ABSTRACT
Brain metastases represent the most common type of brain tumor. These tumors offer a dismal prognosis and significantly impact quality of life for patients. Their capacity for central nervous system (CNS) invasion is dependent upon induced disruptions to the blood–brain barrier (BBB), alterations to the brain microenvironment, and mechanisms for escaping CNS immunosurveillance. In the emerging era of immunotherapy, understanding how metastases are influenced by the immunologic peculiarities of the CNS will be crucial to forging therapeutic advances. In this review, the immunology of brain metastasis is explored.
Abbreviations
| BBB | = | blood–brain barrier |
| CLNs | = | cervical lymph nodes |
| CNS | = | central nervous system |
| COX-2 | = | cyclooxygenase-2 |
| CSF | = | cerebrospinal fluid |
| CTLA-4 | = | cytotoxic T lymphocyte antigen-4 |
| GAMs | = | glioma-associated macrophages/microglia |
| GBM | = | glioblastoma multiforme |
| iNOS | = | inducible nitric oxide synthase |
| LPS | = | lipopolysaccharide |
| MMP-1 | = | matrix metalloproteinase-1 |
| OS | = | overall survival |
| P-gp | = | P-glycoprotein |
| PD-1 | = | programmed cell death-1 |
| PFS | = | progression-free survival |
| TAMs | = | tumor-associated macrophages |
| TCRs | = | T-cell receptors |
| TILs | = | tumor-infiltrating lymphocytes |
| TNF-α | = | tumor necrosis factor α |
| Tregs | = | regulatory T cells |
| VEGF | = | vascular endothelial growth factor |
Introduction
As the complex relationship between cancer ontogeny and the human immune system becomes better understood, interest has increased in elucidating the immunologic interactions between tumor cells and both their microenvironment as well as the systemic immune system. As a tumor confine, the brain represents a special case, both with regard to microenvironmental peculiarities and to immune trafficking. Regarding the latter, despite the long-held belief that the blood–brain barrier (BBB) confers “immune privilege”, a number of groups have demonstrated robust trafficking of immune components through tumors' brain microenvironment, as well as tumor-driven changes in systemic immune function.Citation1 Our understanding of tumor mechanisms of immunosuppression and immune evasion deepens as the roles of the cells and various factors that make up the tumor microenvironment are better characterized. Ultimately, this increased understanding may aid in the era of immunotherapy to shift the balance from tumor escape to tumor destruction.
To date, knowledge of immune trafficking across the BBB comes from investigations of primary brain tumors. However, metastases from non-CNS primary tumors are the most common brain tumor type, nearly 10 times more common than malignant gliomas.Citation2-4 Each year, approximately 150,000 people are diagnosed with brain metastases in the United States.Citation5 It is estimated that 10–30% of all patients with solid tumors develop metastatic CNS disease; due to an aging population and improvements to cancer screening and therapeutic efficacy, it is anticipated that the incidence of brain metastasis will increase. Brain metastases most commonly originate from three primary solid malignancies: lung, breast, and melanoma.Citation3 Regardless of the site of origin, brain metastases are responsible for significant morbidity and quality-of-life impairment; common symptoms include headaches, seizures, focal neurologic deficits (such as weakness or numbness), cognitive changes, and gait disturbances.Citation2,4 The current repertoire of therapy leave patients with a dismal prognosis, with survival typically estimated to be on the order of months.Citation3 Moreover, these therapies cause significant side effects that greatly affect quality of life for patients.Citation6,7 Immunotherapy offers the potential to target tumor cells while avoiding collateral damage to adjacent normal tissue,Citation8 with the additional advantage of built-in surveillance and memory in the event of recurrence. As immunotherapy for a variety of systemic tumors gains momentum and legitimacy,Citation9 there is increased interest in defining and modulating the unique immune environment within the CNS. Consequently, a deeper knowledge of BBB modulation and immune trafficking is needed in order to develop rational immunotherapies for CNS metastases. Herein, we review the current understanding of the immunology of metastasis, as well as current trials of immunotherapy to date.
The blood–brain barrier
The notion of the CNS as an immune-privileged compartment can be traced to Peter Medawar's 1948 study demonstrating the absence of rejection of allogeneic tissue grafted into brains of experimental animals.Citation10 This view has since been revised and the CNS is now known to be “immunologically distinct” rather than isolated.Citation1 This distinction is thought to arise at least in part from the BBB, which isolates blood in the CNS vasculature from the extracellular fluid of the brain.Citation11 Under physiologic circumstances, the BBB is considered a neurovascular unit, consisting of a layer of specialized endothelial cells connected by tight junctions, and maintained by a peripheral cellular network of astrocytes and pericytes.Citation12,13 In contrast to the peripheral vasculature, brain endothelial cells have continuous tight junctions without fenestrations. Though small (< 400–500 Da) and lipophilic molecules cross the BBB via passive diffusion, the integrity of the epithelium restricts most movement to large molecules.Citation14 Active transport mechanisms including receptor-mediated transcytosis of large molecules, efflux pumps, and multi-drug-resistance proteins further regulate transit across the BBB.Citation12,15 This “protective” effect of the BBB can at times preclude or limit the in vivo study of early brain pathology, as contrast agents used in imaging and many drugs are prevented from entering the brain parenchyma.Citation11 However, BBB permeability is increased in certain settings such as systemic inflammation, infection, cancer, and radiotherapy.Citation16-19
The neovasculature within metastatic brain tumors is often deemed more permeable than normal CNS vasculature, thus creating a compromised blood-tumor barrier (BTB).Citation40 Recent work has shown varied degrees of BTB permeability across multiple models of breast cancer brain metastases lacking any correlation with lesion size.Citation41 While this increased permeability has been demonstrated previously, the BTB appears to remain sufficiently intact to bar passage of chemotherapeutic agents. Indeed, Lockman et. al. have shown in two models of breast cancer brain metastasis that uptake of cytotoxic chemotherapeutic agents remained greatly reduced, and in only 10% of lesions did these drugs reach cytotoxic levels.Citation42 Therefore, the extent of BTB disruption and resultant therapeutic implications within brain metastases remain active areas of investigation.
