune responses came from animal studies using tumor cells engineered to express IL-7. These studies demonstrated that high local concentration of IL-7 can recruit CD4+ and CD8+ T cells to the tumor sites, resulting in tumor rejection.Citation25,26 In recent years, considerable efforts have been invested to study the utility of recombinant IL-7 in cancer immunotherapy. Recombinant human IL-7 (rhIL-7) administered to patients with cancer exhibited a favorable safety profile, preferentially expanded circulating naive CD4+ and CD8+ T cells but not Treg cells, and led to increased T-cell receptor (TCR) repertoire diversity.Citation27-29 Based on these clinical trials and other preclinical studies, IL-7 has been considered as a promising immunotherapy drug with anticipated benefits in promoting immune reconstitution in elderly patients and patients with prior lymphodepletive chemotherapy.Citation30,31 In the setting of adoptive T-cell therapy (ACT), IL-7 as a T-cell growth factor has often been used in ex vivo cell culture to expand tumor-reactive T cells.Citation32
In this study, we investigated the role of IL-7 in inducing polyfunctionality in CD4+ T cells. We found that IL-7-driven polyfunctionality in CD4+ T cells is mechanistically dependent on STAT5 activation, and correlates with increased chromatin accessibility in multiple effector genes. From the therapeutic standpoint, we evaluated the efficacy of IL-7-conditioned polyfunctional CD4+ T cells in adoptive cell therapy in murine models of lymphoma and colon cancer. Our data provide insights into the mechanisms underlying the induction of polyfunctional CD4+ T cells, and validate therapeutic strategies that capitalize on the antitumor potential of polyfunctional CD4+ T cells.
Results
IL-7 confers polyfunctionality to activated CD4+ T cells in vitro
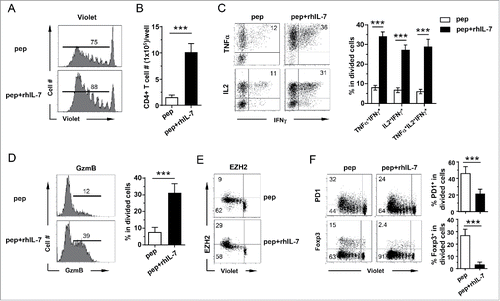
The in vivo conditions in which polyfunctional CD4+ T cells can be induced often involve chemotherapy, or TBI.Citation8,10,17,19 These maneuvers may remove cytokine sinks, making growth factors available to tumor-specific CD4+ T cells.Citation33 Among the cytokines/growth factors induced by chemotherapy or TBI, IL-7, a common γ chain cytokine, is known to regulate T-cell survival, differentiation and memory formation. This prompted us to test whether IL-7 can induce polyfunctionality in CD4+ T cells during in vitro culture. To this end, splenocytes from the 6.5 TCR-Tg mice, which give rise to CD4+ T cells recognizing an epitope derived from influenza hemagglutinin (HA), were stimulated with HA peptide in the presence or absence of exogenous rhIL-7. Addition of rhIL-7 led to enhanced CD4+ T-cell proliferation () and accumulation (). Importantly, divided CD4+ T cells derived from the IL-7-conditioned culture acquired greater polyfunctionality as reflected by the increased frequency of cells that can concomitantly produce two or three Th1-type cytokines including IL-2, TNFα and IFNγ (). Moreover, these CD4+ T cells also had markedly increased granzyme B expression (). Indeed, about 20% of the IL2+ TNFα+ IFNγ+ CD4+ T-cells expressed granzyme B (Fig. S1A), implicating the potential of these cells to concurrently mediate diverse effector functions. Of note, these polyfunctional CD4+ T cells were meager in IL-17A production (Fig. S1B). EZH2, a histone methyltransferase, was recently identified as a key regulatory gene controlling the polyfunctionality of human effector T cells.Citation34 Interestingly, we found that the frequency of highly divided EZH2+ CD4+ T cells increased nearly three-fold in T-cells stimulated in the presence of rhIL-7 compared to T-cells stimulated without rhIL-7 (). Furthermore, acquisition of polyfunctionality by divided CD4+ T cells, as the result of antigenic stimulation in the presence of rhIL-7, was associated with reduced expression of the immune regulatory proteins, PD-1 and Foxp3 (). We further confirmed that OVA-specific CD4+ T cells, derived from either DO11.10 (BALB/c background) or OT-II (C57BL/6 background) TCR-Tg mice, can also acquire polyfunctionality when stimulated with the cognate peptide in the presence of rhIL-7 (Fig. S2). The results suggest that IL-7-driven CD4+ polyfunctionality is not restricted to a particular antigen or mouse strain.
Figure 1. IL-7 confers polyfunctionality to 6.5 TCR-Tg CD4+ T cells upon antigenic stimulation in vitro. Violet-dye-labeled spleen cells from 6.5 TCR-Tg mice were stimulated with 1 μg/mL HA peptide in the absence or presence of 100 ng/mL rhIL-7. After 7 d in culture, cells were harvested, enumerated and analyzed by FACS. (A) CD4+ T cell proliferation status as reflected by violet dye dilution. Histograms shown are representative of CD4+ T cells harvested from the specified culture conditions. Numbers indicate the percentage of divided CD4+ T cells. (B) Absolute number of CD4+ T cells recovered from the specified culture condition. Live CD4+ T cell number is calculated as: total live cells count per well × percent CD4+ T cells. Data are pooled from six independent experiments and shown as mean ± SD. (C) Expression profiles of pro-inflammatory cytokines produced by activated CD4+ T cells. Cells recovered from culture were stimulated with Leukocyte Activation Cocktail containing PMA, ionomycin and GolgiPlug for 4 h before intracellular staining for IL-2, TNFα and IFNγ. Representative dot plots shown are gated on divided CD4+ T cells, and the numbers indicate the percentage of cells in the corresponding quadrant. The results from six independent experiments are summarized in bar graph. Data are shown as mean ± SD. (D) Expression profiles of granzyme B in cultured CD4+ T cells. Histograms shown are gated on divided CD4+ T cells. Numbers in histograms indicate the percent of granzyme B-expressing cells in divided CD4+ T cells. Data are summarized in scatter plots. (E) EZH2 expression in cultured CD4+ T cells. Representative dot plots are shown to indicate EZH2 expression relative to CD4+ T-cell division. Numbers represent percent of cells in the corresponding quadrant. (F) PD1 and Foxp3 expression profiles in cultured CD4+ T cells. Representative dot plots are shown to indicate PD1 and Foxp3 expressions relative to CD4+ T-cell division. Numbers represent percent of cells in the corresponding quadrant. Summary of the results are shown in the bar graphs. Data are pooled from six independent results and shown as mean ± SD. ***, p <0 .001.
The strength of TCR-dependent signaling exerts profound impact on CD4+ T-cell polarity.Citation35 We thus examined whether TCR signal strength affects IL-7-driven polyfunctionality by adding escalating doses of peptide to cell culture in the absence or presence of rhIL-7 (Fig. S3). rhIL-7 was able to increase the frequency of IFNγ+ TNFα+ cells in a wide range of peptide concentrations (0.004–1.0 ug/mL HA peptide for 6.5 CD4+ T cells and 0.04–50 ug/mL OVA peptide for DO11.10 CD4+ T cells). At very high peptide concentrations, rhIL-7 failed to induce polyfunctionality in CD4+ T cells, reminiscent of the results reported by Chiu et al that Ag-specific human CD8+ failed to acquire polyfunctionality at high Ag concentration.Citation36 We chose 1 ug/mL HA peptide or 10 ug/mL OVA peptide as the optimal Ag dose for IL-7 induction of polyfunctionality in 6.5 or DO11.10 CD4+ T cells, respectively. At these Ag doses, addition of rhIL-7 resulted in the highest yield of cells with the polyfunctional phenotype.
