ABSTRACT
Recognition of tumor cells by the immune system is a key step in cancer eradication. Melphalan is an alkylating agent routinely used in the treatment of patients with multiple myeloma (MM), but at therapeutic doses it leads to an immunosuppressive state due to lymphopenia. Here, we used a mouse model of MM to investigate the ability of in vivo treatment with low doses of melphalan to modulate natural killer (NK) cell activity, which have been shown to play a major role in the control of MM growth. Melphalan treatment was able to enhance the surface expression of the stress-induced NKG2D ligands RAE-1 and MULT-1, and of the DNAM-1 ligand PVR (CD155) on MM cells, leading to better tumor cell recognition and killing by NK cells, as highlighted by NK cell increased degranulation triggered by melphalan-treated tumor cells. Remarkably, NK cell population was not affected by the melphalan dose used, but rather displayed activation features as indicated by CD107a and CD69 expression. Furthermore, we showed that low doses of melphalan fail to induce tumor cell apoptosis, but promote the in vivo establishment of a senescent tumor cell population, harboring high levels of the stress-induced ligands RAE-1 and PVR. Taken together our data support the concept of using chemotherapy in order to boost antitumor innate immune responses and report the possibility to induce cellular senescence of tumor cells in vivo.
Abbreviations
| BM | = | bone marrow |
| C12FDG | = | 5-dodecanoylaminofluorescein di-β-D-galactopyranoside |
| DDR | = | DNA damage response |
| DNAM-1 | = | DNAX accessory molecule-1 |
| IMiDs | = | immunomodulatory drugs |
| MFI | = | median fluorescence intensity |
| MM | = | multiple myeloma |
| NK cell | = | natural killer cell |
| NKG2D | = | natural-killer group 2 member D |
| PBS | = | phosphate-buffered saline |
| SA-β-Gal | = | senescence-associated β-galactosidase |
| SASP | = | senescence-associated secretory phenotype |
| X-Gal | = | 5-bromo-4-chloro-3-indolyl β-D-galactopyranoside |
Introduction
Chemotherapy is still today the primary treatment of many types of tumors, and for many years it has been thought to rely on its ability to induce apoptosis of cancer cells. However, multidrug resistance due to the disabling of apoptotic pathways is one of the main features acquired during cancer progression. Furthermore, it is now clear that drug-induced DNA damage, by initiating genetically encoded stress-response pathways, can lead to cell fates other than apoptosis, among which premature cell senescence is now under intensive investigation.Citation1-3
Cellular senescence represents a form of permanent cell cycle arrestCitation4 and it was originally shown in human fibroblasts cultured in vitro upon telomere shortening.Citation5 Currently, it has been well established that the senescent phenotype can be triggered by a variety of conditions including oncogene activation and genotoxic stress.Citation6 More in general, activation of the DNA damage response (DDR) pathway plays a pivotal role in the induction of cellular senescence, and senescent cells have been found associated with pathophysiological conditions such as cancer and aging.Citation7 Notably, a physiological role for cellular senescence has been also recently reported during embryogenesis,Citation8,9 and wound healing,Citation10,11 leading to speculate a more general function of this process in tissue remodeling.