Though the brain appears to offer immunologically distinct substrate,Citation20 both physiologic and pathologic immune trafficking across the BBB and BTB occurs. This has been particularly well-described for T-lymphocytes, which are implicated in modulating immunosurveillance within the CNS.Citation21 Antitumor immune responses begin with the presentation of CNS antigens in draining cervical lymph nodes (CLNs); antigens leave the cerebrospinal fluid (CSF) via the olfactory nerve, cross the cribriform plate to enter the nasal mucosa, and ultimately reach the CLNs.Citation22,23 Recently, functional lymphatic vessels were discovered lining the dural sinuses that offer a newly defined mechanism of antigen and cellular egress from the CNS. These vessels express the characteristic molecular markers of lymphatic endothelial cells and can carry fluid, macromolecules, and immune cells from the CSF to the deep CLNs.Citation24,25
Tumor seeding and progression
The general phases of the metastatic cascade include tumor cell departure from the primary site, travel to the CNS, infiltration across the BBB, and alteration of the CNS microenvironment to allow for evasion of immune defenses. The exact mechanism of tumor seeding within the CNS is poorly understood and limited correlation between in vitro and in vivo analyses make such mechanisms difficult to study.Citation26,27 Although the predilection of metastatic foci for the gray-white junction is well-described,Citation28 few therapeutically relevant mechanistic insights are currently had. Sites of seeding are thought to be primarily mechanical: non-laminar flow at vessel branch points coupled with cellular size exceeding vessel capacity may primarily determine locations for metastatic foci.Citation29-32 It is unknown, however, whether BBB disruption at these loci precedes metastatic spread (or is even a necessary prerequisite) or whether all changes observed in the BBB at metastatic loci are secondary to tumor formation.
Two separate animal models of brain metastasis have shown that the BBB remains intact throughout the early phase of tumor evolution, suggesting that BBB damage occurs secondary to later stage metastatic proliferation.Citation33 Assays of vessel permeability in a murine xenograft model have demonstrated supraphysiologic permeability only in larger metastatic tumors.Citation34 Additionally, in a murine metastatic lung cancer model, BBB integrity was not altered until later stages of development, despite the retained ability of the BBB to functionally exclude solute and drug via expression of the efflux transporter P-glycoprotein (P-gp).Citation35 Moreover, although extravasation of single cells appears to largely determine brain metastasis formation, intravascular tumor cell proliferation can also occur, secondarily disrupting vessels and the BBB.Citation36 Indeed, in an array of animal models, viable cells have been found within the lumen of intracerebral vasculature 3–5 d following intra-carotid injection.Citation11,26,37-39
The reduced shear force at branch points in the vasculature allows metastatic tumor cells to attach to the endothelium through various integrins, selectins, and chemokines.Citation29-32 Following diapedesis, metastatic tumor cells remain in close contact with brain endothelial cells and rely heavily on them for their proliferation.Citation20 Previous observations suggest that transmigrated metastatic cells only proliferate when located on the basolateral surface of brain endothelial cells.Citation43 Furthermore, breast cancer and melanoma cells have been shown to align along the extraluminal surface of brain vasculature Citation36 and endothelium,Citation44 respectively. The vascular basement membrane supports the growth of metastatic cells prior to tumor vasculature formation, a process termed vessel cooption.Citation45
A complex cascade of factors mediating vascular remodeling follows endothelial binding and tumor seeding. Among the cascade is vascular endothelial growth factor (VEGF), which has been extensively investigated and contributes significantly to tumor extravasation. VEGF presence instigates exposure of the vascular basement membrane via disturbance of tight and adherens junctions,Citation46 and mediates parasitization of peritumoral vasculature.Citation43,47-49
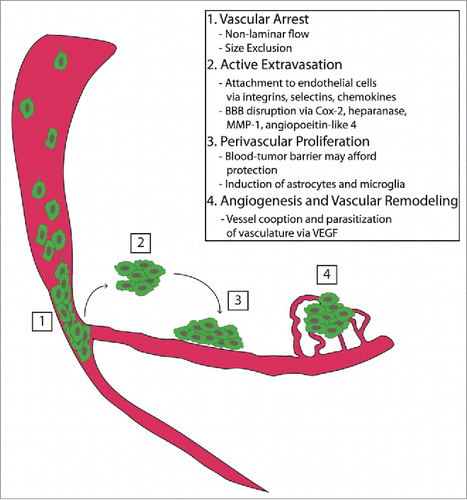
In summary, the four critical steps required for successful metastasis formation include vascular arrest by size exclusion, active extravasation, rigid perivascular position, and growth via angiogenesis and vascular remodelingCitation43 (). Each of these steps represents a potential therapeutic target. For example, the use of anti-angiogenic agents is under pre-clinical investigation in a variety of tumors. Of note, treatment with VEGF blockade in a melanoma metastasis model inhibited angiogenesis while maintaining physiologic BBB permeability.Citation50 Another study compared brain metastasis-selected variants of a breast cancer cell line to the parental cell line, and showed that the metastatic variants had higher levels of VEGF-A expression and contained more CD31+ blood vessels. Furthermore, mice treated with VEGF inhibition had reduced angiogenesis and restricted growth of the brain metastases.Citation51 The effects of these changes on immune trafficking to sites of metastases are unclear, though the increased clinical use of therapeutic VEGF blockade (as with bevacizumab) will prompt further investigation. Though small, a pilot phase II study (NCT01281696) examining the effect of bevacizumab combined with etoposide and cisplatin for patients with breast cancer CNS metastases exhibited promising efficacy with a 60% response rate.Citation52 This same treatment regimen followed by whole brain radiotherapy will be evaluated in a randomized phase II study that is currently enrolling patients (NCT02185352). Another ongoing Phase II study is evaluating the combination of bevacizumab with carboplatin (NCT01004172).
Figure 1. Tumor seeding and progression. The critical steps required for successful metastasis formation within the brain include (1) vascular arrest by size exclusion, (2) active extravasation of metastatic tumor cells, (3) stringent perivascular location and proliferation, and (4) sustained growth via angiogenesis and vascular remodeling.
Tumor microenvironment and immune evasion
Though study of the tumor microenvironment has frequently focused on the populations and phenotypes of tumor-infiltrating lymphocytes (TILs) and monocytes,Citation53 the cytoarchitecture surrounding these cells has also undergone considerable scrutiny. Analysis of intracranial melanoma and breast cancer metastases has identified upregulated microenvironmental expression by the metastatic tumor cells of cyclooxygenase 2 (Cox-2), matrix metalloproteinase-1 (MMP-1), heparanase, and angiopoeitin-like 4,Citation54-56 which further degrade subjacent BBB.Citation54,55 This altered expression may facilitate the development of adjoining micrometastases, which can persist even when the primary metastatic site has been eliminated.Citation28 The dysregulated expression of these mediators in metastatic melanoma cells seems to be driven by expression of activated STAT3, which has been shown to increase angiogenesis and invasion.Citation49 Many other adhesion molecules, soluble factors, proteolytic enzymes, and signaling pathways have been implicated in the interaction between metastatic tumor cells and endothelial cells of the BBB and are reviewed in Wilhelm et. al.Citation20
Metastatic tumor cells also subvert the glial cells in their microenvironment to their advantage. Astrocytes are among the first glial cells encountered by metastasizing cells, and some evidence suggests that they aid in CNS invasion by tumor cells.Citation57,58 Under the influence of the neurotrophin, nerve growth factor, melanoma-adjacent astrocytes contribute to brain colonization of melanoma cells via expression of heparanase.Citation57 Moreover, metastatic brain tumors are highly resistant to chemotherapy, and past work has shown that these tumors can harness the neuroprotective effects of activated astrocytes for chemotherapeutic resistance and survival.Citation58 However, astrocytes may play a dual role in tumor propagation, as astrocyte-secreted plasminogen appears conversely to induce apoptosis in metastatic cells.Citation59 Thus, the ultimate contribution of this glial cell population to tumor progression remains complex and controversial.