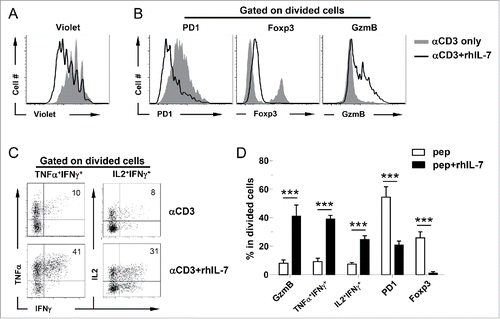
We next examined whether non-TCR transgenic, polyclonal CD4+ T cells can be endowed with polyfunctionality by IL-7 similar to TCR-Tg CD4+ T cells. To this end, BALB/c splenocytes were stimulated with soluble αCD3 Ab in the presence or absence of rhIL-7. Again, rhIL-7 promoted CD4+ T-cell proliferation (), downregulated PD-1 and Foxp3 expressions, and conferred CD4+ T cells the capability to produce IFNγ, IL-2, TNFα, and granzyme B (). We confirmed that the above results can be replicated by replacing rhIL-7 with recombinant mouse IL-7 (data not shown). Altogether, these data indicate that acquisition of polyfunctionality might be a general feature of CD4+ T cells upon antigenic stimulation in the presence of exogenous IL-7.
Figure 2. Polyclonal CD4+ T cells acquire polyfunctionality in the presence of rhIL-7 in vitro. Violet-dye-labeled spleen cells from a BALB/c mouse were stimulated with soluble αCD3 mAb (50 ng/mL) in the absence or presence of rhIL-7. 7 d after culture, cells were harvested and analyzed by FACS. (A) Cell proliferation status of CD4+ T cells under different culture conditions is shown in overlay histograms. (B) Expression profiles of PD1, Foxp3 and granzyme B in CD4+ T cells under different culture conditions. Results shown are representative of two independent experiments. (C) Expression profiles of pro-inflammatory cytokines produced by activated CD4+ T cells. Cells recovered from culture were stimulated with PMA and ionomycin in the presence of GolgiPlug for 4 h before intracellular staining for IL-2, TNFα and IFNγ. Representative dot plots shown are gated on divided CD4+ T cells, and the numbers indicate the percent of cells in the corresponding quadrant. (D) The results of (B) and (C) are summarized in bar graph and shown as mean ± SD. Data are pooled from two independent experiments. ***, p <0 .001.
Induction of polyfunctionality in CD4+ T cells requires the presence of IL-7 in the late phase of T-cell activation
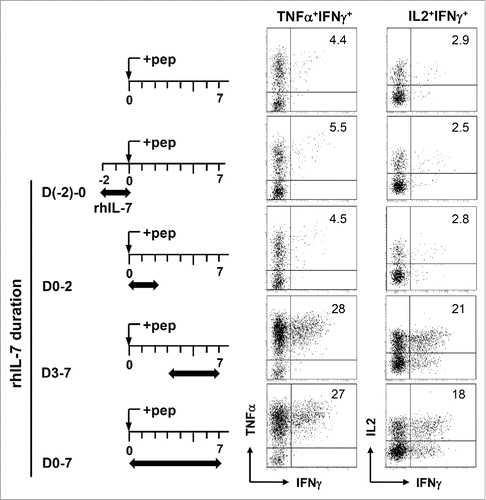
We set out to determine the timing and duration of rhIL-7 needed to induce polyfunctionality in CD4+ T cells. To this end, rhIL-7 was added to cell culture at different stages of T-cell activation (), and acquisition of polyfunctionality by CD4+ T cells was evaluated using concomitant productions of IFNγ, IL-2 and TNFα as the readout. IL-7 is known to regulate the survival and homeostasis of naïve T cells.Citation23 Naïve CD4+ T cells exposed to rhIL-7 in the absence of Ag from D-2 to D0 indeed had improved viability compared to cells resting in media only (data not shown). However, these CD4+ T cells did not acquire polyfunctionality upon subsequent antigenic stimulation without the continuous presence of rhIL-7 (), implying that prior IL-7 exposure does not predispose CD4+ T cells to polyfunctionality induction. Adding rhIL-7 during the early stage of T-cell activation (D0–2) also failed to induce polyfunctional CD4+ T cells (). In contrast, the presence of rhIL-7 in the late phase of T-cell activation (D3–7) was sufficient for polyfunctionality induction ().
Figure 3. Induction of polyfunctionality in CD4+ T cells requires the presence of rhIL-7 during the late phase of T-cell activation. Violet-dye-labeled spleen cells from 6.5 TCR-Tg mice were stimulated with 1 μg/mL HA peptide. rhIL-7 (100 ng/mL) was added to culture following the timeline depicted in the schema. 7 d after peptide stimulation, cells were harvested and restimulated with PMA and ionomycin for 4 h before cytokine intracellular staining. Dot Plots shown are gated on divided CD4+ T cells. Data shown are representative of three independent experiments.
IL-7 induces differential epigenetic modifications in the promoters of effector cytokine genes, granzyme B and Foxp3
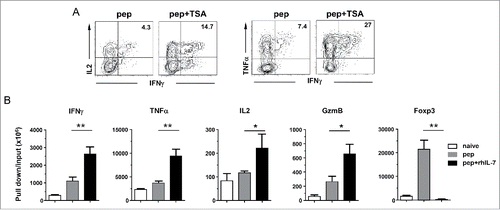
It has been shown that IL-7 signaling can regulate gene expression by inducing epigenetic modifications, including histone acetylation, that result in increased chromatin accessibility.Citation37-39 To test if polyfunctionality can be induced by pharmacologically increasing histone acetylation, trichostatin A (TSA), a pan histone deacetylase (HDAC) inhibitor, was added to CD4+ T cells during antigenic stimulation. We found that TSA can mimic rhIL-7 in inducing polyfunctional CD4+ T cells, as demonstrated by a two-fold increase in the percent of IFNγIL2TNFα-producing CD4+ T cells after TSA treatment (). The data raised the possibility that alteration of histone acetylation may underlie IL-7-driven polyfunctionality. To further test this hypothesis, we conducted ChIP assays to evaluate the impact of IL-7 signaling on histone 3 acetylation in the promoters of a panel of genes relevant to the polyfunctional phenotype. shows that antigenic stimulation in the presence of rhIL-7 (pep+ rhIL-7) led to markedly increased levels of H3 acetylation in the promoters of IFNγ, TNFα,IL-2 and granzyme B, whereas Ag alone (pep) only modestly increased H3 acetylation in these genes compared to unstimulated naïve CD4+ T cells. In contrast, H3 acetylation in Foxp3 promoter significantly increased in CD4+ T cells stimulated with peptide only, but was rather low in CD4+ T cells stimulated with pep+ rhIL-7. The results provide evidence that IL-7 signaling may simultaneously increase chromatin accessibility in multiple genes, and that this effect is selective because increased histone acetylation only occurs in the promoters of effector cytokine genes and granzyme B but not in Foxp3.
Figure 4. IL-7-induced polyfunctional CD4+ T cells exhibit increased histone acetylation in the promoters of effector cytokine genes and granzyme B but not Foxp3. (A) TSA recapitulates rhIL-7 in inducing polyfunctionality in CD4+ T cells. Spleen cells from 6.5 TCR-Tg mice were labeled with violet dye and stimulated with 1 ug/mL HA peptide. TSA (20 uM) was added to cell culture on days 2 and 3. Cells were harvested on day 4 and subjected to ICS for IFNγ, TNFα and IL-2. Dot plots shown are representative of three independent experiments. (B) Histone 3 acetylation ChIP assay. Spleen cells from 6.5 TCR-Tg mice were stimulated with 1 μg/mL HA peptide in the absence or presence of rhIL-7. 7 d later, CD4+ T cells were FACS-sorted and subjected to ChIP analysis using an antibody specific for acetylated histone 3 (H3) for immunoprecipitation. Purified naïve CD4+ T cells were included as control. The immuneprecipitated DNA was analyzed by real-time PCR to assess H3 acetylation in the promoter regions of IFNγ, TNFα, IL-2, granzyme B and Foxp3. Results are normalized to input DNA. Data shown are representative of two independent experiments with similar results. *, p <0 .05; **, p <0 .01.
