ABSTRACT
Interleukin 12 (IL12) is a key inflammatory cytokine critically influencing Th1/Tc1-T-cell responses at the time of initial antigen encounter. Therefore, it may be exploited for cancer immunotherapy. Here, we investigated how IL12, and other inflammatory cytokines, shape effector functions of human T-cells. Using a defined culture system, we followed the gradual differentiation and function of antigen-specific CD8+ T cells from their initial activation as naïve T cells through their expansion phase as early memory cells to full differentiation as clonally expanded effector T cells. The addition of IL12 8 days after the initial priming event initiated two mechanistically separate events: First, IL12 sensitized the T-cell receptor (TCR) for antigen-specific activation, leading to an approximately 10-fold increase in peptide sensitivity and, in consequence, enhanced tumor cell killing. Secondly, IL12 enabled TCR/HLA-independent activation and cytotoxicity: this “non-specific” effect was mediated by the NK cell receptor DNAM1 (CD226) and dependent on ligand expression of the target cells. This IL12 regulated, DNAM1-mediated killing is dependent on src-kinases as well as on PTPRC (CD45) activity. Thus, besides enhancing TCR-mediated activation, we here identified for the first time a second IL12 mediated mechanism leading to activation of a receptor-dependent killing pathway via DNAM1.
Introduction
Harnessing the cytotoxic armamentarium of T-cells to improve on cancer therapy has become a major focus in translational medicine.Citation1 Several strategies are aimed to remove the brakes of T-cell activation, while maintaining high specificity and function, in order to provide a selective attack on tumor cells without the risk of negative side-effects. However, clinical data on cytokine-release-syndrome using genetically engineered T cells or T-cell engaging antibody constructs,Citation2,3 or simply GvHD following donor lymphocyte infusions, illustrate that non-specific, off-target effects of T cells are a major clinical hurdle and risk factor for effective T-cell therapies.
Inflammatory cytokines such as IL6, TNF-α, IL1ß, IL18 and IL12 not only serve as effector cytokines—as seen in cytokine-release-syndrome—but may feed back onto T-cell effector function during this process. In addition, (co-)administration of a pro-inflammatory cytokines to improve efficacy has been a long-standing strategy in cancer immunology. To this end, murine studies specifically explored the role of IL12, and revealed that IL12, in concert with IL18, was responsible for enhanced memory T-cell activation and heightened TCR-sensitivity toward cognate antigen, leading to faster viral clearance.Citation4,5 How IL12 affects human memory T cells; however, is less well understood. These effects are clearly distinguished from effects during the priming phase of naïve T cells, where IL12 has been identified as the cytokine classically delivering the so-called “signal 3” to primed T cells rendering them fully functional.Citation6,7
Cytotoxic T cells not only get activated through their TCR-signal, but also receive signals through additional (death-) receptors, e.g. the Fas/FasL system. Besides the Fas/FasL system, CD8+-T-cells express so-called “NK-cell receptors” as NKG2D (CD314) and DNAM-1 (DNAX accessory molecule-1; CD226). The latter belongs to the family of nectin-binding receptors and is expressed on T- and NK-cells and known to be an adhesion molecule.Citation8,9 However, for NK-cells DNAM1 is a major contributor of NK-cell mediated cytotoxicity against various human tumors including melanoma and Ewing's sarcoma.Citation10,11 In addition, the efficacy of DNAM1-based chimeric antigen receptor engineered T-cells has been demonstrated in a murine melanoma system.Citation12 Still, little is known on the regulation and origin function of DNAM-1 in T cells, but it has been attributed to stabilization of the immunological synapse in concert with LFA1.Citation13
In this paper, we evaluate how an inflammatory cytokine milieu contributes to TCR-dependent and TCR-independent effector functions in a well-defined antigen-specific system of human CD8+-T-cells. We show that IL12 has the unique capacity to increase TCR-avidity in human effector T-cells leading to heightened antigen-specific cytotoxicity. At the same time and depending on the tumor target, TCR-independent cytotoxicity can be massively increased in IL12 treated T cells. We here identified DNAM-1 as the main effector pathway for such heightened lytic capacity and explore the signaling pathways involved in this process. These results are relevant for translational approaches aiming to boost T-cell effector function with the help of IL12 as an immunotherapeutic strategy against cancer and to elucidate “unexpected” off-target effects of T-cells mediated by IL12.
Results
The magnitude and the quality of a T-cell response are critically influenced by the cytokine milieu during the first antigen encounter, called the priming phase. It is less clear, how the cytokine microenvironment after the initial priming event influences the resulting T-cell response. Especially in the human setting, this question is difficult to assess for responses to defined antigens, as these responses are either of very low frequency, requiring repetitive stimulation or may only be described as part of complex processes such as an ongoing infection. Using an in vitro priming system, we are able to recapitulate antigen-specific priming and expansion of naïve CD8+ T cells.Citation14,15 This system allows reliable quantitative and qualitative analysis of peptide-specific T cells after a single stimulation of naïve T cells, thus excluding confounding variables related to re-stimulation procedures. The 10 d period of the protocol can be divided into an initial priming phase using peptide-loaded, IL12 producing, autologous DCs (day 0–3) as antigen presenting cells. This is followed by an expansion phase (day 3–10) in response to low dose IL7 and IL15 (). When using the melanosomal, HLA-A02:01-restricted heteroclitic peptide antigen Melan-A26–35(A27L) as a model antigen, a surprisingly strong T-cell response can be elicited with cells from almost any HLA-A02:01+ healthy donor (). In previous work, standard conditions have been established that now allow us to compare against this positive control of antigen-specific T-cell expansion.Citation15
Figure 1. IL12 increases TCR sensitivity toward cognate antigen. (A) Experimental setting. After priming of Melan-A(26–35(27L)) specific T cells various cytokines were added after pooling of wells on day +8. IL7 and IL15 was present throughout the assay to ensure survival. 48 h later cells were evaluated for overall count, the percentage of Melan-A-multimer+ CD8+ T-cells and cytokine production upon restimulation. (B) Upper row: Representative dot plots of MHC-multimer-staining with no addition of inflammatory cytokines (standard, left), the addition of 10 ng/mL IL12 (middle) and interferon-α 450 IU/mL (right). Middle row: Staining of intracellular cytokines of CD8+ T cells stimulated with Melan-A(26–35(27L)) peptide (10 ng/mL; 2nd row) or bottom row: irrelevant peptide CYP1B1(239–247) (103 ng/mL; 3rd row), gated on CD8+. (C) Absolute cell counts (left) and percentages of multimer+ T-cells (right) on d + 10 of un- or IL12 treated cells in indicated dosages (Mean and SD, results from more than five experiments). (D) MFI values on d + 11 of interferon-γ and TNF-α in untreated or IL12 treated T cells stimulated with Melan-A(26–35(27L)) peptide loaded on autologous monocytes (103 ng/mL). Results are from five independent experiments. MFI of irrelevant peptide-pulsed monocytes is subtracted. (E) Log EC50 of interferon-γ and TNF-α was calculated from the response curves for each indicated cytokine. Indicated is the difference to “standard” (= no additional inflammatory cytokine, only IL7/IL15) treatment. Results are from five independent experiments, each cytokine was tested at least three times. *p < 0.05; **p < 0.01.(F) Upper panel: representative response curves of interferon-γ and TNF-α, gated on CD8+ T cells. The percentage of cytokine+ T cells is put in relation to the respective percentage of MHC-multimer+CD8+ T cells in each sample. Lower panel: Changes in logEC50 from five independent experiments of interferon-γ and TNF-α no IL12 vs. IL12 (10 ng/mL) normalized for CD8+multimer+ T cells. Log EC50 of interferon-γ and TNF-α was calculated from the response curves for each indicated cytokine. Indicated is the difference to “standard” (= no additional cytokine) treatment.