As cellular senescence limits the replicative potential of cells and senescent cells can be recognized by the immune system, senescence is thought to be a major barrier to tumor formation.Citation12 This finding has attracted the attention of the scientific community, leading to explore the possibility of inducing senescence in tumors by chemotherapy.Citation13,14 Furthermore, many widely used anticancer drugs have been recently shown to exert immuno-modulatory effects, supporting the idea of using chemotherapy in order to boost the immune system.Citation15,16
Natural killer (NK) cells are innate lymphocytes with marked cytotoxic activity toward cells expressing stress signals.Citation17 Their activation requires the engagement of specific activating receptors on cell surface, among which NKG2D (natural-killer group 2, member D) and DNAM-1 (DNAX accessory molecule-1) are the best characterized.Citation18-22 The cognate ligands are poorly expressed by normal cells while they are often induced on cancer and virus-infected cells as the result of stress-response pathway activation.Citation23-25 Recently, a role for NK cells in the immune surveillance of senescent cells has been pointed out in liver fibrosis,Citation26,27 multiple myeloma (MM),Citation28 and hepatocellular carcinoma.Citation29,30
MM is a neoplastic plasma cell disease, characterized by the clonal proliferation of malignant plasma cells mostly in the bone marrow (BM) and less frequently in extra-medullary tissues.Citation31 Current therapies rely on autologous haematopoietic stem cell transplantation and/or administration of several classes of drugs, including alkylating agents (melphalan), IMiDs (thalidomide, lenalidomide), or proteasome inhibitors (bortezomib). Nevertheless, this disease is still classified as incurable and there is a need to identify new strategies of intervention. Previous studies have disclosed a pivotal role for NK cells in the immune response against MM.Citation32-37 To this regard, our group has demonstrated that a number of therapeutic drugs, including genotoxic agents, can boost the expression of NKG2D and DNAM-1 ligands on MM cells in vitro and ex vivo, and can enhance NK cell antitumor activity.Citation25,28,38-41 Moreover, our findings also show that the enhanced expression of activating ligands on MM cells after the administration of genotoxic drugs such as doxorubicin and melphalan at doses that fail to induce apoptosis, is mediated by the DDR activation via ATM/ATR, and is associated with a drug-induced tumor cell senescent phenotype.Citation28,41
In this study, we explored the possibility to induce in vivo cellular senescence of tumor cells upon genotoxic drug treatment in a mouse model of MM resembling the human disease, and we evaluated the contribution of NK cells to the immune surveillance of MM cells in mice treated with low doses of melphalan. We showed that the treatment with melphalan can promote in vivo the establishment of a senescent tumor cell population that exhibits increased levels of NKG2D and DNAM-1 ligands, leading to better NK cell recognition of MM cells.
Results
NKG2D and DNAM-1 ligand expression is enhanced on MM cells by low doses of melphalan
Melphalan is an alkylating agent routinely used in the treatment of patients with MM.Citation42 Our group has previously demonstrated that low doses of melphalan can enhance the expression of NKG2D and DNAM-1 ligands on MM cells in vitro.Citation28 To strengthen the in vivo relevance of our evidence, we decided to investigate whether treatment with low doses of melphalan could have similar immune-stimulatory effects by taking advantage of an already established mouse model of MM.Citation43 C57BL/KaLwRiJ mice were intravenously injected with syngeneic 5TGM1 MM cells and tumor progression in the BM was followed by evaluating the number of IgG2b+ cells. We chose 3 weeks after tumor injection as experimental end point to avoid the impairment of NK cell effector functions due to MM-derived alteration of NK cell trafficking as previously reported by our group.Citation44 Fifty micrograms of melphalan in phosphate-buffered saline (PBS), or PBS alone as sham, were intraperitoneally administered to tumor-bearing mice as illustrated in . Melphalan treatment significantly increased the surface expression levels of the NKG2D ligands RAE-1 and MULT-1, and of the DNAM-1 ligand PVR (CD155) on MM cells as evaluated by immunostaining and flow cytometry (). The average fold increase was 1.4 for RAE-1, 1.5 for MULT-1, and 1.2 for PVR. The dose of 50 μg per mouse was the lowest dose among those tested, able to induce the up-regulation of RAE-1 on MM cells (Fig. S1). In parallel, in vitro treatment of 5TGM1 cells showed similar results upon 24, 48, and 72 h of melphalan treatment (Fig. S2).
Figure 1. Study design. Fifty micrograms of melphalan in PBS (or PBS alone as sham) were intraperitoneally administered every 3 d until day 20 beginning at day 11 after 5TGM1 cell injection, to tumor-bearing and age-matched healthy mice.