Subversion of endogenous immune responses, long thought to be a hallmark of primary gliomas, is also a characteristic of brain metastases. Importantly, one of the main physiologic roles of microglia, the resident CNS antigen presenting cells, is the dampening of cell-mediated immune responses, a function that is frequently embraced by metastases. The nitric oxide-mediated tumoricidal activity of metastatic tumor-infiltrating microglia has been well-described in vitro.Citation60 However, in vivo models have demonstrated microglial recruitment by tumor cells and subsequent secretion of pro-mitotic factors contributing to tumor cell proliferation.Citation61 Microglia have also been shown to enhance invasion and colonization of brain parenchyma by acting as WNT signaling-dependent guiding rails and active transporters. These findings suggest inhibition of pro-invasive microglia may serve as a therapeutic strategy for metastatic tumors.Citation62
Impairments in the antitumor functions of glioma-associated macrophages/microglia (GAMs) are well known. Gliomas appear to secrete various cytokines that skew GAMs toward the alternatively activated (M2) phenotype and suppress the classically activated (M1) phenotype, producing a tumor-supportive environment. Similar impairments have been reported in brain metastases, as well.Citation63 In response to metastatic lung cancer cells in brain tissue, microglia in the tumor microenvironment display characteristic signs of activation, including proliferation, migration, amoeboid appearance, and formation of a dense capsule about the metastatic tumor mass. However, these microglia lack both inducible nitric oxide synthase (iNOS) and tumor necrosis factor-α (TNF-α), deeming them unable to mediate phagocytosis, cytotoxicity, and antigen presentation. Additionally, supernatants from lipopolysaccharide (LPS)-activated microglia induce apoptosis of metastatic cells in vitro in a time- and dose-dependent manner, but at lower concentrations, trophic effects on cancer cells are seen. These results suggest that the various factors released by microglia may create a favorable niche allowing metastatic cells to thrive.Citation64 This may be linked to calmodulin and integrin expression on both astrocytes and tumor cells,Citation20 the therapeutic implications of which have yet to be explored.
Early experiments indicated that macrophage-deficient mice continue to develop normal primary neoplasms but that metastasis formation is inhibited.Citation65 Both macrophage and mast cell populations can induce inflammatory responses that lead to retraction of astrocytic end feet and comprise the glia limitans.Citation66 This retraction can allow for metastatic cells in the perivascular space to gradually gain access to the brain parenchyma, mirroring observations from experimental animal models. Indeed, tumor-associated macrophages (TAMs) are often observed at later stages of metastasis when cell extravasation has already occurred.Citation11
The greatest “selective pressure” on CNS tumors is proffered by T lymphocytes. Cancer immunosurveillance has long been shown to be a function of cell-mediated immunity, with T-cells, the enforcers. Likewise the goals of many immune-based cancer therapies are the priming and tumor-infiltration of tumor-specific T-cells. Past experiments have indeed demonstrated that depletion of both CD4+ and CD8+ T cells abrogates immune responses against brain metastases.Citation67 With regard to immune evasion, TILs in both gliomas and CNS metastases consistently exhibit deficits tied to tumor and microenvironment-induced immunosuppression. Such immunosuppression is particularly well documented in glioblastoma multiforme (GBM).Citation68,69 Immunotherapies rely on a precursor frequency of T cells; however, T-cell lymphopenia is an immunologic shortcoming in GBM patients,Citation70 and regulatory T cells (Tregs) persist at disproportionate levels leading to further T-cell inhibition.Citation71 Activated T cells successfully trafficking to the CNS tumor site encounter a milieu of immunosuppressive factors within the tumor microenvironment that serve to thwart immune effectors. These elements include more Tregs,Citation72 indoleamine 2,3-dioxygenase expression,Citation73 MHC and B7 family protein downregulation,Citation74,75 PTEN loss, Citation76 STAT3 expression/activation,Citation77 production of TGF-β and IL-10,Citation78 MICA/B secretion,Citation79,80 and HLA-E expression.Citation81 Additionally, sampling of glioma TILs reveals high levels of CD95, PD-1, PD-L1, CTLA-4, LAG3, and TIM3,Citation68 indicative of immune cell exhaustion, characterized by poor effector function, obstinate expression of inhibitory receptors, and an altered transcriptional state.Citation82 These findings in GBM indicate that delivering immune cells to the CNS is only a beginning step in the path to tumor eradication.
The systemic and local immunologic consequences of CNS metastasis are not as well characterized as in primary brain tumors. However, their location within the brain suggests contributions by many of the same environmental immunosuppressive factors. For instance, Tregs have been shown to infiltrate experimental models of metastatic melanoma, breast, and colon cancer within the brain and have also been seen in human metastatic brain lesions at autopsy.Citation83 Additionally, expression of PD-1 has been seen in approximately 63% of TILs in melanoma brain metastasis, most highly associated with areas of the tumor showing positive expression of PD-L1.Citation84 PD-1 positive TILs have been found in a variety of brain metastases, with the highest levels in melanoma and renal cell carcinoma.Citation85 Debate remains about the prognostic relevance of PDL1 expression by brain tumor metastases; one study found no association between high or low PD-L1 expression and overall survival (OS) in a mixed brain metastasis cohort as well as in subsets of lung and breast cancer brain metastases. However, patients with melanoma brain metastases with higher expression levels of PD-L1 showed a trend toward better survival.Citation85 Another study determined high PD-L1 expression was associated with prolonged survival in melanoma brain metastases irrespective of prior systemic immune therapy, although it did not retain independent prognostic value on multivariate analysis.Citation86 High tumor PD-L1 expression predicting higher response rates has been seen in a prior trial of anti-PD1 therapy in patients with various solid tumors.Citation87 Thus, whether PD-L1 expression is utilized by the tumor to mediate immune escape or if it predicts treatment response to therapeutic blockade remains in question.
T-cell exhaustion also appears to be relevant in intracranial metastases. Though the mechanisms underlying the movement and clonal expansion of T cells systemically and within the tumor microenvironment remain under investigation, numerous studies document T-cell ineffectiveness as a result of tumor-induced exhaustion and stand-downs at immune checkpoints. As such, strategies fostering immune checkpoint blockade (described below) have resulted in clinically significant improvements in treatment and prognosis.Citation88 Similarly, it is possible that CNS-specific elements – for example, microglia – are responsible for tumor-driven induction of T-cell anergy, differential recruitment, or expansion. This has not yet been studied, though it has been shown that CD8+ T-cell responses in the setting of tumor xenografts are partially contingent on a unique and specific pattern of integrin signaling, Citation89-91 which can theoretically be blunted either by tumor or microglial signaling.