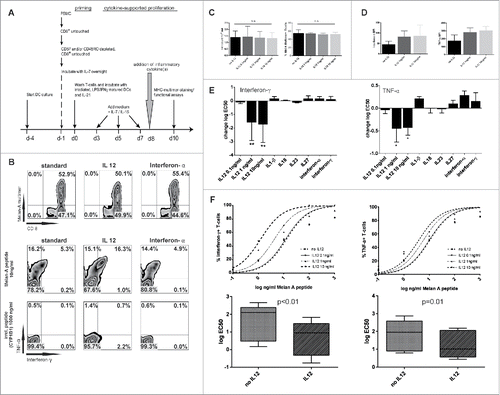
IL12 increases TCR sensitivity toward cognate antigen
To assess the impact of inflammatory cytokines on memory/effector T cells, we focused on the expansion phase of the resulting T-cell response which peaks on day +8 of culture. At this time point, any one of selected inflammatory cytokines was added to the culture. T-cell responses were analyzed 48 h resp. 72 h later (experimental setup ). For quantitation, cells were counted and the percentage of MHC-multimer+ CD8+ T cells was determined (representative dot plot ). Functionally, cells were analyzed for their capacity to produce Tc1 cytokines (interferon-γ and TNF-α) upon stimulation against titrated amounts of peptide. None of the added cytokines significantly altered the total and antigen-specific cell count (for IL12: , for other cytokines: data not shown). However, when IL12 was added on day +8 of culture, and T cells were functionally evaluated 48 h later, IL12 was the only cytokine that increased Tc1-effector cytokine production at amounts as low as 1 ng/mL (). T cells remained highly specific and responded only to cognate antigen presented by autologous monocytes: incubation with autologous monocytes loaded with irrelevant control peptides elicited no relevant reactivity ( and Fig. S1A).
It is well known that IL12 critically influences TCR-sensitivity during the priming phase.Citation16,17 Our results suggested that IL12 also enhances TCR-sensitivity during the proliferative phase following the initial TCR-trigger. To better document this process, we determined the EC50 of responses to titrated amounts of peptide. IL12 was the only cytokine that induced a log10 shift in the peptide dose required to achieve half-maximal responses (log EC50 mean: interferon-γ: 1.56 ± 1.03 vs. 0.64 ± 0.99, p < 0.01; TNF-α: 1.79 ± 0.87 vs. 1.26 ± 0.77, p = 0.011; ). On a semi-quantitative, per-cell level, the MFI for interferon-γ and TNF-α was higher in IL12-pre-incubated T-cells (MFI no IL12 vs. IL12 (10 ng/mL): interferon-γ 47 ± 14 vs. 87 ± 51; TNF-α 175 ± 73 vs. 305 ± 52; ). The maximum effect of IL12 pre-incubation was evident after 48 h of incubation and was subsequently lost over prolonged incubation periods (Fig. S1B).
Next, we asked whether a combination of selected inflammatory cytokines (interferon-α, interferon-γ, TNF-α, IL18, IL23 and IL33) with IL12 would further enhance the observed TCR sensitization. No synergistic, additive effects were observed (Fig. S1C). However, combining IL12 with IL18 or IL33 induced TCR-independent activation and high production of interferon-γ with only little amounts of TNF-α (Fig. S1D), which is in line with recent findings in mice.Citation18,19
From this data, we conclude that IL12 not only plays a crucial role at the time of priming, but also enhances effector function by modulating TCR sensitivity in human CD8+ T cells.
IL12 enhances antigen-specific cytotoxicity and retains CD62L expression
We next focused on T-cell mediated cytotoxicity of cancer cells, endogenously expressing the antigen, and wanted to know, if IL12 pre-incubation enhances the cytolytic capacity of the T-cells. To exclude bias based on differing cell numbers or few contaminating NK-cells, we first purified MHC-multimer+ cells (representative dot plot ), before incubating them with or without IL12 for 48 h (IL7 and IL15 were present in all groups to ensure survival). Using a low effector-to-target ratio (2:1) we assessed induction of apoptosis, by probing for activated caspase-3, in a Melan-A+, HLA-A02:01+ melanoma cell line after 4 h incubation with the T-cells. IL12 pre-incubated Melan-A-sp. T cells clearly and significantly killed tumor cells better than the untreated T cell population (% Casp-3+ cells: 16.8 ± 7.6% vs. 57.2 ± 6.4%; 5 experiments, p < 0.01). MHC-Class I blockade using the W6/32 antibody demonstrated that the process was MHC-dependent ().
Figure 2. IL12 enhances antigen-specific cytotoxicity and retains CD62L expression. (A) Representative dot plots with gating strategy for Melan-A-MHC-multimer purified T cells. (B) MHC-multimer-purified Melan-A sp T-cells were cultivated with or without IL12 (10 ng/mL) for 48 h and then used in a caspase-3 apoptosis assay against the HLA-A02:01+ melanoma cell line FM55 (without exogenous peptides). HLA-ABC blocking antibody W6/32 (10 µg/mL) was added to the tumor cells 15 min before the T cells were added and was present during the assay. After 4 h tumor cells were stained for activated caspase-3 (for the gating strategy see ). E/T-ratio 2:1. Mean and SD from three independent experiments. (C) Melan-A sp. T cells in the proliferative phase were incubated for additional 48 h with or without IL12 and analyzed for various surface markers (gated on CD8+ MHC-multimer+ T cells as shown in ). Right panels: Summary of ten experiments, depicted as change in MFI (x-fold over isotype, mean and quartiles). For additional results see Fig. S1E. (D) Representative histograms of T-bet- and Granzyme B-expression. Filled: isotype, dashed line indicates no IL12, continuous line with IL12 treatment. Right: Summary of more than five experiments as MFI results (x-fold over isotype) are from more five experiments, including clonal cell populations (STEAP1, PRAME and clonal Melan-A cells). For all experiments in C and D: box, line and whiskers indicate: 25th to 75th percentile, median and min. to max. Significance was corrected for multiple comparisons.
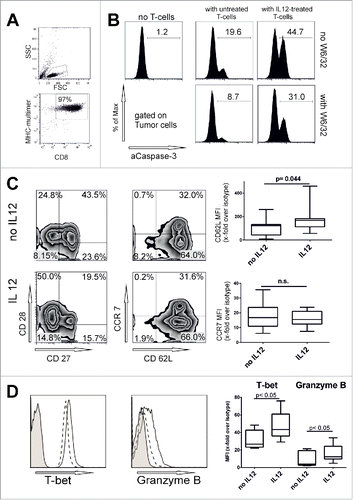
Figure 3. Interleukin 12 enables T-cell-clones for HLA-dependent and independent cytolytic activity. (A) PRAME sp. clonal T cells were stimulated with PRAME(435–443) loaded HLA-A02:01+ monocytes in increasing peptide concentration (101 to 104 ng/mL PRAME(435–443) peptide in the presence of Brefeldin A for 5 h. E/T ratio was 2:1. Cells were then stained for intracellular cytokines and response curve for interferon-γ and TNF-α were calculated. Representative of three independent experiments. (B) Gating strategy and representative dot plots of a caspase-3 assay with STEAP-1 sp. clonal T-cells against Ewing's sarcoma cell line TC71 (HLA-A02:01+). A CD8+-CD45- tumor cell gate was set and activated caspase-3 was evaluated. Where indicated, T cells were added at E/T ratio of 20:1. As positive control, tumor cells were peptide-loaded overnight (103 ng/mL) and washed thoroughly before addition of T cells. (C) Caspase-3 assay of STEAP1 or PRAME sp. clonal T cells against various tumor cell lines. T cells were or were not pre-incubated with IL12 (10 ng/mL) for 48 h. Spontaneous activation of caspase-3 for each tumor cell line is subtracted. Mean of five independent experiments.