IL-7-driven polyfunctionality is STAT5 dependent
STAT5 is a critical mediator of IL-7 signaling.Citation40 To test if STAT5 is required for acquisition of polyfunctionality in CD4+ T cells, a STAT5 inhibitor (STAT5i) or vehicle was added to cells under the pep+ rhIL-7 culture condition. STAT5i greatly diminished IFNγ production in CD4+ T cells without affecting cell proliferation driven by pep+ rhIL-7 (Fig. S4), suggesting the involvement of STAT5 in IL-7-induced polyfunctionality. To further define the role of STAT5 in CD4+ T-cell polyfunctionality, a CA mutant (CA-STAT5) or dominant-negative mutant (DN-STAT5) of STAT5 was introduced into CD4+ T cells from DO11.10 mice using a retroviral vector (RV) carrying Thy1.1 marker (). Comparable transduction efficiency was achieved in CA-STAT5 and DN-STAT5 RV-infected CD4+ T cells (). As shown in , CA-STAT5 rendered CD4+ T cells polyfunctional, as reflected by their ability to produce multiple cytokines, even in the absence of rhIL-7, and addition of rhIL-7 did not further enhance cytokine production; conversely, DN-STAT5-transfected CD4+ T cells failed to acquire polyfunctionality even in the presence of rhIL-7 which conferred polyfunctionality in untransfected CD4+ T cells. Similarly, CA-STAT5 induced granzyme B in CD4+ T cells in the absence of rhIL-7, whereas DN-STAT5 reduced granzyme B in CD4+ T cells stimulated in the presence of rhIL-7 (data not shown). In contrast, CA-STAT5 had opposite effect on Foxp3 expression compared to its effect on cytokines and granzyme B. As shown in , CA-STAT5 significantly reduced Foxp3 expression (6.1 ± 1.8%) in CD4+ T cells that would otherwise acquire considerable Foxp3 expression (20.6 ± 3.3%) when stimulated in the absence of rhIL-7, whereas DN-STAT5-transfected CD4+ T cells retained Foxp3 expression despite the presence of rhIL-7. To address whether STAT5 can induce the epigenetic marks associated with CD4+ T-cell polyfunctionality, we conducted acetyl-histone H3 ChIP assays for a group of representative genes (IFNγ, TNFα and Foxp3) using CD4+ T cells transduced with CA-STAT5. shows that STAT5 overexpression led to increased H3 acetylation, comparable to that in IL-7-conditioned CD4+ T cells, in the promoters of IFNγ and TNFα, whereas H3 acetylation in the Foxp3 promoter was significantly reduced in both IL-7-conditioned and STAT5-overexpressing CD4+ T cells. Altogether, the data provide compelling evidence that STAT5 activation downstream of IL-7 signaling is necessary and sufficient to drive polyfunctionality in CD4+ T cells.
Figure 5. IL-7-driven polyfunctionality is STAT5-dependent. The experimental timeline is depicted in the schema. Spleen cells from DO11.10 TCR-Tg mice were labeled with violet dye and stimulated with the cognate peptide for 24 h. Cells were then harvested and spun infected with CA-STAT5 or DN-STAT5 retrovirus carrying the Thy1.1 marker. 12 h after infection, cells were harvested and restimulated with peptide-pulsed spleen cells from CD45.1 mice as APCs with or without addition of rhIL-7 (100 ng/mL). Cells were subsequently cultured for an additional 4 d and subjected for FACS analysis. (A) Viral transduction efficiency in CD4+ T cells evaluated by expression of Thy1.1 marker. Histograms shown are representative of three independent experiments. Numbers indicate the percent of virus-infected cells in total CD4+ T cell population. (B) Cytokine profiles of virus-infected CD4+ T cells. Cells recovered from culture were subjected to intracellular staining for TNFα and IFNγ. Representative dot plots shown are gated on divided CD4+ T cells. Numbers indicate the percent of TNFα+ IFNγ+ cells. (C) Summary of the results shown in (B). (D) Expression profiles of Foxp3 in virus-infected CD4+ T cells. Numbers indicate the percent of Foxp3-positive cells in divided CD4+ T cells. (E) Summary of the results shown in (D). Data are pooled from three independent experiments and shown as mean ± SD. (F) Histone 3 acetylation ChIP assay. Spleen cells from 6.5 TCR-Tg mice were transduced with CA-STAT5 retrovirus in the absence of rhIL-7. Infected CD4+ T cells were FACS-sorted and subjected to H3ac ChIP assays. CD4+ T cells stimulated with peptide in the absence or presence of rhIL-7 were used for comparison. The immuneprecipitated DNA was analyzed by real-time PCR to assess H3 acetylation in the promoter regions of IFNγ, TNFα and Foxp3. Results are normalized to input DNA. Data shown are representative of two independent experiments with similar results. *, p <0 .05; **, p <0 .01; ***, p <0 .001. n.s., not statistically significant.
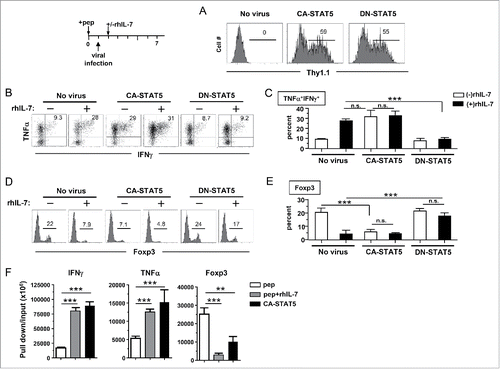
Polyfunctional CD4+ T cells expand intratumorally in response to immunization
The polyfunctional CD4+ T cells exhibited features associated with tumoricidal activities, including expressions of IFNγ, TNFα and granzyme B. We predicted that these CD4+ T cells, generated from peptide-stimulated and rhIL-7-suplemented culture (herein termed p+IL-7CD4+ T cells), should exert strong antitumor effects in vivo. To test this, HA-specific polyfunctional CD4+ T cells were transferred into mice bearing three-day-old A20HA tumors. To our surprise, adoptive transfer of polyfunctional CD4+ T cells alone was unable to reduce tumor growth compared to the no treatment control group (Fig. S5, p+IL-7CD4 vs no Tx). We reasoned that a strong antigenic stimulus, such as a therapeutic vaccine, may trigger the action of polyfunctional CD4+ T cells and result in a favorable antitumor effect. Of note, immunization of mice with a HA-expressing vaccinia vaccine (vacHA) did not thwart tumor growth, whereas immunization following adoptive transfer of polyfunctional CD4+ T cells led to significantly delayed tumor growth (, p+IL7CD4+vacHA vs vacHA). In contrast, adoptive transfer of activated but non-polyfunctional CD4+ T cells, generated from the culture containing peptide but no rhIL-7, did not manifest discernible therapeutic benefit even after vaccination (, pCD4+vacHA). The results indicate that in-vitro-generated polyfunctional CD4+ T cells, upon sufficient stimulation in situ, are capable of mediating robust antitumor effects.