Current immunotherapy trials for intracranial metastasis
Immune checkpoint blockade
As CNS immune-accessibility becomes accepted, and as immunotherapy gains greater momentum among trialed therapies for primary brain tumors, there is now developing interest in immunotherapeutic approaches to brain metastases (see ). As alluded to above, one such emerging approach is immune checkpoint blockade. Immune checkpoints, induced on T cells following their activation, serve to attenuate immune responses (). For example, cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) is a well-characterized inhibitory molecule responsible for downregulating T-cell proliferation and abrogating activated T-cell responses.Citation92 Ipilimumab, a monoclonal anti-CTLA-4 antibody, was approved in 2011 as monotherapy for metastatic melanoma.Citation93 Though data on the efficacy of CTLA-4 antagonism is robust,Citation94 studies of the effects on CNS metastases are less available. Ipilimumab was shown in a phase II prospective trial (NCT00623766) to have an activity in patients with newly diagnosed melanoma brain metastases without causing unexpected toxic effects. At 2 y, however, the OS for patients treated with ipilimumab monotherapy was 26%, while those who required additional treatment with steroids had only 10% OS at 24 mo.Citation95 These results suggested that steroid treatment at the initiation of ipilimumab therapy could abrogate the immune response elicited by checkpoint blockade. This is an important consideration in patients with CNS metastases, given the frequent employment of steroids in this population. Subsequently, in 146 patients with asymptomatic melanoma brain metastases treated with ipilimumab, a global disease control rate of 27% was seen, with median progression-free survival (PFS) and OS of 2.8 and 4.3 mo, respectively.Citation96
Figure 2. Immune checkpoints and checkpoint blockade. T lymphocytes, the effector cells of the immune system, recognize and are activated against metastatic tumor cells. This process is mediated via presentation of peptide antigens displayed on major histocompatibility complex (MHC) molecules of antigen presenting cells (APCs) to the T-cell receptors (TCRs), as well as binding of co-stimulatory molecules (such as CD28 on T cells binding to CD80 or CD86 on APCs). Immune checkpoints, such as CTLA-4 and PD-1, are induced on T cells following their activation. CTLA-4 is also expressed on regulatory T cells (Tregs). These checkpoints attenuate immune responses carried out by T cells. Tumor cells and Tregs utilize these molecules as a mechanism of immunosuppression within the tumor microenvironment. Immune checkpoint blockade is achieved using monoclonal antibodies directed against these molecules, thus relinquishing these mechanisms of immune inhibition. Evaluation of these therapies in patients with brain metastasis is currently underway.
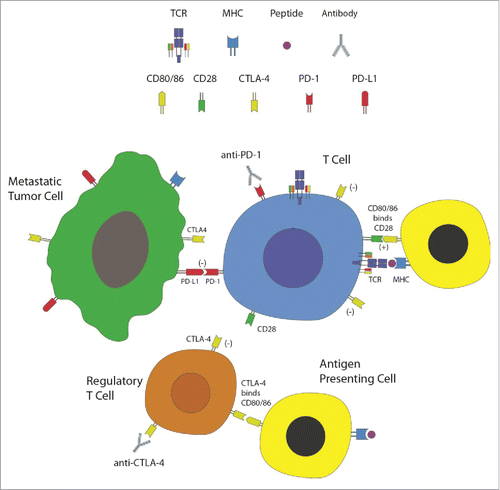
Table 1. Selected clinical trials of immunotherapy for melanoma, lung, and breast cancer brain metastases.
A second well-defined immune checkpoint on T cells is programmed cell death-1 (PD-1). Binding of PD-1 to its ligands (PD-L1 and PD-L2), expressed by many cell types including tumor cells, instigates T-cell shutdown and even apoptosis.Citation92 Nivolumab and pembrolizumab are monoclonal antibodies directed against PD-1 and approved for treatment of patients with metastatic melanoma (nivolumab and pembrolizumab) and metastatic non-small cell-lung carcinoma (nivolumab only).Citation97 The combination of ipilimumab and nivolumab has now been shown in randomized phase II and III trials to have a clinical benefit in untreated melanoma compared to ipilimumab alone.Citation98,99 A phase II study of pembrolizumab is currently underway (NCT02085070). Recently, a single-arm phase II trial of ipilimumab in combination with fotemustine in patients with melanoma and asymptomatic brain metastases showed intracranial disease control in 50% of patients and a median OS of 13.4 mo.Citation100 Additionally, an objective response to systemic therapy with ipilimumab has been shown to be associated with prolonged survival in patients who undergo surgical resection of melanoma brain metastases.Citation101 Lastly, a triple-arm phase III clinical trial will compare the OS at 2 y of fotemustine monotherapy, combination ipilimumab and fotemustine, and combination ipilimumab and nivolumab in patients with metastatic melanoma with brain metastases. This study is currently recruiting participants and is not expected to reach completion until 2020 (NCT02460068).
Combined radiation therapy
A growing body of data investigating combinatorial radiotherapy and immunotherapy has demonstrated significant potential in melanoma brain metastases (reviewed in Patel et. al.).Citation102 The notion that radiation may synergize with immunomodulation has generated significant interest in recent years. It is known that radiation induces an immune response through initiation of multiple tissue-injury pathways. These can culminate in the production of IFNγ and TNF-α which, in turn, promote lymphocyte tissue infiltration Citation103 and CD8+ T-cell activation.Citation104 This synergy may be derived from the “abscopal (distant bystander) effect,” hypothesized to arise from exposure of previously-unpresented tumor specific epitopes in the setting of local inflammation secondary to radiation therapy. As such, metastatic sites outside the field of radiation may then be recognized and destroyed by the immune system, Citation103 a form of “in-situ vaccination.” Citation105 There is now mounting data supporting the use of combined radiation and immunotherapy. Knisely et. al. retrospectively reviewed a prospectively collected cohort of 77 patients with melanoma brain metastases and found that combination ipilimumab and radiosurgery was associated with an increased median survival of 21.3 mo compared to 4.9 mo with radiosurgery alone.Citation106 These results were corroborated in another series of 70 patients in which the median survival of combined treatment increased to 19.9 mo compared to 5.3 mo with stereotactic radiosurgery (SRS) alone.Citation107 Further prospective studies are ongoing ().