Effector function is generally linked to the differentiation level of the cell: as we observed stronger effector functions in IL12-preincubated T-cells, we expected increased differentiation toward an effector phenotype. When using standard expansion conditions, Melan-A sp. T-cells acquire central-memory-like phenotype (CCR7+/−, CD62L+, CD27+/− and CD28+/−) (). Interestingly, CD62L and CCR7 remained expressed after IL12 incubation; even more, CD62L MFI was higher in cells incubated with IL12 (), which is in line with previous findings in murine CD4+ T-cells.Citation20 Other markers such as CD27 and CD183 (CXCR3) were gradually lost following IL12 incubation (Fig. S1E with detailed phenotypic analysis). Differences in CD62L were statistically significant (MFI x-fold over Isotype: 109 ± 71 vs. 172 ± 103 p < 0.05). For other surface markers, the level of significance could not be reached when corrected for multiple comparisons. More Granzyme B protein was expressed in IL12 pre-incubated cells (p < 0.05, ). Increased T-bet protein expression (p < 0.05, ) indicated stronger skewing toward a Tc1 phenotype.
Interleukin 12 enables T-cells for HLA-independent cytolytic activity
The Melan-A-sp. T-cells analyzed so far had been studied as a polyclonal, central-memory like T-cell population. We suspected that IL12 mediates its effect on a cellular level and wanted to exclude that IL12 leads to selection of T-cell-subpopulations with a high-affinity receptor. In addition, we wanted to exclude the possibility that the Melan-A antigen represents a biologic exception, reflected by the higher precursor frequency seen in healthy donors.Citation21 In consequence, we generated T-cell clones using limiting dilution technique against Melan-A, but also against known tumor-antigens STEAP-1(292−300(293L))Citation22 and PRAME(435–443).Citation23 Due to the prolonged culture time and repetitive stimulation, the resulting T-cell clones were now fully differentiated effector cells (CD27−, CD28−, CD62L−, CCR7−, CD45RA+; data not shown).
When T-cell clones were pre-incubated with IL12 for 48 h, the same increase in responsiveness was observed on the level of cytokine production against peptide-loaded targets resulting in an EC50 shift comparable to what had been seen in central memory like T-cells (), showing that the TCR sensitization mediated by IL12 is indeed on a single cell level and not due to changes in the clonal repertoire of polyclonal Ag-sp. T-cells and the selection of TCRs with a high affinity. Irrelevant peptide-pulsed autologous monocytes elicited again no response (data not shown). For immunotherapy purposes, however, recognition of endogenously processed and presented antigen on HLA-matched tumor cells is the key requirement to show biological meaningful activity. Many melanoma, rhabdomyosarcoma and Ewing's sarcoma cell lines express the PRAME and STEAP1 antigens, and, therefore, served as target cell lines. However, despite clear recognition of exogenous peptide, HLA-A02:01+ tumor cells previously testing positive for PRAME or STEAP1 mRNA were not recognized by the T-cell clones (representative dot plot ). This phenomenon has indeed been described for both tumor-antigens before.Citation24 Apart from the possibility of insufficient processing or presentation, this missing lytic capacity can be explained either by selection of T-cell clones with low TCR affinity or a reduced fitness of the T cells to form a functional synapse—called functional avidity. We hypothesized, that IL12 pre-incubation may particularly benefit these T-cells to restore their lytic function. Indeed, IL12 pre-incubation of T-cell clones with IL12 enhanced lysis in HLA-matched, antigen+ tumor cells, corroborating our initial hypothesis at first glance. However, HLA-A02:01 negative and/or respective antigen negative tumor cell lines included as negative controls were also lysed, sometimes even to a higher degree. This suggested that IL12 also enhanced HLA/antigen-independent lysis of tumor cells (). Blocking MHC-class I with an antibody (W6/32) did not prevent or diminish the IL12 pre-incubated T-cell clones from killing any of the tumor cell lines.
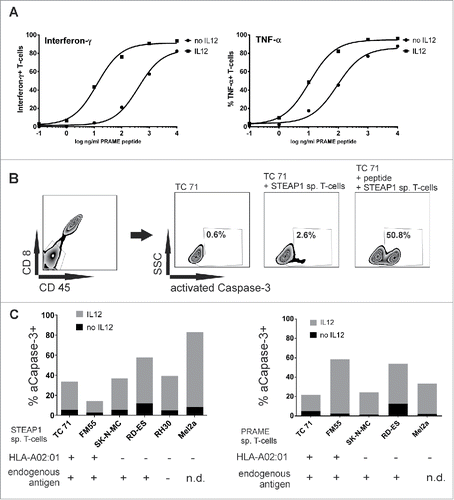
HLA-independent tumor-cell lysis is mediated by DNAM1 (CD226)
HLA-independent/antigen-independent lytic activity by T cells is generally termed “non-specific” and, apart from the Fas/FasL system, is rarely studied. In general, it is a phenomenon regarded to be multi-factorial, depending on the cell line used as a target, as well as non-specified conditions inherent in the T-cell culture. Our data suggested that this “non-specific” lytic activity was reproducibly induced by IL12, arguing for a more defined mechanism. We, therefore, chose to evaluate mechanisms for T-cell activation more closely. Experiments with MHC class I blocking antibodies had ruled out that binding of the TCR to an HLA-molecule, even if an allo-response was suspected, is the key mechanism in this response (). Nevertheless, we wanted to block potential TCR-triggering downstream of the TCR. For this purpose, we added the src-kinase inhibitor dasatinib to the killing assay. Dasatinib is known to block TCR-triggering by blockade of LCK, a key src-kinase in the TCR-signaling pathway.Citation25 Surprisingly, the HLA-independent lytic activity induced by IL12, was completely abolished by dasatinib co-incubation, as well as incubation with another src-inhibitor, src-inhibitor 1, which is a more specific src-family inhibitor (). Thus, IL12 induces an HLA-independent lytic activity whose signaling is dependent on src-kinases. This result seemingly contradicted our hypothesis, as LCK-signaling is downstream of TCR-triggering, whereas the observed lytic activity was TCR-independent. In search for an alternative lytic pathway regulated by another src kinase, we hypothesized that DNAM1 (CD226) may play a role in the observed MHC-independent lysis. DNAM1 is a receptor for nectin and nectin-like proteins and has been mostly studied in NK-cells. In these cells, DNAM1 in concert with LFA-1 acts as an activating NK-cell receptor, mediating HLA-independent lysis in targets expressing CD155 (the main ligand) or CD112. Signaling through DNAM1 is dependent on the src-kinase FYN and, therefore, would be blocked by src-kinase inhibitors as well. We then asked, whether IL12-mediated, HLA-independent lysis could be blocked by targeting DNAM1 with a DNAM1-specific inhibitory antibody (clone DX11).Citation8 Indeed, inhibiting DNAM1 specifically with the antibody, abolished HLA-independent lysis almost at the same level as was observed with src-kinase inhibitors. Additionally, blocking of NKG2D (CD314), which is another NK-cell receptor known to be present on CD8+ T cells playing a relevant role in so-called “cytokine-induced killer cells (CIKs)”Citation26 had no effect on cytotoxicity induced by IL12 (). This data suggests that IL12 mediated sensitization of CD8+ T-cell clones specifically increases DNAM1-mediated activity against tumor cells.