Figure 6. In vitro-generated polyfunctional CD4+ T cells expand intratumorally in response to immunization. (A) Adoptive transfer of rhIL-7-conditioned polyfunctional CD4+ T cells followed by vaccination exerts robust antitumor effects in vivo. The schema outlines the timeline of experimental procedures. Mice bearing three-day-old subcutaneous A20HA-luci tumors were not treated or treated as indicated. Tumor growth curves of each group are shown. Results are presented as mean ± SD of tumor area. Mice were also subjected to BLI periodically to visualize the tumor burden. Representative images of mice in each group before and after treatment are shown in (B). (C) Intratumoral expansion of the transferred polyfunctional CD4+ T cells in response to immunization. Following the procedures depicted in the schema, luciferase-transduced polyfunctional CD4+ T cells (p+IL7CD4+-luci) were transferred to tumor-bearing mice followed by PBS or vacHA injection. BLI was conducted to detect luciferase-tagged donor CD4+ T cells. Representative images of mice in each group at specified time are shown. Results of T-cell luciferase signal intensity quantified as photon/sec are summarized in (D). Each symbol in plot represents one mouse. The lines represent the average value of T-cell signal intensity.
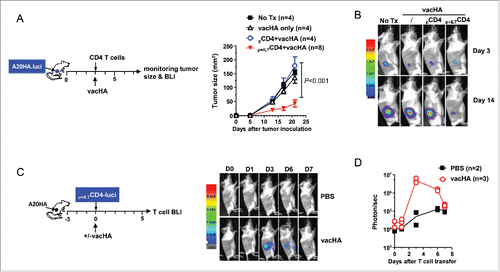
The necessity of immunization to enable polyfunctional CD4+ T cells prompted us to examine whether vacHA can drive the expansion of the donor CD4+ T cells. To this end, HA-specific CD4+ T cells cultured under the polyfunctional condition (HA+rhIL-7) were transduced with a RV containing luciferase. These luciferase-transduced CD4+ T cells were adoptively transferred into mice bearing three-day-old A20HA tumors, followed by vacHA or PBS injection. Mice were imaged thereafter to detect the presence of luciferase-tagged donor CD4+ T cells. 3 d after vacHA immunization, there was a marked expansion of the transferred CD4+ T cells within the tumor loci, whereas T-cell signal was barely detectable in unimmunized mice (). The T-cell signal abated in immunized mice after day 3, and was hardly detected by day 7 (). The results clearly indicate that IL-7-conditioned polyfunctional CD4+ T cells can home to the sites of the tumor, and can expand in vivo in response to immunization.
Adoptive transfer of polyfunctional CD4+ T cells following lymphodepletive chemotherapy leads to tumor rejection, antigen epitope spreading and persistence of stem cell-like memory T cells
We noticed that the combination of polyfunctional CD4+ T cells and vaccination was not effective in controlling more advanced tumors (>10-day-old A20HA, data not shown). We asked if large tumors can be treated efficaciously by transferring polyfunctional CD4+ T cells after pre-conditioning the hosts with lymphodepletive chemotherapy, which has become a widely adopted procedure in adoptive cell therapy. To test this, mice with large subcutaneous A20HA tumors received cyclophosphamide (CTX) preparative chemotherapy (). The next day, some mice further received adoptive transfer of activated, non-polyfunctional HA-specific CD4+ T cells (pCD4+), while some other mice received IL-7-conditioned polyfunctional HA-specific CD4+ T cells (p+IL7CD4+). Adoptive transfer of polyfunctional CD4+ T cells after CTX led to complete regression of established tumors, whereas transfer of activated, non-polyfunctional CD4+ T cells after CTX did not exhibit any benefit compared to CTX alone (). Of note, the transferred polyfunctional CD4+ T cells retained the polyfunctional phenotype throughout the experimental timeframe, as reflected by their ability to concurrently produce IFNγ, IL-2 and TNFα (), and by their reduced expressions of PD-1 and Foxp3 ().
Figure 7. Adoptive transfer of polyfunctional CD4+ T cells following lymphodepletive chemotherapy leads to complete regression of large tumors, accompanied by antigen epitope spreading and persistence of stem cell-like memory T cells. The schema outlines the timeline of experimental procedures. Mice were inoculated with A20HA subcutaneously in the flank. When tumor sizes reached ∼170 mm2, mice were treated with CTX (150 mg/kg). The next day, some mice received adoptive transfer of in-vitro-activated, non-polyfunctional HA-specific CD4+ T cells (pCD4+), while some other mice received IL-7-conditioned polyfunctional HA-specific CD4+ T cells (p+IL7CD4+). (A) Tumor growth curves of mice in each group are shown. Results are presented as mean ± SD of tumor area. (B) Representative images of tumors in mice under each condition before and after treatment. (C) Polyfunctional status of the transferred CD4+ T cells. Tail blood was collected from mice 17 d after T-cell transfer. Leukocytes in blood samples were stimulated with PMA/ionomycin in the presence of GolgiPlug for 4 h before cytokine ICS. The cytokine profiles of the donor CD4+ T cells, revealed by FACS, are shown by representative dot plots. Bar graph summarizes the percent of donor CD4+ T cells capable of simultaneously producing two or three cytokines. (D) Percent donor CD4+ T cells expressing PD-1 or Foxp3. Results are presented as mean ± SD of at least three samples per group. (E) Persisting donor CD4+ T cells exhibit features of Tem, Tcm and Tscm cells. Tail blood from cured mice receiving CTX+p+IL7CD4+ was collected and examined for expressions of CD44, CD62L, Sca-1 and CCR7 in donor and host CD4+ T cells. Three subsets are gated based on distinct CD44CD62L patterns. Percent of the gated populations is summarized in bar graph. Expression patterns of Sca-1 and CCR7 of the gated cells are shown in (F). Numbers in histogram represent the values of MFI of Sca-1 or CCR7. (G) Cytokine expression relative to CCR7 in donor CD4+ T cells. Leukocytes from the peripheral blood of cured mice were stimulated with PMA/ionomycin and subjected to ICS to reveal the relation of IL-2 and IFNγ to CCR7 in donor CD4+ T cells. Numbers represent percent of cytokine-producing cells in CCR7+ or CCR7− donor CD4+ T cells. (H) CTX+p+IL7CD4+ treatment leads to tumor antigen epitope spreading and activation of host CD8+ T cells. 20 days after T-cell transfer, mice treated with CTX+pCD4+ or CTX+p+IL7CD4+ were sacrificed. Purified splenic CD8+ T cells were labeled with violet dye and incubated with irradiated A20HA, A20WT or MOPC315 cells. 7 d later, CD8+ T cells were harvested and examined for proliferation/activation by evaluating violet-dye-dilution and CD25 expression. ***, p <0 .001.
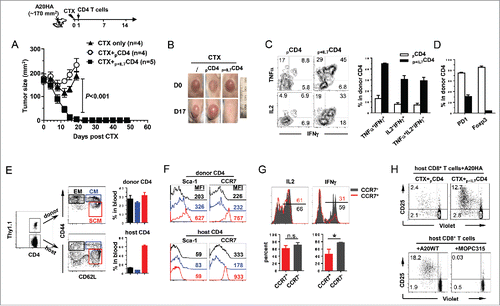
Mounting evidence indicates that memory T cells are more efficacious than terminally differentiated effector cells in mediating tumor rejection, and that the antitumor potency of memory T cells increases in the order of effector memory cells (Tem) < central memory cells (Tcm) <memory stem cells (Tscm).Citation41 To examine whether the transferred polyfunctional CD4+ T cells had given rise to memory cells, peripheral blood was collected from the cured mice and stained for CD44 and CD62L. As shown in , both the donor and host CD4+ T cells appeared to contain four distinct populations: CD44hiCD62L−, CD44hiCD62Lhi, CD44loCD62Lhi and CD44loCD62L−. Interestingly, there were higher frequencies of CD44hiCD62L− and CD44hiCD62Lhi cells, which correspond to Tem and Tcm, respectively, in the donor CD4+ T cells than in the host CD4+ T cells (). Further phenotypic analysis of the donor cells showed that Sca-1 expression was high in CD44loCD62Lhi cells, but was reduced in CD44hiCD62Lhi and CD44hiCD62L− cells (). Furthermore, CCR7 was high in CD44loCD62Lhi cells but low in CD44hiCD62Lhi and CD44hiCD62L− cells (). In contrast, the host CD4+ T cells barely expressed Sca-1, and only the CD44loCD62Lhi cells expressed CCR7 (). Intracellular cytokine staining (ICS) showed that the CCR7hi cells (mainly CD44loCD62Lhi) were proficient in IL-2 production but less abundant in IFNγ, while CCR7lo cells were good producers of both IL-2 and IFNγ (). Altogether, the results suggest that the persisting CD4+ donor T cells contained a heterogeneous population of memory cells exhibiting features of Tscm (CD44loCD62LhiSca1+CCR7hi), Tcm (CD44hiCD62Lhi) and Tem (CD44hiCD62L−) cells.