Other immunotherapies
Immunotherapy can also include active, passive, and adoptive modalities, but these have not generally been tested for the specific indication of brain metastasis. Generally, experience is limited to subgroup analysis when patients with brain metastases were not excluded from immunotherapy trials for systemic cancer. For instance, Hong et. al. retrospectively assessed the objective metastatic response rate to a lymphodepletive regimen followed by adoptive cellular therapy with IL-2 and autologous TILs or peripheral blood lymphocytes that expressed T-cell receptors (TCRs) recognizing melanocyte differentiation antigens. The authors found that 41% of patients treated with TILs and 22% of patients treated with TCR-transduced lymphocytes achieved complete responses, suggesting that activated lymphocytes can traffic to the CNS to effectively target melanoma metastases.Citation108
Conclusions and future directions
Metastatic disease to the central nervous system (CNS), despite its dismal prognosis, high prevalence, and increasing incidence, is almost entirely indirectly studied. The complex interactions between tumor and its microenvironment, which include both native CNS and immune componentry, are poorly understood. Moreover, the regulation of the BBB with respect to the kinetics of immune cell transit must be further investigated. Our knowledge is also limited by logistics, particularly with regard to study of the tumor microenvironment. Normal tumor margins are not typically taken during surgical resection, and thus, any in vitro study of metastatic tumor cells will inherently preclude successful duplication of the native milieu. Study of this disease in humans has also been limited as patients with a diagnosis of brain metastasis have historically been excluded from clinical trials.Citation109 Only now, with the advent of immune checkpoint blockade has this trend begun to be reversed. The therapeutic challenge, then, will now appropriately shift to maintenance of immune responses within the CNS, presenting no paucity of further research endeavors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Dunn GP, Fecci PE, Curry WT. Cancer immunoediting in malignant glioma. Neurosurgery 2012; 71:201-22; PMID:22353795; http://dx.doi.org/10.1227/NEU.0b013e31824f840d
- Gallego Perez-Larraya J, Hildebrand J. Brain metastases. Handb Clin Neurol 2014; 121:1143-57; PMID:24365409; http://dx.doi.org/10.1016/B978-0-7020-4088-7.00077-8
- Ranjan T, Abrey LE. Current management of metastatic brain disease. Neurotherapeutics 2009; 6:598-603; PMID:19560748; http://dx.doi.org/10.1016/j.nurt.2009.04.012
- Lu-Emerson C, Eichler AF. Brain metastases. Continuum 2012; 18:295-311; PMID:22810128
- Chan AW, Loeffler JS. Controversies in the management of patients with brain metastases. Cancer J. 2001; 7:105-7; PMID:11324762
- Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011; 29:134-41; PMID:21041710; http://dx.doi.org/10.1200/JCO.2010.30.1655
- Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009; 10:1037-44; PMID:19801201; http://dx.doi.org/10.1016/S1470-2045(09)70263-3
- Mitchell DA, Fecci PE, Sampson JH. Immunotherapy of malignant brain tumors. Immunol Rev. 2008; 222:70-100; PMID:18363995; http://dx.doi.org/10.1111/j.1600-065X.2008.00603.x
- Gedeon PC, Riccione KA, Fecci PE, Sampson JH. Antibody-based immunotherapy for malignant glioma. Semin Oncol. 2014; 41:496-510; PMID:25173142; http://dx.doi.org/10.1053/j.seminoncol.2014.06.004
- Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948; 29:58-69; PMID:18865105
- Hamilton A, Sibson NR. Role of the systemic immune system in brain metastasis. Mol Cell Neurosci. 2013; 53:42-51; PMID:23073146; http://dx.doi.org/10.1016/j.mcn.2012.10.004
- Woodworth GF, Dunn GP, Nance EA, Hanes J, Brem H. Emerging insights into barriers to effective brain tumor therapeutics. Front Oncol. 2014; 4:126; PMID:25101239; http://dx.doi.org/10.3389/fonc.2014.00126
- Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011; 14:1398-405; PMID:22030551; http://dx.doi.org/10.1038/nn.2946
- Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx 2005; 2:3-14; PMID:15717053; http://dx.doi.org/10.1602/neurorx.2.1.3
- Groothuis DR, Vavra MW, Schlageter KE, Kang EW, Itskovich AC, Hertzler S, Allen CV, Lipton HL. Efflux of drugs and solutes from brain: the interactive roles of diffusional transcapillary transport, bulk flow and capillary transporters. J Cereb Blood Flow Metab 2007; 27:43-56; PMID:16639426; http://dx.doi.org/10.1038/sj.jcbfm.9600315
- Brightman MW, Klatzo I, Olsson Y, Reese TS. The blood-brain barrier to proteins under normal and pathological conditions. J Neurol Sci. 1970; 10:215-39; PMID:4909727; http://dx.doi.org/10.1016/0022-510X(70)90151-6
- Pollay M, Roberts PA. Blood-brain barrier: a definition of normal and altered function. Neurosurgery 1980; 6:675-85; PMID:7001265; http://dx.doi.org/10.1227/00006123-198006000-00014
- Sjogren AM, Thelestam M, Blomqvist L, Linda H, Remahl S, Risling M. Extravasation of staphylococcal alpha-toxin in normal and injured CNS regions lacking blood-brain barrier function: observations after ventral root replantation. Brain Res. 1991; 559:276-82; PMID:1794101; http://dx.doi.org/10.1016/0006-8993(91)90012-K
- d'Avella D, Cicciarello R, Albiero F, Mesiti M, Gagliardi ME, Russi E, d'Aquino A, Tomasello F, d'Aquino S. Quantitative study of blood-brain barrier permeability changes after experimental whole-brain radiation. Neurosurgery 1992; 30:30-4; PMID:1738452; http://dx.doi.org/10.1227/00006123-199201000-00006
- Wilhelm I, Molnar J, Fazakas C, Hasko J, Krizbai IA. Role of the blood-brain barrier in the formation of brain metastases. Int J Molecular Sci. 2013; 14:1383-411; PMID:23344048; http://dx.doi.org/10.3390/ijms14011383
- Owens T, Renno T, Taupin V, Krakowski M. Inflammatory cytokines in the brain: does the CNS shape immune responses?. Immunol Today 1994; 15:566-71; PMID:7848517; http://dx.doi.org/10.1016/0167-5699(94)90218-6
- Cserr HF, Knopf PM. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunol Today 1992; 13:507-12; PMID:1463583; http://dx.doi.org/10.1016/0167-5699(92)90027-5