Figure 4. HLA-independent tumor-cell lysis is mediated by DNAM-1. (A) Caspase-3 assay against HLA-A02:01+, STEAP1+ Ewing's sarcoma cell line TC71. T-cell clones were pre-incubated with IL12 for 48 h. IL12 (10 ng/mL), Dasatinib (50 nM) or HLA-class I blocking antibody W6/32 (10 µg/mL) were present during the assay when indicated. Mean of three different experiments. (B) Caspase-3 assay in HLA-A02:01-melanoma cell line Mel2a (right), HLA-A02:01+, antigen positive Ewing's sarcoma cell line TC71 (left) with STEAP1 specific clonal T cells. E/T ratio 20:1. T cells were or were not pre-incubated for 48 h with 10 ng/mL IL12. Src-inhibitor-1 (100 nM), DNAM-1 blocking antibody DX11 (10 µg/mL) and NKG2D blocking antibody 1D11 (10 µg/mL) was added were indicated. Results from five independent experiments. (C) Purified Melan-A sp. T cells were pre-incubated for 48 h with or without 10 ng/mL IL12. Mel2a melanoma cells were used as targets. As indicated, dasatinib (50 nM), or various blocking antibodies (10 µg/mL) were added throughout the caspase-3 assay(E/T ratio was 2:1). (D) Summary of similar experiments as in (C) with different tumor cell lines Results are from eight different experiments, 2/8 with clonal Melan-A population (two different clones).
Next, we re-focused on our initial model using early, central memory like polyclonal Melan-A-sp. T cells as central memory and effector memory T-cells differ in their effector functions. In addition, we wanted to exclude that the observed DNAM1 mediated killing pathway is an artifact due to long-term in vitro culture. We had clearly seen a sensitizing effect of IL12 on the TCR-mediated antigen-specific lysis of these T cells, but no unspecific activation when autologous monocytes were loaded with irrelevant antigen. Nevertheless, we observed HLA-independent lysis even at E/T ratios as low as 2:1 against selected tumor cell lines (). Blocking of src-kinases through dasatinib abolished the antigen-independent lysis while blocking MHC-I molecules through W6/32 had again no effect ().
The main known ligand for DNAM1 is the poliovirus receptor (CD155) and, with a possible restriction to humans, CD112, the poliovirus-receptor related two protein (PVRL2), albeit with a lower binding affinity.Citation27,28 Blockade of these ligands on tumor targets using monoclonal antibodies blocked the increase in lysis, whereas blockade of another nectin-binding receptor (CD96) had no effect (). The maximal blocking effect was achieved by using the blocking antibody against CD155, whereas blocking CD112 alone achieved inconsistent results (Fig. S2). Biologically, this is fitting, as CD155 is known as the main receptor for DNAM1 in human cells.Citation27 However, cautious interpretation of the results is required, as differences may also arise from the limited efficacy of the available blocking antibodies as has been described previously.Citation29
We next evaluated several tumor cell lines for expression of the ligands for nectin-like proteins CD155 and CD112. Considerable variation in the expression of these molecules exists (). Most importantly, we found that the HLA-A02:01 negative melanoma cell line Mel624 lacked CD155 and CD112 expression. When using this tumor cell line as a target, we observed no antigen-independent activity of IL12 pre-incubated T-cells (early central memory like Melan-A sp. T cells or Melan-A sp. T-cell clones)(), further corroborating the hypothesis, that IL12 sensitizes T-cells to increased DNAM1 activity.
Table 1. CD112 and CD115 expression of various tumor cell lines.
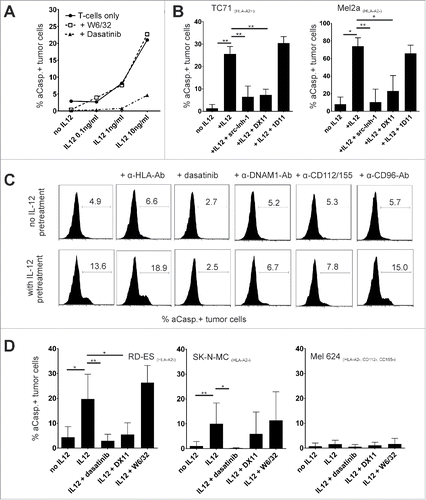
IL12 enabled DNAM1 mediated cytotoxicity is dependent on CD45 phosphatase activity
DNAM1 is constitutively expressed on the majority of T cellsCitation8 while relatively little is known about its regulation. Evaluation of DNAM1 expression on IL12 pre-incubated T cells showed a significant, albeit moderate upregulation (∼1.25 fold) of DNAM1 () while other receptors known to be part of HLA-independent cytotoxicity as NKG2D and Fas/FasL were unaltered (Fig. S1E).
Figure 5. IL12 enabled DNAM-1 mediated cytotoxicity is dependent on CD45 phosphatase activity. (A) Left: Change in DNAM-1 MFI mediated by IL12. Summary from different T-cell populations (early central memory T cells (Melan A), effector memory T cells (clonal STEAP1-sp. and PRAME-sp. Populations)). Results are from more than 10 experiments. Right: representative histogram of DNAM-1 on Melan-A sp. T cells (d + 10) with (empty) and without (tinted) IL12 pre-incubation. Dark-gray: isotype control. (B) Left: change of MFI over isotype in CD45 and subtype expression in STEAP1-sp. clonal populations incubated or not with 10 ng/mL IL12. Right: representative histograms, dark gray is isotype control, light gray no IL12 and black line IL12 incubation. (C) STEAP1 sp. clonal T cells were or were not pre-incubated with IL12 and tested against TC71 Ewing's sarcoma cell line. Reagents were present during assay as indicated (dosages as in 4(B), CD45-inhibitor 3.5 µM) after a pre-incubation time of 90 min on the T cells. Results are from three different experiments, spontaneous apoptosis is subtracted. E/T-ratio 20:1.
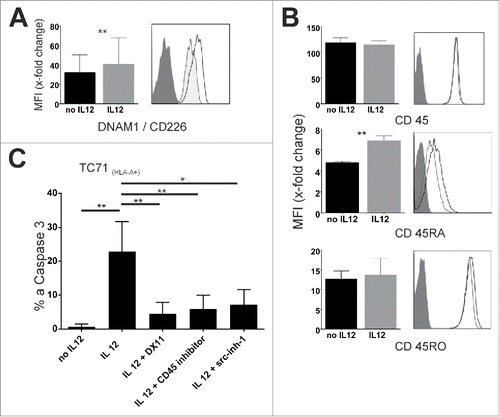
To better understand the underlying mechanism we generated global gene expression profiles of clonal tumor-antigen specific effector T cells (STEAP1 and PRAME) incubated or not incubated with 10 ng/mL IL12 for 48 h. IL12 incubation caused significant changes in various gene sets. For example IL12 incubated cells showed significant changes in gene expression relevant metabolic activation. Genes necessary for inflammatory-, IL2-, TNF-α- and interferon-γ-responses showed (expectable) significant changes, too. A more detailed overview of pathways influenced by IL12 signaling is given in Fig. S3. However, we searched for changes in gene expression influencing src-kinase family activity. Src-family kinases possess several phosphorylation sites, some inhibiting and some promoting their kinase activity.Citation30 When analyzing possible kinases or phosphatases relevant for changes in src-family activity, only mRNA of protein tyrosine phosphatase, receptor type C (PTPRC; CD45) was upregulated after a period of 48 h (.). CD45 regulates TCR- and BCR-signaling mainly through dephosphorylating LCK (TCR) and Lyn (BCR) and is expressed in high density on all lymphocytes.Citation30 Its role in modulation of immune cell function has been extensively described.Citation31,32 Interestingly, CD45 is expressed in several isoforms depending on the splicing of the transcripts and the different expression of the isoforms is also used to classify T-cell subsets. Here, CD45RA is a marker for naïve, antigen inexperienced T-cells as well as for terminally differentiated, antigen-experienced effector T-cells.Citation31,33 We analyzed total CD45 and isoform expression on protein level via surface staining of IL12 incubated T-cells. No significant change in overall protein expression of CD45 or CD45RO occurred; while CD45RA was slightly (∼1.4-fold), but significantly upregulated (). This might be indicative of further cellular differentiation as CD45RA is re-expressed in terminally differentiated effector cells. However, we also wanted to test, whether CD45 affected DNAM1 mediated cytotoxicity functionally. Indeed, when using a specific CD45-phosphatase inhibitor, it completely abolished DNAM1 mediated cytotoxicity observed in IL12 pre-incubated T-cells (). Thus, IL12 incubation leads to increased DNAM1 mediated cytotoxicity, which is dependent on src-kinase activity, most likely FYN, and also depends on CD45 phosphatase activity.