Clinical studies using tumor-specific CD4+ T cells for adoptive cell therapy indicate that the efficacy of CD4+ ACT is associated with activation of the endogenous CD8+ T cells via antigen epitope spreading.Citation11,12 To examine the tumor reactivity of the host CD8+ T cells, A20HA-bearing mice that had received either CTX+pCD4+ or CTX+p+IL7CD4+ were sacrificed on day 20, when the former mice had progressing tumors whereas the latter mice appeared to be tumor-free. CD8+ T cells purified from the spleens of mice were labeled with violet dye and cultured with irradiated A20HA tumor cells. 7 d after culture, CD8+ T cells were harvested and evaluated for cell proliferation (violet dye dilution) and activation (CD25 expression). upper panel shows that CD8+ T cells from mice receiving CTX+pCD4+ marginally proliferated; in contrast, a significant fraction (15%) of CD8+ T cells from mice receiving CTX+p+IL7CD4+ had undergone multiple rounds of cell division, and those divided cells exhibited high levels of CD25, implying the priming of the endogenous CD8+ T cells. To determine if the primed CD8+ T cells can respond to tumor-associated antigens other than HA, CD8+ T cells purified from mice receiving CTX+p+IL7CD4+ were co-cultured with irradiated A20 wild-type tumor cells (A20WT) or irrelevant MOPC315 plasmacytoma cells. Interestingly, these CD8+ T cells responded to A20WT cells but not MOPC315 cells (), indicating the occurrence of antigen epitope spreading beyond the antigen targeted by the donor CD4+ T cells. The persistence of memory donor CD4+ T cells, together with the activation of the endogenous CD8+ population, was associated with 100% protection of the cured mice from tumor re-challenge (data not shown).
To test the generality of the robust antitumor effects of ACT using polyfunctional CD4+ T cells, we extended studies to the CT26HA colorectal tumor model. Host pre-conditioning with CTX led to enhanced expansion of the transferred CD4+ T cells (Fig. S6A), which retained their polyfunctionality (Fig. S6B). Again, the persisting donor cells in mice receiving CTX plus polyfunctional CD4+ T cells contained a heterogeneous memory population consisting of Tscm (CD44loCD62LhiSca1+CCR7hi), Tcm (CD44hiCD62Lhi) and Tem (CD44hiCD62L−) cells (Fig. S6C). Similar to the results seen in the lymphoma model, CTX plus polyfunctional CD4+ T cells resulted in complete regression of CT26HA tumors; in contrast, CTX alone only led to transient tumor growth delay, whereas transfer of polyfunctional CD4+ T cells without CTX pre-conditioning had no therapeutic benefit compared to untreated mice (Fig. S6D).
Discussion
It is well-recognized that polyfunctional T cells are more effective in controlling viral and bacterial infections.Citation42 It is tempting to speculate that tumor-reactive polyfunctional T cells would be more efficacious in controlling tumor growth. However, this premise has not been experimentally demonstrated. In addition, the mechanisms underlying the induction of tumor-reactive polyfunctional T cells are poorly defined. Here, we report that IL-7 can confer polyfunctionality to activated CD4+ T cells. Importantly, the in vitro-generated, IL-7-conditioned polyfunctional CD4+ T cells, when used for ACT, manifested robust antitumor effects which correlated with antigen epitope spreading and persistence of Tscm. Our results are in line with the recent report by Zhao et al showing that the presence of EZH2+ polyfunctional T cells correlates with better survival in patients with ovarian cancer.Citation34 Interestingly, our data indicate that IL-7-conditioned polyfunctional CD4+ T cells also express high level of EZH2 (), suggesting a possible link between IL-7-signaling and EZH2 induction. Whether EZH2 plays a critical role in programming murine T-cell polyfunctionality similar to its function in polyfunctional human T cells warrants further investigation.
Besides IL-7, IL-2 and IL-15, two other common γ-chain cytokines often used in cancer immunotherapy, can also activate STAT5. However, we found that IL-15 was incapable of, and IL-2 was not as efficient as IL-7 in inducing polyfunctionality in activated CD4+ T cells (data not shown). Our focus on IL-7-driven CD4+ T-cell polyfunctionality has direct clinical implications because several lines of evidence indicate that IL-7 is superior to IL-2 in suppressing Treg expansion, retaining T-cell viability and effector functions during ex vivo expansion of tumor-specific T cells used for adoptive cellular therapy.Citation43,44 Indeed, in a number of ACT clinical trials IL-7 was included in the cytokine cocktail used to expand donor T cells ex vivo.Citation32 Our study provides new evidence that IL-7 signaling may lead to inhibition of Foxp3 expression through epigenetic gene regulation (), suggesting that the use IL-7 rather than IL-2 in culturing T cells for adoptive immunotherapy has additional advantage of generating fewer Foxp3+ T cells.
Even though most studies regard IL-7 as a T-cell growth factor, an early study by Murphy et al suggested that rhIL-7 can potentiate the efficacy of adoptive immunotherapy by enhancing T-cell functionality.Citation45 We show that in two different tumor models, one for B-cell lymphoma and one for colon cancer, adoptive transfer of IL-7-conditioned polyfunctional CD4+ T cells following lymphodepletive chemotherapy resulted in impressive antitumor effects. It is worth noting that unlike A20 tumor cells, CT26 tumor cells do not express MHC-II molecules,Citation46 and cannot be induced to express MHC-II molecules by IFNγ,Citation18 thus they are unable to directly interact with CD4+ T cells via antigen recognition. Polyfunctional CD4+ T cells may directly attack A20HA tumor cells and mediate tumor destruction through perforin and granzyme B. For CT26HA tumors, polyfunctional CD4+ T cells may drive tumor senescence through IFNγ and TNFα,Citation47 or target tumor stroma by eliciting CD8+ T-cell cytotoxicity.Citation5 Even though polyfunctional CD4+ T cells may be versatile in mediating tumor rejections, in both models the persisting donor CD4+ T cells contained subpopulations exhibiting attributes of Tem, Tcm and Tscm cells. CD8+ Tscm cells have been well characterized.Citation48,49 A recent study reported that IL-7 promotes the induction and IL-15 facilitates the expansion of human CD8+ Tscm cells from naïve precursors upon antigenic stimulation.Citation50 CD4+ T cells with stem cell-like properties have also been found in mice and humans.Citation41 It has been shown that Th17 cells are long-lived cells in vivo and exhibit stem cell-like features.Citation51,52 Interestingly, we showed that IL-7-conditioned CD4+ T cells barely expressed IL-17A (Fig. S1B). Instead, our study indicates for the first time that IL-7-induced Th1-type polyfunctional CD4+ T cells have the potential to form memory cells with stem cell attributes in vivo. It is possible that Tscm cells continuously self-renew and give rise to Tem and Tcm cells, which in turn mediate effective and durable immunosurveillance.