- Cserr HF, Harling-Berg CJ, Knopf PM. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol 1992; 2:269-76; PMID:1341962; http://dx.doi.org/10.1111/j.1750-3639.1992.tb00703.x
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015; 523:337-41; PMID:26030524
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015; 212:991-9; PMID:26077718; http://dx.doi.org/10.1084/jem.20142290
- Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol. 2010; 176:2958-71; PMID:20382702; http://dx.doi.org/10.2353/ajpath.2010.090838
- Lorger M, Lee H, Forsyth JS, Felding-Habermann B. Comparison of in vitro and in vivo approaches to studying brain colonization by breast cancer cells. J Neuro-oncol. 2011; 104:689-96; PMID:21359851; http://dx.doi.org/10.1007/s11060-011-0550-4
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006; 12:895-904; PMID:16892035; http://dx.doi.org/10.1038/nm1469
- Gorantla V, Kirkwood JM, Tawbi HA. Melanoma brain metastases: an unmet challenge in the era of active therapy. Curr Oncol Rep. 2013; 15:483-91; PMID:23954973; http://dx.doi.org/10.1007/s11912-013-0335-3
- Brayton J, Qing Z, Hart MN, VanGilder JC, Fabry Z. Influence of adhesion molecule expression by human brain microvessel endothelium on cancer cell adhesion. J Neuroimmunol 1998; 89:104-12; PMID:9726832; http://dx.doi.org/10.1016/S0165-5728(98)00127-1
- Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert opinion on therapeutic targets 2007; 11:1473-91; PMID:18028011; http://dx.doi.org/10.1517/14728222.11.11.1473
- Kusters B, Westphal JR, Smits D, Ruiter DJ, Wesseling P, Keilholz U, de Waal RM. The pattern of metastasis of human melanoma to the central nervous system is not influenced by integrin alpha(v)beta(3) expression. Int J of Cancer Journal international du cancer 2001; 92:176-80; PMID:11291042; http://dx.doi.org/10.1002/1097-0215(200102)9999:9999%3c::AID-IJC1173%3e3.0.CO;2-L
- Serres S, Soto MS, Hamilton A, McAteer MA, Carbonell WS, Robson MD, Ansorge O, Khrapitchev A, Bristow C, Balathasan L et al. Molecular MRI enables early and sensitive detection of brain metastases. Proc Natl Acad Sci USA. 2012; 109:6674-9; PMID:22451897; http://dx.doi.org/10.1073/pnas.1117412109
- Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 2002; 3:53-7; PMID:11905606; http://dx.doi.org/10.1016/S1470-2045(01)00622-2
- On NH, Mitchell R, Savant SD, Bachmeier CJ, Hatch GM, Miller DW. Examination of blood-brain barrier (BBB) integrity in a mouse brain tumor model. J Neuro-oncol. 2013; 111:133-43; PMID:23184143; http://dx.doi.org/10.1007/s11060-012-1006-1
- Lu W, Bucana CD, Schroit AJ. Pathogenesis and vascular integrity of breast cancer brain metastasis. Int J Cancer Journal international du cancer 2007; 120:1023-6; PMID:17187362; http://dx.doi.org/10.1002/ijc.22388
- Ballinger WE, Jr., Schimpff RD. An experimental model for cerebral metastasis: preliminary light and ultrastructural studies. J Neuropathol Exp Neurol 1979; 38:19-34; PMID:430105; http://dx.doi.org/10.1097/00005072-197901000-00003
- Kawaguchi T, Tobai S, Nakamura K. Extravascular migration of tumor cells in the brain: an electron microscopic study. Invasion metastasis 1982; 2:40-50; PMID:7188390
- Paku S, Dome B, Toth R, Timar J. Organ-specificity of the extravasation process: an ultrastructural study. Clin Exp Metastasis 2000; 18:481-92; PMID:11592305; http://dx.doi.org/10.1023/A:1011858925376
- Gerstner ER, Fine RL. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. J Clin Oncol. 2007; 25:2306-12; PMID:17538177; http://dx.doi.org/10.1200/JCO.2006.10.0677
- Adkins CE, Mohammad AS, Terrell-Hall TB, Dolan EL, Shah N, Sechrest E, Griffith J, Lockman PR. Characterization of passive permeability at the blood-tumor barrier in five preclinical models of brain metastases of breast cancer. Clin Exp Metastasis 2016; 33:373-83; PMID:26944053
- Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, Adkins CE, Roberts A, Thorsheim HR, Gaasch JA et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010; 16:5664-78; PMID:20829328; http://dx.doi.org/10.1158/1078-0432.CCR-10-1564
- Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010; 16:116-22; PMID:20023634; http://dx.doi.org/10.1038/nm.2072
- Fazakas C, Wilhelm I, Nagyoszi P, Farkas AE, Hasko J, Molnar J, Bauer H, Bauer HC, Ayaydin F, Dung NT et al. Transmigration of melanoma cells through the blood-brain barrier: role of endothelial tight junctions and melanoma-released serine proteases. PloS One 2011; 6:e20758; PMID:21674054
- Carbonell WS, Ansorge O, Sibson N, Muschel R. The vascular basement membrane as “soil” in brain metastasis. PloS One 2009; 4:e5857; PMID:19516901; http://dx.doi.org/10.1371/journal.pone.0005857
- Fan J, Cai B, Zeng M, Hao Y, Giancotti FG, Fu BM. Integrin beta4 signaling promotes mammary tumor cell adhesion to brain microvascular endothelium by inducing ErbB2-mediated secretion of VEGF. Ann Biomed Eng. 2011; 39:2223-41; PMID:21556948; http://dx.doi.org/10.1007/s10439-011-0321-6
- Kusters B, Leenders WP, Wesseling P, Smits D, Verrijp K, Ruiter DJ, Peters JP, van Der Kogel AJ, de Waal RM. Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res. 2002; 62:341-5; PMID:11809675
- Kusters B, de Waal RM, Wesseling P, Verrijp K, Maass C, Heerschap A, Barentsz JO, Sweep F, Ruiter DJ, Leenders WP. Differential effects of vascular endothelial growth factor A isoforms in a mouse brain metastasis model of human melanoma. Cancer Res. 2003; 63:5408-13; PMID:14500375
- Xie TX, Huang FJ, Aldape KD, Kang SH, Liu M, Gershenwald JE, Xie K, Sawaya R, Huang S. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006; 66:3188-96; PMID:16540670; http://dx.doi.org/10.1158/0008-5472.CAN-05-2674
- Leenders WP, Kusters B, Verrijp K, Maass C, Wesseling P, Heerschap A, Ruiter D, Ryan A, de Waal R. Antiangiogenic therapy of cerebral melanoma metastases results in sustained tumor progression via vessel co-option. Clin Cancer Res. 2004; 10:6222-30; PMID:15448011; http://dx.doi.org/10.1158/1078-0432.CCR-04-0823
- Kim LS, Huang S, Lu W, Lev DC, Price JE. Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. Clin Exp Metastasis 2004; 21:107-18; PMID:15168728; http://dx.doi.org/10.1023/B:CLIN.0000024761.00373.55