Table 2. Change in gene expression relevant for src-family kinases through IL12 incubation.
Discussion
Exploiting T cells to target cancerous cells has become infinitely more tangible due to recent advances in cellular and antibody engineering.Citation34 While more and more patients benefit from these new therapies, the search to broaden the spectrum of potential targets and the concern for possible detrimental side effects due to non-specific activity are key topics in the field.
In this context, we examined the impact of inflammatory cytokines on human, tumor-antigen-specific memory T-cells during the expansion phase following the initial priming. We found that IL12 distinctively influences the antigen-specific lytic capacity of memory/effector cells by enhancing the TCR-sensitivity about one log fold. Previous work from murine models has shown that “inflammation” improves functional TCR avidity, pointing to a feed-back loop between an inflammatory environment/innate immunity and T-cell function.Citation4,5 Our data thus confirm these findings; but despite testing a broad range of pro-inflammatory cytokines and their combinations, IL12 was the only one leading to increased TCR-sensitivity in human T cells. Of note, this heightened TCR-mediated sensitivity was observed despite the retained CD62L expression, at least in T-cells cultured for a limited period only. This contrasts results reported by Yang et al., suggesting a link between CD62L shedding and the acquisition of (lytic) effector functions.Citation35 Additional functional studies will be necessary to mechanistically link CD62L-shedding to TCR-mediated lytic activity.
IL12 has long been recognized as an “endogenous adjuvant” to boost T-cell responses and promising pre-clinical data suggested to use this cytokine for cancer therapy.Citation36-38 However, clinical trials showed only modest efficacy, but severe side effects,Citation37,39 which led to abandoning the systemic administration of IL12 in patients.
Still, at least two strategies for using IL12 are pursued furtherCitation38,39: pre-incubation of T-cells with IL12 prior to adoptive transfer might enhance efficacy, especially when effects are mediated via the TCR. However, in our study, the effects of pre-incubation were fading after few days. Genetic engineering of those T-cells with prolonged or durable IL12 signaling would, therefore, be more effective.Citation40,41 Secondly, providing IL12 locally at the tumor site would reduce systemic toxicity to some extent.Citation42-44 In addition to the effects mediated by immune cells, a direct differentiating, senescence-inducing effect on certain cancer types has been described recently.Citation45 However, our results also show that enhanced TCR-reactivity is not the only mechanism by which IL12 exerts its tumor-cytotoxic effects. We identified a second, receptor-mediated killing pathway via DNAM1. In this context, it is noteworthy that one of the dominant mechanisms by which NK-cells exert their killing of the tumors used in this study is DNAM1-mediated; indicating that theses tumor entities are very sensitive toward DNAM1-mediated cytotoxicity.Citation10,11 However, with regard to the safety of cellular therapeutics additional activated pathways of cytotoxicity are no minor issues: DNAM1 ligands are expressed in healthy cells as well, and whether unwanted cell damage in healthy tissue may occur through exacerbated IL12 signaling of those T-cells requires cautious evaluation.
TCR-independent/MHC-independent activity of T-cells is usually regarded as “non-specific” and inherent to ill-defined variables of in vitro cultures and the use of tumor cell lines. We here discovered a second IL12-mediated mechanism, which greatly increased such “non-specific” cytotoxicity. However, this activity is based specifically on heightened DNAM1 mediated cytotoxicity, moving a defined “innate mechanism of defense” into the focus of our research on antigen-specific T-cells. It is known that DNAM1 signals through the src-kinase FYN, which led us to identify this pathway in the first place. mRNA and protein expression; however, pointed toward a functional gain, as DNAM1 surface protein expression was only slightly enhanced. Data from microarray analyzes showed selective upregulation of PTPRC(CD45). Given the key role of phosphatases in regulating src-kinases, we suspected a link between CD45 and DNAM1 activity. Thus, we functionally describe—for the first time—a DNAM1-mediated, IL12-induced mechanism of HLA-independent cytotoxicity which is dependent on CD45 activity. CD45 has been shown to be regulated on the cell surface with regard to its proximity to the TCR-synapse; after synapse initiation it is first transferred out of the central region into the periphery, and later on transferred back into the central part of the synapse to sustain TCR signaling.Citation32 In addition, IL12 may play a role in regulating the membrane compartmentation including CD45 accessibility.Citation46 Therefore, it seems plausible for IL12 to increase CD45 density near the TCR synapse, leading to a higher TCR signal and increased TCR sensitivity. Higher CD45 accessibility might also influence FYN and, therefore, DNAM1 signaling, leading to an additional receptor-mediated killing pathway. Further studies are needed to test our hypothesis that higher CD45 accessibility through IL12 boosts DNAM1 signaling via FYN, even if no TCR signal is present.
Our findings show similarities but also clear differences to what is known about CIKs, which are induced by very high doses of IL2, IL15 and interferon-γ in combination with OKT3 stimulation. Tumor cell killing by such cells is mediated predominantly via NKG2D,Citation47 which was not the case in our setting. However, a role for DNAM1 has also been reported in CIK cells.Citation26 Interestingly IL12 administration in vivo had synergistic effects with CIK cell therapy in murine models.Citation48 Thus, the evaluation of IL12 for current CIK protocols, especially if expanded with IL15, may be worthwhile.
Different hypotheses regarding the biological relevance can be derived from this data: in inflamed tissue, that is, in the presence of IL12, low avidity CD8+ T cells may contribute to the clearance of the pathogen, be it a viral infection or a malignant transformed cell, in a dual way: if cognate antigen is present, TCR-sensitization will be useful for HLA-dependent activation, if CD155/CD112 are expressed as ligands for DNAM1, IL12 will enhance the HLA-independent, DNAM1 mediated defense mechanism. Fitting, it is known, that these ligands for DNAM1 are upregulated in infected and/or malignant cells as stress signals, i.e. DNA damage.Citation9,27,49 In addition, it has been previously shown that tissue-specific infection results in nonspecific recruitment of memory T cells to inflamed sites.Citation5,50 Our results substantiate that such “useless” T cells in terms of their TCRs can play a relevant role in pathogen clearance not only as bystander cytokine producers: interferon-γ production of those T cells is therefore not only induced by the combination of inflammatory cytokines (such as IL12 and IL18/IL33 together) but may also be signaled through DNAM1 mediated activation.
In summary, this work describes a dual effect of IL12 on human CD8+ T-cells influencing both, antigen-dependent and -independent T-cell activation. We show that IL12 enhances TCR sensitivity on the one hand, and also enables a receptor-mediated killing pathway executed by DNAM1 which is dependent on src-kinase-family- and CD45-activity.
Material and methods
Cells
Peripheral blood mononuclear cells (PBMC) were obtained from leukapheresis products from healthy donors and stored in liquid nitrogen (consent and collection guidelines were in accordance with institutional regulations). The melanoma cell lines FM55, Mel397, T6217, Mel624 and Mel2a were kind gifts of Dr J. Becker, University of Würzburg. TC71 (Ewing's Sarcoma) was purchased from the German Collection of Cell Cultures (DSMZ, Braunschweig, Germany). Ewing's sarcoma cell lines RD-ES and rhabdomyosarcoma cell line RH30 were kind gifts of Dr K. Schilbach, University of Tübingen. Ewing's sarcoma cell lines SK-N-MC and SK-ES-1 were kind gifts of Dr C. Rössig, University of Münster.