It should be noted that in our study, chemotherapy pre-conditioning is a prerequisite to allow the transferred polyfunctional CD4+ T cells to be effective in rejecting large tumors. It is known that preparative chemotherapy can transiently reset the immunosuppressive tumor microenvironment by reducing Tregs and MDSCs, and making “space” for donor cells to expand.Citation53,54 Chemotherapy can also temporarily increase the bioavailability of endogenous IL-7 by reducing its consumption by host cells.Citation33 Intriguingly, our previous work using the same B-cell lymphoma model showed that CTX chemotherapy induces monocytic MDSCs which act to tolerize polyfunctional CD4+ T cells through a PD-1-dependent mechanism.Citation55 The results from the current study suggest that rhIL-7 may overcome this immunosuppressive mechanism by amplifying polyfunctional CD4+ T cells that outnumber MDSCs, and/or by rendering polyfunctional CD4+ T cells resistant to suppression. Along this line, a recent study reported that rhIL-7 administration not only expanded IL-7Rα-overexpressing, GD2-specific chimeric antigen receptor (CAR) T cells in vivo, but also made these cells refractory to Treg-mediated suppression.Citation56 Given the recent advances in T-cell engineering technology, generating synthetic, antigen-specific T cells for adoptive immunotherapy has become a reality.Citation32 Our study using TCR-Tg CD4+ T cells may have clinical relevance to ACT using T cells with genetically engineered Ag-specific TCRs or CARs.
In summary, in this study we show that beyond its utility as a T-cell growth factor, IL-7 can be used to generate polyfunctional CD4+ T cells. We demonstrate that adoptive cell therapy using IL7-conditioned polyfunctional CD4+ T cells led to tumor eradication, antigen epitope spreading and persistence of Tscm. These findings reveal a previously unappreciated role of IL-7 in regulating CD4+ T cell function, and support the use of recombinant IL-7 in combination with antitumor CD4+ T cells in the setting of ACT.
Materials and methods
Mice
BALB/c mice (Thy1.2+/+) of four to six weeks of age were purchased from Charles River. 6.5 TCR-Tg mice on a BALB/c (Thy1.1+/+) background expressing an αβTCR specific for amino acids 110–120 from influenza HA presented by MHC class II molecule IEd were described previously.Citation17 DO11.10 mice and OT-II mice were purchased from the Jackson Laboratory. All mice were housed under specific pathogen-free (SPF) conditions by Laboratory Animal Services of the Augusta University. All animal experiments were approved by the Institutional Animal Care and Use Committee of Augusta University.
Antibodies and reagents
The following fluorochrome-conjugated antibodies were used for flow cytometry: anti-mouse PD1-PE (RMP1-30), CD4+-FITC (GK1.5), CD4+-APC/Cy7 (RM4-5), CD45.2-APC (104), CD44-FITC (IM7), CD62L-PE (MEL-14), Sca-1-PE/Cy7 (D7), CCR7-APC (4B12), IFNγ-APC (XMG1.2), IFNγ-FITC (XMG1.2), TNFα-PE (MP6-XT22), TNFα-PE/Cy7 (MP6-XT22), IL2-PE (JES6-5H4), and control IgG mAbs were purchased from Biolegend. Granzyme B-Alexa Fluor 647 (GB11), IL17A-PE (TC11-18H10), EZH2-APC (clone 11) and Thy1.1-perCP (OX-7) were purchased from BD. Foxp3-APC staining kit was purchased from eBiosciences. Violet dye was purchased from Invitrogen. Recombinant human interleukin 7 (rhIL-7, CYT107) was provided by Cytheris. STAT5i (CAS 285986-31-4) and TSA were purchased from EMD Millipore Corporation. CTX was purchased from Tokyo Chemical Industry (TCI).
In vitro CD4+ T cells stimulation and intracellular cytokine staining (ICS)
Spleen cells from 6.5 TCR-Tg mice were labeled with 1 μM violet dye and stimulated with 1 μg/mL HA peptide, in the absence or presence of 100 ng/mL rhIL-7, in a round-bottom-96-well plate (1.5 × 105 cells/well in 200 uL medium). 7 d after culture, cells were harvested for FACS analysis. For ICS, cells from in vitro culture were stimulated with Leukocyte Activation Cocktail containing PMA, ionomycin and GolgiPlug (BD Biosciences) for 4 h at 37°C. Cells were harvested and stained for surface molecules, followed by intercellular cytokine staining according to manufacturer's instruction. Flow cytometry data were acquired on a LSRII (BD Biosciences) and analyzed with Flowjo software (Treestar inc.) or BD FACSDiva software (BD Biosciences).
Chromatin immunoprecipitation assay (ChIP)
Cultured splenocytes were stained with CD4+-FITC and subjected to cell sorting using a FACSAaria (BD Biosciences). The purity of sorted CD4+ T cells was normally greater than 98%. ChIP analysis was performed using the acetyl-Histone H3 Immunoprecipitation Assay kit (Millipore) according to the manufacturer's instruction with slight modifications. Briefly, purified CD4+ T cells were crosslinked with 0.5% paraformaldehyde for 10 min at room temperature and quenched with glycine (final concentration 125 mM) for 5 min. After washing twice with cold PBS containing protease inhibitor, cells were lysed in ChIP lysis buffer on ice for 10 min. Cell lysates were then sonicated on ice to shear the genomic DNA into 200–500 bp fragments using a Misonix S3000 sonicator. Sonicated samples were diluted in dilution buffer, pre-cleared with protein A agarose beads and immunoprecipitated with α-acetylated histone H3 at 4°C overnight. Protein A agarose beads were added and incubated at 4°C for 60 min. After washes, the DNA-protein complexes were eluted with elution buffer (1% SDS and 0.1 M NaHCO3). The DNA-protein crosslinks were reversed, followed by protein digestion and DNA extraction with Qiagen PCR-purification kit. Immunoprecipitated DNA was subjected to quantitative real-time PCR using SYBR Green Master Mixture (Biorad), and normalized to input DNA.
CD4+ T cell retroviral transduction
The RVs encoding CA-STAT5 or DN-STAT5 with Thy1.1 as a marker were provided by Dr Susan Kaech (Yale University).Citation57,58 RV MSCV-luciferase-IRES-Thy1.1 was a gift from Dr Hyam Levitsky (Johns Hopkins University). 293 T cells were transfected with retroviral construct and pCL-Eco using Lipofectamine 2000 (Invitrogen) according to manufacturer's instruction. Splenocytes from 6.5 or DO11.10 TCR-Tg mice were stimulated with 1 μg/mL HA peptide (6.5 splenocytes) or 10 ng/mL OVA peptide (DO11.10 splenocytes) for 24 h. Cells were harvested and spin infected with retrovirus at 2,500 rpm for 120 min at 24°C in the presence of 8 μg/mL polybrene. 12 h later, cells were collected, and mixed with equal number of fresh splenocytes from a naïve mouse as APCs. The cognate peptide was added to culture with or without 100 ng/mL rhIL-7. Cells were subsequently cultured for an additional 4 d and subjected for FACS analysis.
Bioluminescent imaging (BLI)
BLI was performed on a Spectral Advanced Molecular Imaging X (Ami X) system (Spectral Instruments Imaging). Each mouse received an intraperitoneal injection of 150 mg/kg luciferin and anesthetized by inhalation of 2% isoflurane. Mice were then placed into the camera chamber, where a controlled flow of 2% isofluane was administered via a nose cone. The photographic images were acquired and overlaid with pseudocolor luminescent images. All BLI data were analyzed with AMI View (Spectral Instruments Imaging) software. The luminescence was quantified as photon/sec as an indicator of tumor burden.