- Wu PF, Lin CH, Kuo CH, Chen WW, Yeh DC, Liao HW, Huang SM, Cheng AL, Lu YS. A pilot study of bevacizumab combined with etoposide and cisplatin in breast cancer patients with leptomeningeal carcinomatosis. BMC Cancer 2015; 15:299; PMID:25928457; http://dx.doi.org/10.1186/s12885-015-1290-1
- Dunn GP, Dunn IF, Curry WT. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun. 2007; 7:12; PMID:17691714
- Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA et al. Genes that mediate breast cancer metastasis to the brain. Nature 2009; 459:1005-9; PMID:19421193; http://dx.doi.org/10.1038/nature08021
- Izraely S, Sagi-Assif O, Klein A, Meshel T, Tsarfaty G, Pasmanik-Chor M, Nahmias C, Couraud PO, Ateh E, Bryant JL et al. The metastatic microenvironment: brain-residing melanoma metastasis and dormant micrometastasis. Int J Cancer 2012; 131:1071-82; PMID:22025079; http://dx.doi.org/10.1002/ijc.27324
- Ridgway LD, Wetzel MD, Marchetti D. Modulation of GEF-H1 induced signaling by heparanase in brain metastatic melanoma cells. J Cell Biochem 2010; 111:1299-309; PMID:20803552; http://dx.doi.org/10.1002/jcb.22854
- Marchetti D, Li J, Shen R. Astrocytes contribute to the brain-metastatic specificity of melanoma cells by producing heparanase. Cancer Res. 2000; 60:4767-70; PMID:10987284
- Lin Q, Balasubramanian K, Fan D, Kim SJ, Guo L, Wang H, Bar-Eli M, Aldape KD, Fidler IJ. Reactive astrocytes protect melanoma cells from chemotherapy by sequestering intracellular calcium through gap junction communication channels. Neoplasia 2010; 12:748-54; PMID:20824051; http://dx.doi.org/10.1593/neo.10602
- Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 2014; 156:1002-16; PMID:24581498; http://dx.doi.org/10.1016/j.cell.2014.01.040
- Brantley EC, Guo L, Zhang C, Lin Q, Yokoi K, Langley RR, Kruzel E, Maya M, Kim SW, Kim SJ et al. Nitric oxide-mediated tumoricidal activity of murine microglial cells. Transl Oncol. 2010; 3:380-8; PMID:21151477; http://dx.doi.org/10.1593/tlo.10208
- Fitzgerald DP, Palmieri D, Hua E, Hargrave E, Herring JM, Qian Y, Vega-Valle E, Weil RJ, Stark AM, Vortmeyer AO et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metastasis 2008; 25:799-810; PMID:18649117; http://dx.doi.org/10.1007/s10585-008-9193-z
- Pukrop T, Dehghani F, Chuang HN, Lohaus R, Bayanga K, Heermann S, Regen T, Van Rossum D, Klemm F, Schulz M et al. Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia 2010; 58:1477-89; PMID:20549749
- Wei J, Gabrusiewicz K, Heimberger A. The controversial role of microglia in malignant gliomas. Clin Dev Immunol 2013; 2013:285246; PMID:23983766; http://dx.doi.org/10.1155/2013/285246
- He BP, Wang JJ, Zhang X, Wu Y, Wang M, Bay BH, Chang AY. Differential reactions of microglia to brain metastasis of lung cancer. Mol Med. 2006; 12:161-70; PMID:17088948; http://dx.doi.org/10.2119/2006-00033.He
- Lin EY, Pollard JW. Macrophages: modulators of breast cancer progression. Novartis Found Sym. 2004; 256:158-68; PMID:15027489; http://dx.doi.org/10.1002/0470856734.ch12
- Theoharides TC, Rozniecki JJ, Sahagian G, Jocobson S, Kempuraj D, Conti P, Kalogeromitros D. Impact of stress and mast cells on brain metastases. J Neuroimmunol 2008; 205:1-7; PMID:18977036; http://dx.doi.org/10.1016/j.jneuroim.2008.09.014
- Lu W, Su J, Kim LS, Bucana CD, Donawho C, He J, Fidler IJ, Dong Z. Active specific immunotherapy against occult brain metastasis. Cancer Res. 2003; 63:1345-50; PMID:12649197
- Fecci PE, Heimberger AB, Sampson JH. Immunotherapy for primary brain tumors: no longer a matter of privilege. Clin Cancer Res. 2014; 20:5620-9; PMID:25398845; http://dx.doi.org/10.1158/1078-0432.CCR-14-0832
- Rolle CE, Sengupta S, Lesniak MS. Mechanisms of immune evasion by gliomas. Adv Exp Med Biol. 2012; 746:53-76; PMID:22639159; http://dx.doi.org/10.1007/978-1-4614-3146-6_5
- Brooks WH, Roszman TL, Mahaley MS, Woosley RE. Immunobiology of primary intracranial tumours. II. Analysis of lymphocyte subpopulations in patients with primary brain tumours. Clin Exp Immunol 1977; 29:61-6; PMID:330067
- Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, Herndon JE, 2nd, Bigner DD, Dranoff G, Sampson JH. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006; 66:3294-302; PMID:16540683; http://dx.doi.org/10.1158/0008-5472.CAN-05-3773
- El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro-oncology 2006; 8:234-43; PMID:16723631; http://dx.doi.org/10.1215/15228517-2006-006
- Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, Tobias AL, Han Y, Lesniak MS. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012; 18:6110-21; PMID:22932670; http://dx.doi.org/10.1158/1078-0432.CCR-12-2130
- Facoetti A, Nano R, Zelini P, Morbini P, Benericetti E, Ceroni M, Campoli M, Ferrone S. Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin Cancer Res. 2005; 11:8304-11; PMID:16322289; http://dx.doi.org/10.1158/1078-0432.CCR-04-2588
- Anderson RC, Anderson DE, Elder JB, Brown MD, Mandigo CE, Parsa AT, Goodman RR, McKhann GM, Sisti MB, Bruce JN. Lack of B7 expression, not human leukocyte antigen expression, facilitates immune evasion by human malignant gliomas. Neurosurgery 2007; 60:1129-36; PMID:17538388; http://dx.doi.org/10.1227/01.NEU.0000255460.91892.44
- Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007; 13:84-8; PMID:17159987; http://dx.doi.org/10.1038/nm1517
- Abou-Ghazal M, Yang DS, Qiao W, Reina-Ortiz C, Wei J, Kong LY, Fuller GN, Hiraoka N, Priebe W, Sawaya R et al. The incidence, correlation with tumor-infiltrating inflammation, and prognosis of phosphorylated STAT3 expression in human gliomas. Clin Cancer Res. 2008; 14:8228-35; PMID:19088040; http://dx.doi.org/10.1158/1078-0432.CCR-08-1329
- Roussel E, Gingras MC, Grimm EA, Bruner JM, Moser RP. Predominance of a type 2 intratumoural immune response in fresh tumour-infiltrating lymphocytes from human gliomas. Clin Exp Immunol 1996; 105:344-52; PMID:8706344; http://dx.doi.org/10.1046/j.1365-2249.1996.d01-753.x
- Friese MA, Platten M, Lutz SZ, Naumann U, Aulwurm S, Bischof F, Buhring HJ, Dichgans J, Rammensee HG, Steinle A et al. MICA/NKG2D-mediated immunogene therapy of experimental gliomas. Cancer Res. 2003; 63:8996-9006; PMID:14695218