Reagents, media, cytokines and antibodies
T cells and DCs were cultured in Cellgenix DC medium, supplemented with human AB serum (5%, PAA or Biochrome), 25 IU/mL Penicillin, 25 IU/mL Streptomycin (PAA). Tumor cells were maintained in culture with RPMI 1640 medium (Biochrome) containing 10% FCS (Gibco) and 25 IU/mL Penicillin + 25 IU/mL Streptomycin. The cytokines IL1b, IL2, IL4, IL7, IL12, IL18, IL23, IL15, IL21, IL23, IL27, IL33, TNF-α, interferon-α and interferon-γ were purchased from Peprotech. Granulocyte-macrophage colony-stimulating factor (GM-CSF) was purchased from Gentaur. LPS (E. coli O:15) was purchased from Sigma-Aldrich. Peptides were supplied by JPT Peptide Technologies. Src-Inhibitor-1 was purchased from Sigma (cat. no. S2075), dasatinib was purchased from Sellekchem (cat. no. S1021). CD45-inhibitor was purchased from Merck Chemicals (cat. no. 540215). Antibodies used for flow cytometry: CD28-PerCPCy5.5 (clone CD28.2), CD62L-FITC (Dreg56), CD95-FITC (DX2), CD95L-PE (NOK-1), CD184-PE (12G5), CD195-PE (T218), Perforin-FITC (dG9), T-bet-PerCPCy5.5 (4B10), PD1-FITC (MIH4) from eBioscience, CD8+-PB (HIT8a), CD8+-FITC (RPA-T8), CD8+-PerCPCy5.5 (HIT8a), CD27-FITC (0323), CD45-APC (2D1), CD45RO-PE (UCHL1), CD45RA-PE (HI100), CD96-PE (NK92.36), CD112-PE (TX31), CD155-PE (SKIL4), CD183-PerCPCy5.5 (G025H7), CD194-PerCPCy5.5 (TG6), CD195-FITC (HEK/1/85a), CD226-FITC (TX25), CCR7-BV421 (G043H7), Granzyme B-PE (GB11), TIGIT-PE (MBSA43) from BioLegend, CD14-PE (MP9) and CD 274-FITC (MH1) from BD. CD314-PE (BAT221) antibodies were from Miltenyi. HLA-A02:01-restricted APC-MHC-multimers for Melan-A(26–35(27L)), STEAP1(292–300(293L)) and PRAME(435–443) were obtained from Immudex. MHC-multimer staining was performed at room temperature (20 min) with 100 nM dasatinib added for stabilized staining.
Cell isolation
Isolation of monocytes, CD8+ and CD8+ naïve T cells was performed using isolation kits from Miltenyi according to the manufacturer's instructions. Anti-APC-beads were also purchased from Miltenyi and used according to the manufacturer's instructions, detailed description appears elsewhere.Citation15
Priming protocol
The detailed priming protocol appears elsewhere.Citation15 Briefly, DCs were generated from plastic-adherent monocytes. After 72 h of culture in GM-CSF/IL4-containing Cellgenix DC medium, DCs were matured in medium containing 100 ng/mL IL4, 800 IU/mL GM-CSF, 10 ng/mL LPS, and 100 U/mL interferon-γ plus peptide (Melan-A(26–35(27L)): ELAGIGILTV; STEAP1(292–300(293L)): MLAVFLPIV; PRAME(435–443): NLTHVLYPV) respectively at 2.5 μg/mL. After 16 h, DCs were irradiated (30 Gy), washed and co-incubated with CD45RO-, CD57- naïve CD8+ T cells at a 1:4 ratio in medium containing 5% AB serum and 30 ng/mL IL21. Fresh medium, IL7 and IL15 were added on days +3, +5 and +7 of culture. In some experiments, feeding of d +7 was performed on d +8 and additional cytokine were added.
Generation of T-cell clones
T-cell clones were generated using limiting dilution techniqueCitation51: 0.3/well MHC-multimer-enriched T cells were seed on 96 well plates and cultured with CellGro Cellgenix DC medium, 5% human serum, 25 IU IL2 and 5 ng/mL IL15 and 30 ng/mL soluble OKT3 (OrthocloneTM, Janssen-Cilag) together with 2 × 105 feeder cells/well (PBMCs from three different, HLA-miss matched donors, donor ratios 1:1:1) and cultivated for 14 d. After 14 d plates were screened for Ag-sp. T-cell clones by MHC-multimer-staining and then re-stimulated using irradiated feeder cells with OKT3. Cells were then used for experiments or cryo-conserved. When thawed, cells were re-stimulated on irradiated feeder cells with OKT3; experiments were done at least 10 d after re-stimulation, T-cell clones were cultured in CellGro Medium containing 5% human serum, 25 IU/mL IL2 and 5 ng/mL IL15. For experiments, cells were least >97% CD8+; otherwise, T cells were additionally purified using CD8+ Micro-Beads (Miltenyi) according to manufacturer's protocol.
Cell counting
Cell counting was performed with trypan blue using a Neubauer chamber or alternatively with an automated cell counter (Countess, Invitrogen).
Intracellular cytokine staining
Staining was performed after an incubation period under stimulating conditions of 5 h in an effector/target ratio of 2:1 against autologous CD14+ monocytes. Brefeldin A (BioLegend) was added at the beginning of the stimulation period. Surface staining against CD8+ was performed first, followed by fixation and permeabilization (Fix+Perm, BD, Heidelberg). Staining was then carried out using the following antibodies: TNF-α-PerCPCy5.5 (clone Mab11, BioLegend); and interferon-γ-APC (4S.B3, BioLegend). When indicated, several control peptides were used (all purchased from JPT Peptide Technologies): gp100(25–33), Her2neu(369–377), IL13Ra(345–354(354L), MUC(950–958), pp65(485–493), Survivin(96–104(97M)), CYP1B1(239–247), Aurora(207–215).
Activated caspase-3 assay
Detailed assay has been described elsewhere.Citation15 Briefly, tumor cells were plated at 5 × 104/well in a 96-well plate, for positive controls appropriate peptide was added at 5 µg/mL. Ewing's sarcoma and rhabdomyosarcoma tumor cell lines were pretreated with 100 IU/mL interferon-γ for >24 h prior to the assay to upregulate MHC-class I molecules in experiments where TCR-engagement was expected. After overnight incubation at 37°C, tumor cells were washed once with PBS/HS5% and T cells at indicated E/T ratios were added and cultured for 4 h at 37°C. Adherent tumor cell lines were then incubated with Accutase (PAA) for 5–10 min at 37°C. Surface staining with CD45 (clone HI30, BioLegend) and CD8+ (clone HIT8a, BioLegend) was done and followed by fixation and permeabilization (Fix+Perm, BD). Intracellular caspase-3 was then stained with PE-labeled rabbit anti-active caspase-3 (BD) for 30 min at room temperature. DNAM1 blocking antibody was purchased from BD (clone DX11, 10 µg/mL). Blocking antibodies against the following antigens were purchased from Biolegend (standard concentration 10 µg/mL): HLA-ABC (clone W6/32), CD112 (cloneTx31), CD155 (clone SKII4), CD96 (clone 92.39), NKG2D (clone 1D11) was a gift from J. Wischhusen, University of Würzburg).
mRNA microarray
CD8+ tumor-antigen-specific T-cell clones (PRAME and STEAP1; Dextramer+ >97%) were or were not pre-incubated with 10 ng/mL IL12 for 48 h. Total RNA from T-cell clones was isolated using the mirVana miRNA isolation kit (Thermo Fisher) according to the manufacturer's protocol. RNA integrity numbers (RIN, 1 = worst, 10 = best) for total RNA samples were determined by a Bioanalyzer using the RNA 6000 Nano kit (Agilent Technologies), these consistently ranged between 9.4 and 9.7. Of each sample, 100 ng total RNA were used for single-step in vitro transcription, chemical fragmentation and biotin labeling using the IVT PLUS kit (Affymetrix) following the manufacturer's recommendations. Samples were hybridized to GeneChip Human PrimeView microarrays (Affymetrix), cRNA binding to complementary probes was quantified with streptavidin-phycoerythrin conjugate and a GeneChip Scanner 3000 7G (Affymetrix). Raw microarray readout was obtained with the GeneChip Command Console (Affymetrix) and further processed in the statistical language environment R version 3.2.1 along with Bioconductor packages “affy” for data import, “vsn” for normalization, “made4” for explorative data analysis and visualization and “limma” for the detection of differentially expressed genes (requiring a false discovery rate (FDR) < 0.05 and a log2 fold change (logFC) ≥ 1 or logFC ≤ −1). Global-scale functional categorization of expression changes was performed with Gene Set Enrichment Analysis. Pathway mapping and visualization of differentially expressed genes was performed with the Bioconductor package “pathview” . Microarray data generated for the present study has been deposited at Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) in entry GSE75720.