Tumor cells and animal tumor models
WT A20 (A20WT) B-cell lymphoma cell line was purchased from ATCC. HA-expressing A20 tumor cell line (A20HA) and HA-expressing colorectal tumor cell line CT26HA were generated and maintained as described previously.Citation59 A20HA-luciferase cells (A20HA-luci) were generated by electroporating A20HA cells with a luciferase-encoding plasmid. A20HA-luci tumor cells were subcutaneously injected to the right flank of BALB/c mice (5 × 105 per mouse). 3 d after tumor inoculation, a total of approximately of 4 × 106 in vitro cultured 6.5 TCR-Tg CD4+ T cells were adoptively transferred into each recipient. For immunization, mice were i.p. injected with 107 plaque-forming units (pfu) of recombinant vaccinia virus encoding HA (vacHA) in 100 μL Hanks buffer. The growth of subcutaneous tumors was monitored by caliper measurement of the tumor area twice a week, and expressed as the product of two perpendicular diameters in square millimeters. For large established tumor models, A20HA or CT26HA cells were subcutaneously injected to the right flank of BALB/c mice. When tumor sizes reach 120–170 mm2, mice were i.p. injected with 150 mg/kg CTX. The next day, 2.5–4.0 × 106 in vitro cultured HA-specific CD4+ T cells were injected to mice via tail vein.
Statistical analysis
Data were analyzed using Prism 4.0 (GraphPad Software, Inc.). The statistical significance of the results was determined using the Student's t test. Differences in tumor sizes among different treatment groups were analyzed using the Mann-Whitney U test. P values less than 0.05 were considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
Z-C.D. and C.F.L. performed research, analyzed results and wrote the paper; C.Y., T.H., M.K., W.P., H.K. and E.C. performed research; S.F.G., Y.C., B.R.B. and D.H.M. provided critical reagents and edited the paper; G.Z. designed and performed research, analyzed results and wrote the paper.
KONI_A_1171445_s02.pdf
Download PDF (329.5 KB)Funding
This work is funded by National Institutes of Health grant R01CA158202 and the American Cancer Society Research Scholar Grant (RSG-12-169-01-LIB) to G.Z. NIH R01 CA72669 to B.R.B.
References
- Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med 1998; 188:2357-68; PMID:9858522; http://dx.doi.org/10.1084/jem.188.12.2357
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002; 298:850-4; PMID:12242449; http://dx.doi.org/10.1126/science.1076514
- Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW, Scott B. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. J Immunol 2000; 165:6047-55; PMID:11086036; http://dx.doi.org/10.4049/jimmunol.165.11.6047
- Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med 1998; 187:693-702; PMID:9480979; http://dx.doi.org/10.1084/jem.187.5.693
- Schietinger A, Philip M, Liu RB, Schreiber K, Schreiber H. Bystander killing of cancer requires the cooperation of CD4(+) and CD8(+) T cells during the effector phase. J Exp Med 2010; 207:2469-77; PMID:20921286; http://dx.doi.org/10.1084/jem.20092450
- Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood 2007; 109:5346-54; PMID:17327412; http://dx.doi.org/10.1182/blood-2006-10-051318
- Corthay A, Skovseth DK, Lundin KU, Rosjo E, Omholt H, Hofgaard PO, Haraldsen G, Bogen B. Primary antitumor immune response mediated by CD4+ T cells. Immunity 2005; 22:371-83; PMID:15780993; http://dx.doi.org/10.1016/j.immuni.2005.02.003
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 2010; 207:637-50; PMID:20156971; http://dx.doi.org/10.1084/jem.20091918
- Qui HZ, Hagymasi AT, Bandyopadhyay S, St Rose MC, Ramanarasimhaiah R, Menoret A, Mittler RS, Gordon SM, Reiner SL, Vella AT et al. CD134 plus CD137 dual costimulation induces Eomesodermin in CD4 T cells to program cytotoxic Th1 differentiation. J Immunol 2011; 187:3555-64; PMID:21880986; http://dx.doi.org/10.4049/jimmunol.1101244
- Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med 2010; 207:651-67; PMID:20156973; http://dx.doi.org/10.1084/jem.20091921
- Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med 2008; 358:2698-703; PMID:18565862; http://dx.doi.org/10.1056/NEJMoa0800251
- Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014; 344:641-5; PMID:24812403; http://dx.doi.org/10.1126/science.1251102
- Casazza JP, Betts MR, Price DA, Precopio ML, Ruff LE, Brenchley JM, Hill BJ, Roederer M, Douek DC, Koup RA. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med 2006; 203:2865-77; PMID:17158960; http://dx.doi.org/10.1084/jem.20052246
- Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 2007; 13:843-50; PMID:17558415; http://dx.doi.org/10.1038/nm1592
- Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med 2007; 204:1405-16; PMID:17535971; http://dx.doi.org/10.1084/jem.20062363
- Brown DM, Lee S, Garcia-Hernandez Mde L, Swain SL. Multifunctional CD4 cells expressing gamma interferon and perforin mediate protection against lethal influenza virus infection. J Virol 2012; 86:6792-803; PMID:22491469; http://dx.doi.org/10.1128/JVI.07172-11
- Ding ZC, Blazar BR, Mellor AL, Munn DH, Zhou G. Chemotherapy rescues tumor-driven aberrant CD4+ T-cell differentiation and restores an activated polyfunctional helper phenotype. Blood 2010; 115:2397-406; PMID:20118405; http://dx.doi.org/10.1182/blood-2009-11-253336
- Ding ZC, Huang L, Blazar BR, Yagita H, Mellor AL, Munn DH, Zhou G. Polyfunctional CD4(+) T cells are essential for eradicating advanced B-cell lymphoma after chemotherapy. Blood 2012; 120:2229-39; PMID:22859605; http://dx.doi.org/10.1182/blood-2011-12-398321
- Hirschhorn-Cymerman D, Budhu S, Kitano S, Liu C, Zhao F, Zhong H, Lesokhin AM, Avogadri-Connors F, Yuan J, Li Y et al. Induction of tumoricidal function in CD4+ T cells is associated with concomitant memory and terminally differentiated phenotype. J Exp Med 2012; 209:2113-26; PMID:23008334; http://dx.doi.org/10.1084/jem.20120532
- Ayyoub M, Dojcinovic D, Pignon P, Raimbaud I, Schmidt J, Luescher I, Valmori D. Monitoring of NY-ESO-1 specific CD4+ T cells using molecularly defined MHC class II/His-tag-peptide tetramers. Proc Natl Acad Sci USA 2010; 107:7437-42; PMID:20368442; http://dx.doi.org/10.1073/pnas.1001322107
- Lin Y, Gallardo HF, Ku GY, Li H, Manukian G, Rasalan TS, Xu Y, Terzulli SL, Old LJ, Allison JP et al. Optimization and validation of a robust human T-cell culture method for monitoring phenotypic and polyfunctional antigen-specific CD4 and CD8 T-cell responses. Cytotherapy 2009; 11:912-22; PMID:19903103; http://dx.doi.org/10.3109/14653240903136987
- Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, Ku GY, Jungbluth AA, Segal NH, Rasalan TS et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci USA 2008; 105:20410-5; PMID:19074257; http://dx.doi.org/10.1073/pnas.0810114105
- Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol 2005; 174:6571-6; PMID:15905493; http://dx.doi.org/10.4049/jimmunol.174.11.6571
- Bradley LM, Haynes L, Swain SL. IL-7: maintaining T-cell memory and achieving homeostasis. Trends Immunol 2005; 26:172-6; PMID:15745860; http://dx.doi.org/10.1016/j.it.2005.01.004
- Hock H, Dorsch M, Diamantstein T, Blankenstein T. Interleukin 7 induces CD4+ T cell-dependent tumor rejection. J Exp Med 1991; 174:1291-8; PMID:1683890; http://dx.doi.org/10.1084/jem.174.6.1291