- Eisele G, Wischhusen J, Mittelbronn M, Meyermann R, Waldhauer I, Steinle A, Weller M, Friese MA. TGF-beta and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain 2006; 129:2416-25; PMID:16891318; http://dx.doi.org/10.1093/brain/awl205
- Wischhusen J, Friese MA, Mittelbronn M, Meyermann R, Weller M. HLA-E protects glioma cells from NKG2D-mediated immune responses in vitro: implications for immune escape in vivo. J Neuropathol Exp Neurol 2005; 64:523-8; PMID:15977644; http://dx.doi.org/10.1093/jnen/64.6.523
- Wherry EJ. T cell exhaustion. Nat Immunol 2011; 12:492-9; PMID:21739672; http://dx.doi.org/10.1038/ni.2035
- Sugihara AQ, Rolle CE, Lesniak MS. Regulatory T cells actively infiltrate metastatic brain tumors. Int J Oncol. 2009; 34:1533-40; PMID:19424570
- Berghoff AS, Ricken G, Widhalm G, Rajky O, Dieckmann K, Birner P, Bartsch R, Holler C, Preusser M. Tumour-infiltrating lymphocytes and expression of programmed death ligand 1 (PD-L1) in melanoma brain metastases. Histopathology 2015; 66:289-99; PMID:25314639; http://dx.doi.org/10.1111/his.12537
- Harter PN, Bernatz S, Scholz A, Zeiner PS, Zinke J, Kiyose M, Blasel S, Beschorner R, Senft C, Bender B et al. Distribution and prognostic relevance of tumor-infiltrating lymphocytes (TILs) and PD-1/PD-L1 immune checkpoints in human brain metastases. Oncotarget 2015; 6:40836-49; PMID:26517811
- Kluger HM, Zito CR, Barr ML, Baine MK, Chiang VL, Sznol M, Rimm DL, Chen L, Jilaveanu LB. Characterization of PD-L1 expression and associated T-cell infiltrates in metastatic melanoma samples from variable anatomic sites. Clin Cancer Res. 2015; 21:3052-60; PMID:25788491; http://dx.doi.org/10.1158/1078-0432.CCR-14-3073
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/10.1056/NEJMoa1200690
- Stadler S, Weina K, Gebhardt C, Utikal J. New therapeutic options for advanced non-resectable malignant melanoma. Adv Med Sci. 2015; 60:83-8; PMID:25596540; http://dx.doi.org/10.1016/j.advms.2014.12.002
- Masson F, Calzascia T, Di Berardino-Besson W, de Tribolet N, Dietrich PY, Walker PR. Brain microenvironment promotes the final functional maturation of tumor-specific effector CD8+ T cells. J Immunol 2007; 179:845-53; PMID:NOT_FOUND; http://dx.doi.org/10.4049/jimmunol.179.2.845
- Nishimura F, Dusak JE, Eguchi J, Zhu X, Gambotto A, Storkus WJ, Okada H. Adoptive transfer of type 1 CTL mediates effective anti-central nervous system tumor response: critical roles of IFN-inducible protein-10. Cancer Res. 2006; 66:4478-87; PMID:16618775; http://dx.doi.org/10.1158/0008-5472.CAN-05-3825
- Sasaki K, Zhu X, Vasquez C, Nishimura F, Dusak JE, Huang J, Fujita M, Wesa A, Potter DM, Walker PR et al. Preferential expression of very late antigen-4 on type 1 CTL cells plays a critical role in trafficking into central nervous system tumors. Cancer Res. 2007; 67:6451-8; PMID:17616706; http://dx.doi.org/10.1158/0008-5472.CAN-06-3280
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015; 348:56-61; PMID:25838373; http://dx.doi.org/10.1126/science.aaa8172
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010; 363:711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015; 33:1889-94; PMID:25667295; http://dx.doi.org/10.1200/JCO.2014.56.2736
- Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, Wolchok JD, Clark JI, Sznol M, Logan TF et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012; 13:459-65; PMID:22456429; http://dx.doi.org/10.1016/S1470-2045(12)70090-6
- Queirolo P, Spagnolo F, Ascierto PA, Simeone E, Marchetti P, Scoppola A, Del Vecchio M, Di Guardo L, Maio M, Di Giacomo AM et al. Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. J Neuro-oncol. 2014; 118:109-16; PMID:24532241
- Sunshine J, Taube JM. PD-1/PD-L1 inhibitors. Curr Opin Pharmacol 2015; 23:32-8; PMID:26047524; http://dx.doi.org/10.1016/j.coph.2015.05.011
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015; 372:2006-17; PMID:25891304; http://dx.doi.org/10.1056/NEJMoa1414428
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015; 373:23-34; PMID:26027431; http://dx.doi.org/10.1056/NEJMoa1504030
- Di Giacomo AM, Ascierto PA, Pilla L, Santinami M, Ferrucci PF, Giannarelli D, Marasco A, Rivoltini L, Simeone E, Nicoletti SV et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol. 2012; 13:879-86; PMID:22894884; http://dx.doi.org/10.1016/S1470-2045(12)70324-8
- Lonser RR, Song DK, Klapper J, Hagan M, Auh S, Kerr PB, Citrin DE, Heiss JD, Camphausen K, Rosenberg SA. Surgical management of melanoma brain metastases in patients treated with immunotherapy. J Neurosurg 2011; 115:30-6; PMID:21476810; http://dx.doi.org/10.3171/2011.3.JNS091107
- Patel KR, Lawson DH, Kudchadkar RR, Carthon BC, Oliver DE, Okwan-Duodu D, Ahmed R, Khan MK. Two heads better than one? Ipilimumab immunotherapy and radiation therapy for melanoma brain metastases. Neuro-oncology 2015; 17:1312-21; PMID:26014049; http://dx.doi.org/10.1093/neuonc/nov093
- Frey B, Rubner Y, Kulzer L, Werthmoller N, Weiss EM, Fietkau R, Gaipl US. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother 2014; 63:29-36; PMID:24052136; http://dx.doi.org/10.1007/s00262-013-1474-y
- Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009; 114:589-95; PMID:19349616; http://dx.doi.org/10.1182/blood-2009-02-206870
- Vanpouille-Box C, Pilones KA, Wennerberg E, Formenti SC, Demaria S. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine 2015; 33:7415-22; PMID:26148880
- Knisely JP, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VL. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg 2012; 117:227-33; PMID:22702482; http://dx.doi.org/10.3171/2012.5.JNS111929
- Silk AW, Bassetti MF, West BT, Tsien CI, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013; 2:899-906; PMID:24403263; http://dx.doi.org/10.1002/cam4.140
- Hong JJ, Rosenberg SA, Dudley ME, Yang JC, White DE, Butman JA, Sherry RM. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin Cancer Res. 2010; 16:4892-8; PMID:20719934; http://dx.doi.org/10.1158/1078-0432.CCR-10-1507
- Bedikian AY, Richards J, Kharkevitch D, Atkins MB, Whitman E, Gonzalez R. A phase 2 study of high-dose Allovectin-7 in patients with advanced metastatic melanoma. Melanoma Res. 2010; 20:218-26; PMID:20354459