Software and statistics
FlowJo 7.6.5 software was used for flow cytometry data. Graph Pad Prism 6 software was used for statistical analysis. Significance was calculated using paired student's t-test, error bars indicate standard deviation. Correction for multiple comparisons was done using Bonferroni–Holm method.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contribution
MB and MW wrote the manuscript, MB, MLR, YEY, MW performed and analyzed the experiments, CJS generated and analyzed microarray data, ME and PGS helped writing the manuscript.
KONI_A_1188245_s02.zip
Download Zip (4.6 MB)Funding
This study was supported and funded by the Else Kröner-Fresenius Stiftung, by the Interdisziplinäres Zentrum für Klinische Forschung (IZKF) Würzburg, by the parent initiative “Aktion Regenbogen für leukämie- und tumorkranke Kinder Main-Tauber e.V.” and by the Vogel Stiftung Dr. Eckernkamp, Würzburg as well as by the German José Carreras Leukaemia Foundation (R14/09).
References
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013; 342:1432-3; PMID:24357284; http://dx.doi.org/10.1126/science.342.6165.1432
- Teachey DT, Rheingold SR, Maude SL, Zugmaier G, Barrett DM, Seif AE, Nichols KE, Suppa EK, Kalos M, Berg RA et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 2013; 121:5154-7; PMID:23678006; http://dx.doi.org/10.1182/blood-2013-02-485623
- Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J 2014; 20:119-22; PMID:24667956; http://dx.doi.org/10.1097/PPO.0000000000000035
- Slifka MK, Whitton JL. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat Immunolgy 2001; 2:711-7; PMID:11477407; http://dx.doi.org/10.1038/90650
- Richer MJ, Nolz JC, Harty JT. Pathogen-specific inflammatory milieux tune the antigen sensitivity of CD8(+) T cells by enhancing T cell receptor signaling. Immunity 2013; 38:140-52; PMID:23260194; http://dx.doi.org/10.1016/j.immuni.2012.09.017
- Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL12 or type I IFN. J Immunol 2009; 182:2786-94; PMID:19234173; http://dx.doi.org/10.4049/jimmunol.0803484
- Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol 1999; 162:3256-62; PMID:10092777
- Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T, Kitamura T, Nicholl J, Sutherland GR, Lanier LL et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity 1996; 4:573-81; PMID:8673704; http://dx.doi.org/10.1016/S1074-7613(00)70060-4
- de Andrade LF, Smyth MJ, Martinet L. DNAM-1 control of natural killer cells functions through nectin and nectin-like proteins. Immunol Cell Biol 2014; 92:237-44; PMID:24343663; http://dx.doi.org/10.1038/icb.2013.95
- Verhoeven DHJ, de Hooge ASK, Mooiman ECK, Santos SJ, ten Dam MM, Gelderblom H, Melief CJ, Hogendoorn PC, Egeler RM, van Tol MJ et al. NK cells recognize and lyse Ewing sarcoma cells through NKG2D and DNAM-1 receptor dependent pathways. Mol Immunol 2008; 45:3917-25; PMID:18657862; http://dx.doi.org/10.1016/j.molimm.2008.06.016
- Lakshmikanth T, Burke S, Ali TH, Kimpfler S, Ursini F, Ruggeri L, Capanni M, Umansky V, Paschen A, Sucker A et al. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest 2009; 119:1251-63; PMID:19349689; http://dx.doi.org/10.1172/JCI36022
- Wu MR, Zhang T, Alcon A, Sentman CL. DNAM-1-based chimeric antigen receptors enhance T cell effector function and exhibit in vivo efficacy against melanoma. Cancer Immunol Immunother 2015; 64:409-18; PMID:25549845; http://dx.doi.org/10.1007/s00262-014-1648-2
- Shibuya K, Lanier LL, Phillips JH, Ochs HD, Shimizu K, Nakayama E, Nakauchi H, Shibuya A. Physical and functional association of LFA-1 with DNAM-1 adhesion molecule. Immunity 1999; 11:615-23; PMID:10591186; http://dx.doi.org/10.1016/S1074-7613(00)80136-3
- Wölfl M, Merker K, Morbach H, Van Gool SW, Eyrich M, Greenberg PD, Schlegel PG. Primed tumor-reactive multifunctional CD62L+ human CD8+ T cells for immunotherapy. Cancer Immunol Immunother 2011; 60:173-86; PMID:20972785; http://dx.doi.org/10.1007/s00262-010-0928-8
- Wölfl M, Greenberg PD. Antigen-specific activation and cytokine-facilitated expansion of naive, human CD8+ T cells. Nat Protoc 2014; 9:950-66; PMID:24675735; http://dx.doi.org/10.1038/nprot.2014.064
- Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol 2010; 22:333-40; PMID:20363604; http://dx.doi.org/10.1016/j.coi.2010.02.013
- Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol 2005; 174:4465-9; PMID:15814665; http://dx.doi.org/10.4049/jimmunol.174.8.4465
- Freeman BE, Hammarlund E, Raué H-P, Slifka MK. Regulation of innate CD8+ T-cell activation mediated by cytokines. Proc Natl Acad Sci U S A 2012; 109:9971-6; PMID:22665806; http://dx.doi.org/10.1073/pnas.1203543109
- Beadling C, Slifka MK. Differential regulation of virus-specific T-cell effector functions following activation by peptide or innate cytokines. Blood 2005; 105:1179-86; PMID:15471952; http://dx.doi.org/10.1182/blood-2004-07-2833
- van Wely CA, Beverley PC, Brett SJ, Britten CJ, Tite JP. Expression of L-selectin on Th1 cells is regulated by IL12. J Immunol 1999; 163:1214-21; PMID:10415016
- Romero P, Speiser DE, Rufer N. Deciphering the unusual HLA-A2/Melan-A/MART-1-specific TCR repertoire in humans. Eur J Immunol 2014; 44:2567-70; PMID:25154881; http://dx.doi.org/10.1002/eji.201445004
- Rodeberg DA, Nuss RA, Elsawa SF, Celis E. Recognition of six-transmembrane epithelial antigen of the prostate-expressing tumor cells by peptide antigen-induced cytotoxic T lymphocytes. Clin Cancer Res 2005; 11:4545-52; PMID:15958640; http://dx.doi.org/10.1158/1078-0432.CCR-04-2235
- Quintarelli C, Dotti G, Hasan ST, Angelis Bd, Hoyos V, Errichiello S, Mims M, Luciano L, Shafer J, Leen AM et al. High-avidity cytotoxic T lymphocytes specific for a new PRAME-derived peptide can target leukemic and leukemic-precursor cells. Blood 2011; 117:3353-62; PMID:21278353; http://dx.doi.org/10.1182/blood-2010-08-300376
- Altvater B, Pscherer S, Landmeier S, Kailayangiri S, Savoldo B, Juergens H, Rossig C. Activated human γδ T cells induce peptide-specific CD8+ T-cell responses to tumor-associated self-antigens. Cancer Immunol Immunother 2012; 61:385-96; PMID:21928126; http://dx.doi.org/10.1007/s00262-011-1111-6