- Aoki T, Tashiro K, Miyatake S, Kinashi T, Nakano T, Oda Y, Kikuchi H, Honjo T. Expression of murine interleukin 7 in a murine glioma cell line results in reduced tumorigenicity in vivo. Proc Natl Acad Sci USA 1992; 89:3850-4; PMID:1570303; http://dx.doi.org/10.1073/pnas.89.9.3850
- Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, Stetler-Stevenson M, Morton KE, Mavroukakis SA, Morre M et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother 2006; 29:313-9; PMID:16699374; http://dx.doi.org/10.1097/01.cji.0000210386.55951.c2
- Sportes C, Babb RR, Krumlauf MC, Hakim FT, Steinberg SM, Chow CK, Brown MR, Fleisher TA, Noel P, Maric I et al. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin Cancer Res 2010; 16:727-35; PMID:20068111; http://dx.doi.org/10.1158/1078-0432.CCR-09-1303
- Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med 2008; 205:1701-14; PMID:18573906; http://dx.doi.org/10.1084/jem.20071681
- Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev 2008; 222:357-68; PMID:18364014; http://dx.doi.org/10.1111/j.1600-065X.2008.00604.x
- Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol 2011; 11:330-42; PMID:21508983; http://dx.doi.org/10.1038/nri2970
- Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity 2013; 39:49-60; PMID:23890063; http://dx.doi.org/10.1016/j.immuni.2013.07.002
- Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol 2005; 26:111-7; PMID:15668127; http://dx.doi.org/10.1016/j.it.2004.12.003
- Zhao E, Maj T, Kryczek I, Li W, Wu K, Zhao L, Wei S, Crespo J, Wan S, Vatan L et al. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nat Immunol 2016; 17:95-103; PMID:26523864; http://dx.doi.org/10.1038/ni.3313
- van Panhuys N, Klauschen F, Germain RN. T-cell-receptor-dependent signal intensity dominantly controls CD4(+) T cell polarization in vivo. Immunity 2014; 41:63-74; PMID:24981853; http://dx.doi.org/10.1016/j.immuni.2014.06.003
- Chiu YL, Shan L, Huang H, Haupt C, Bessell C, Canaday DH, Zhang H, Ho YC, Powell JD, Oelke M et al. Sprouty-2 regulates HIV-specific T cell polyfunctionality. J Clin Invest 2014; 124:198-208; PMID:24292711; http://dx.doi.org/10.1172/JCI70510
- Schlissel MS, Durum SD, Muegge K. The interleukin 7 receptor is required for T cell receptor gamma locus accessibility to the V(D)J recombinase. J Exp Med 2000; 191:1045-50; PMID:10727465; http://dx.doi.org/10.1084/jem.191.6.1045
- Stanton ML, Brodeur PH. Stat5 mediates the IL-7-induced accessibility of a representative D-Distal VH gene. J Immunol 2005; 174:3164-8; PMID:15749844; http://dx.doi.org/10.4049/jimmunol.174.6.3164
- Ye SK, Agata Y, Lee HC, Kurooka H, Kitamura T, Shimizu A, Honjo T, Ikuta K. The IL-7 receptor controls the accessibility of the TCRgamma locus by Stat5 and histone acetylation. Immunity 2001; 15:813-23; PMID:11728342; http://dx.doi.org/10.1016/S1074-7613(01)00230-8
- Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol 2009; 9:480-90; PMID:19543225; http://dx.doi.org/10.1038/nri2580
- Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer 2012; 12:671-84; PMID:22996603; http://dx.doi.org/10.1038/nrc3322
- Boyd A, Almeida JR, Darrah PA, Sauce D, Seder RA, Appay V, Gorochov G, Larsen M. Pathogen-specific T cell polyfunctionality is a correlate of T cell efficacy and immune protection. PLoS One 2015; 10:e0128714; PMID:26046523; http://dx.doi.org/10.1371/journal.pone.0128714
- Cha E, Graham L, Manjili MH, Bear HD. IL-7 + IL-15 are superior to IL-2 for the ex vivo expansion of 4T1 mammary carcinoma-specific T cells with greater efficacy against tumors in vivo. Breast Cancer Res Treat 2010; 122:359-69; PMID:19826947; http://dx.doi.org/10.1007/s10549-009-0573-0
- Caserta S, Alessi P, Basso V, Mondino A. IL-7 is superior to IL-2 for ex vivo expansion of tumour-specific CD4(+) T cells. Eur J Immunol 2010; 40:470-9; PMID:19950184; http://dx.doi.org/10.1002/eji.200939801
- Murphy WJ, Back TC, Conlon KC, Komschlies KL, Ortaldo JR, Sayers TJ, Wiltrout RH, Longo DL. Antitumor effects of interleukin-7 and adoptive immunotherapy on human colon carcinoma xenografts. J Clin Invest 1993; 92:1918-24; PMID:8408644; http://dx.doi.org/10.1172/JCI116785
- Castle JC, Loewer M, Boegel S, de Graaf J, Bender C, Tadmor AD, Boisguerin V, Bukur T, Sorn P, Paret C et al. Immunomic, genomic and transcriptomic characterization of CT26 colorectal carcinoma. BMC Genomics 2014; 15:190; PMID:24621249; http://dx.doi.org/10.1186/1471-2164-15-190
- Braumuller H, Wieder T, Brenner E, Assmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013; 494:361-5; PMID:23376950; http://dx.doi.org/10.1038/nature11824
- Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med 2005; 11:1299-305; PMID:16288282; http://dx.doi.org/10.1038/nm1326
- Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C et al. A human memory T cell subset with stem cell-like properties. Nat Med 2011; 17:1290-7; PMID:21926977; http://dx.doi.org/10.1038/nm.2446
- Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, Bondanza A, Bordignon C, Peccatori J, Ciceri F et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 2013; 121:573-84; PMID:23160470; http://dx.doi.org/10.1182/blood-2012-05-431718
- Wei S, Zhao E, Kryczek I, Zou W. Th17 cells have stem cell-like features and promote long-term immunity. Oncoimmunology 2012; 1:516-9; PMID:22754771; http://dx.doi.org/10.4161/onci.19440
- Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 2011; 35:972-85; PMID:22177921; http://dx.doi.org/10.1016/j.immuni.2011.09.019
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol 2008; 8:59-73; PMID:18097448; http://dx.doi.org/10.1038/nri2216
- Ding ZC, Zhou G. Cytotoxic chemotherapy and CD4+ effector T cells: an emerging alliance for durable antitumor effects. Clin Dev Immunol 2012; 2012:890178; PMID:22400040; http://dx.doi.org/10.1155/2012/890178
- Ding ZC, Lu X, Yu M, Lemos H, Huang L, Chandler P, Liu K, Walters M, Krasinski A, Mack M et al. Immunosuppressive myeloid cells induced by chemotherapy attenuate antitumor CD4+ T-cell responses through the PD-1-PD-L1 axis. Cancer Res 2014; 74:3441-53; PMID:24780756; http://dx.doi.org/10.1158/0008-5472.CAN-13-3596
- Perna SK, Pagliara D, Mahendravada A, Liu H, Brenner MK, Savoldo B, Dotti G. Interleukin-7 mediates selective expansion of tumor-redirected cytotoxic T lymphocytes (CTLs) without enhancement of regulatory T-cell inhibition. Clin Cancer Res 2014; 20:131-9; PMID:24097874; http://dx.doi.org/10.1158/1078-0432.CCR-13-1016
- Hand TW, Cui W, Jung YW, Sefik E, Joshi NS, Chandele A, Liu Y, Kaech SM. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc Natl Acad Sci USA 2010; 107:16601-6; PMID:20823247; http://dx.doi.org/10.1073/pnas.1003457107
- Onishi M, Nosaka T, Misawa K, Mui AL, Gorman D, McMahon M, Miyajima A, Kitamura T. Identification and characterization of a constitutively active STAT5 mutant that promotes cell proliferation. Mol Cell Biol 1998; 18:3871-9; PMID:9632771; http://dx.doi.org/10.1128/MCB.18.7.3871
- Lu X, Ding Z, Cao Y, Liu C, Habtetsion T, Yu M, Lemos H, Salman H, Xu H, Mellor AL et al. Alkylating agent melphalan augments the efficacy of adoptive immunotherapy using tumor-specific CD4+ T cells. J Immunol 2015; 194(4):2011-21