- Blake S, Hughes TP, Mayrhofer G, Lyons AB. The Src/ABL kinase inhibitor dasatinib (BMS-354825) inhibits function of normal human T-lymphocytes in vitro. Clin Immunol 2008; 127:330-9; PMID:18395492; http://dx.doi.org/10.1016/j.clim.2008.02.006
- Pievani A, Borleri G, Pende D, Moretta L, Rambaldi A, Golay J, Introna M. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood 2011; 118:3301-10; PMID:21821703; http://dx.doi.org/10.1182/blood-2011-02-336321
- Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med 2003; 198:557-67; PMID:12913096; http://dx.doi.org/10.1084/jem.20030788
- Martinet L, Smyth MJ. Balancing natural killer cell activation through paired receptors. Nat Rev Immunol 2015; 15:243-54; PMID:25743219; http://dx.doi.org/10.1038/nri3799
- Tahara-Hanaoka S, Shibuya K, Onoda Y, Zhang H, Yamazaki S, Miyamoto A, Honda S, Lanier LL, Shibuya A. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112). Int Immunol 2004; 16:533-8; PMID:15039383; http://dx.doi.org/10.1093/intimm/dxh059
- Roskoski R. Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun 2005; 331:1-14; PMID:15845350; http://dx.doi.org/10.1016/j.bbrc.2005.03.012
- Saunders AE, Johnson P. Modulation of immune cell signalling by the leukocyte common tyrosine phosphatase, CD45. Cell Signal 2010; 22:339-48; PMID:19861160; http://dx.doi.org/10.1016/j.cellsig.2009.10.003
- Johnson KG, Bromley SK, Dustin ML, Thomas ML. A supramolecular basis for CD45 tyrosine phosphatase regulation in sustained T cell activation. Proc Natl Acad Sci U S A 2000; 97:10138-43; PMID:10963676; http://dx.doi.org/10.1073/pnas.97.18.10138
- Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999; 401:708-12; PMID:10537110; http://dx.doi.org/10.1038/44385
- Stromnes IM, Schmitt TM, Chapuis AG, Hingorani SR, Greenberg PD. Re-adapting T cells for cancer therapy: from mouse models to clinical trials. Immunol Rev 2014; 257:145-64; PMID:24329795; http://dx.doi.org/10.1111/imr.12141
- Yang S, Liu F, Wang QJ, Rosenberg SA, Morgan RA. The shedding of CD62L (L-selectin) regulates the acquisition of lytic activity in human tumor reactive T lymphocytes. PloS One 2011; 6:e22560; PMID:21829468; http://dx.doi.org/10.1371/journal.pone.0022560
- Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev 2002; 13:155-68; PMID:11900991; http://dx.doi.org/10.1016/S1359-6101(01)00032-6
- Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, Anichini A. Interleukin-12: biological properties and clinical application. Clin Cancer Res 2007; 13:4677-85; PMID:17699845; http://dx.doi.org/10.1158/1078-0432.CCR-07-0776
- Devaud C, John LB, Westwood JA, Darcy PK, Kershaw MH. Immune modulation of the tumor microenvironment for enhancing cancer immunotherapy. Oncoimmunology 2013; 2:e25961; PMID:24083084; http://dx.doi.org/10.4161/onci.25961
- Lasek W, Zagożdżon R, Jakobisiak M. Interleukin 12: still a promising candidate for tumor immunotherapy? Cancer Immunol Immunother 2014; 63:419-35; PMID:24514955; http://dx.doi.org/10.1007/s00262-014-1523-1
- Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, Brentjens RJ. Tumor-targeted T cells modified to secrete IL12 eradicate systemic tumors without need for prior conditioning. Blood 2012; 119:4133-41; PMID:22354001; http://dx.doi.org/10.1182/blood-2011-12-400044
- Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ. IL12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology 2015; 4:e994446; PMID:25949921; http://dx.doi.org/10.4161/2162402X.2014.994446
- Rubinstein MP, Su EW, Suriano S, Cloud CA, Andrijauskaite K, Kesarwani P, Schwartz KM, Williams KM, Johnson CB, Li M et al. Interleukin-12 enhances the function and anti-tumor activity in murine and human CD8(+) T cells. Cancer Immunol Immunother 2015; 64(5):539-49; PMID:25676709; http://dx.doi.org/10.1007/s00262-015-1655-y
- Weinstein-Marom H, Pato A, Levin N, Susid K, Itzhaki O, Besser MJ, Peretz T, Margalit A, Lotem M, Gross G. Membrane-attached Cytokines Expressed by mRNA Electroporation Act as Potent T-Cell Adjuvants. J Immunother 2016; 39:60-70; PMID:26849075; http://dx.doi.org/10.1097/CJI.0000000000000109
- Yang S, Ji Y, Gattinoni L, Zhang L, Yu Z, Restifo NP, Rosenberg SA, Morgan RA. Modulating the differentiation status of ex vivo-cultured anti-tumor T cells using cytokine cocktails. Cancer Immunol Immunother 2013; 62:727-36; PMID:23207483; http://dx.doi.org/10.1007/s00262-012-1378-2
- Schilbach K, Alkhaled M, Welker C, Eckert F, Blank G, Ziegler H, Sterk M, Müller F, Sonntag K, Wieder T et al. Cancer-targeted IL12 controls human rhabdomyosarcoma by senescence induction and myogenic differentiation. Oncoimmunology 2015; 4:e1014760; PMID:26140238; http://dx.doi.org/10.1080/2162402X.2015.1014760
- Salgado FJ, Lojo J, Alonso-Lebrero JL, Lluis C, Franco R, Cordero OJ, Nogueira M. A role for interleukin-12 in the regulation of T cell plasma membrane compartmentation. J Biol Chem 2003; 278:24849-57; PMID:12676959; http://dx.doi.org/10.1074/jbc.M212978200
- Geldern Mv, Simm B, Braun M, Weiss EH, Schendel DJ, Falk CS. TCR-independent cytokine stimulation induces non-MHC-restricted T cell activity and is negatively regulated by HLA class I. Eur J Immunol 2006; 36:2347-58; PMID:16909431; http://dx.doi.org/10.1002/eji.200535387
- Helms MW, Prescher JA, Cao Y-A, Schaffert S, Contag CH. IL12 enhances efficacy and shortens enrichment time in cytokine-induced killer cell immunotherapy. Cancer immunol Immunother 2010; 59:1325-34; PMID:20532883; http://dx.doi.org/10.1007/s00262-010-0860-y
- Cerboni C, Fionda C, Soriani A, Zingoni A, Doria M, Cippitelli M, Santoni A. The DNA Damage Response: A Common Pathway in the Regulation of NKG2D and DNAM-1 Ligand Expression in Normal, Infected, and Cancer Cells. Front Immunol 2014; 4:508; PMID:24432022; http://dx.doi.org/10.3389/fimmu.2013.00508
- Ely KH, Cauley LS, Roberts AD, Brennan JW, Cookenham T, Woodland DL. Nonspecific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J Immunol 2003; 170:1423-9; PMID:12538703; http://dx.doi.org/10.4049/jimmunol.170.3.1423
- Ho WY, Nguyen HN, Wolfl M, Kuball J, Greenberg PD. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naïve repertoire. J Immunol Methods 2006; 310:40-52; PMID:16469329; http://dx.doi.org/10.1016/j.jim.2005.11.023