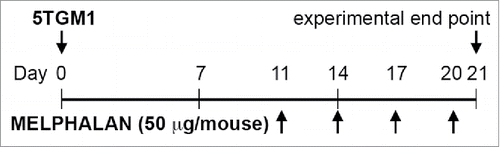
Figure 2. Modulation of RAE-1, MULT-1, and PVR expression on BM MM cells from tumor-bearing mice after melphalan treatment. RAE-1, MULT-1, and PVR surface expression was analyzed as median fluorescence intensity (MFI) by immunostaining and flow cytometry. (A) Representative histograms. Gray histogram and black line represent PBS- and melphalan-treated tumor-bearing mice, respectively; dotted and dashed lines represent PBS- and melphalan-treated tumor-bearing mice isotype controls, respectively. (B) Dot plots and statistical analysis. Each circle or square represents a mouse. The horizontal bar represents the mean value. * p < 0.05, ** p < 0.01.
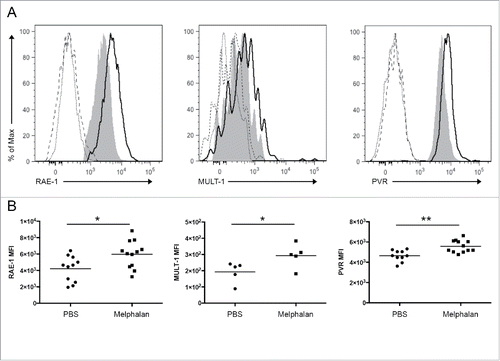
As high doses of melphalan lead to an immunosuppressive state due to lymphopenia,Citation42 changes in the number of BM NK cells identified as CD3− NK1.1+ lymphocytes, were evaluated after melphalan treatment by immunostaining and flow cytometry. No significant variation was detected after drug treatment at the selected dosage in both healthy and tumor-bearing mice (). Similar results were obtained regarding BM CD3+CD4+ and CD3+CD8+ T lymphocytes, and CD11b+F4/80+ macrophages, while CD19+ B lymphocytes were markedly reduced (data not shown). In addition, we analyzed the ability of melphalan to induce BM tumor cell apoptosis by flow cytometry. Tumor cells were identified by the expression of syndecan-1 (CD138), which is a trans-membrane heparan sulfate-bearing proteoglycan expressed by most myeloma plasma cells.Citation45 Percentage of AnnexinV+ tumor cells was similar between melphalan- and PBS-treated tumor-bearing mice, showing the sublethal feature of melphalan at the selected dosage (). This finding was confirmed by Western blot analysis of cleaved PARP-1, a molecular marker of apoptosis. CD138+ MM cells were isolated by fluorescence activated cell sorting from melphalan- and PBS-treated tumor-bearing mice, and only minor cleavage of PARP-1 was detected (). Accordingly, no significant differences were observed between melphalan- and PBS-treated mice regarding tumor growth, as evaluated by the number of IgG2b+ cells (). To evaluate the long-term outcome of the melphalan low dose regime, we analyzed tumor growth also after two additional melphalan administrations at 4 weeks after tumor injection, and we observed reduced amount of IgG2b+ cells in melphalan-treated mice compared with PBS-treated mice (). In addition, we pointed out a significant reduction in the circulating levels of the paraprotein, as evidenced by the ELISA assay of soluble IgG2b in serum (), indicating that drug treatment impacts on tumor progression. Similarly, tumor burden was reduced in the spleen, extra-medullary site of MM growth (data not shown).
Figure 3. BM NK cell number, MM apoptotic rate, and tumor growth after melphalan treatment. (A) The number of BM NK cells from healthy and tumor-bearing mice was evaluated by immunostaining and flow cytometry, NK cells identified as CD3− NK1.1+ lymphocytes. (B and C) MM apoptotic rate was evaluated as the percentage of AnnexinV+ cells and by the cleavage of PARP1 among the CD138+ tumor cells. (D and E) BM tumor growth as evaluated by the number of IgG2b+ cells at 3 and 4 weeks after tumor injection. (F) Concentration of soluble IgG2b in serum of PBS- and melphalan-treated tumor-bearing mice as estimated by ELISA assay. Each circle, square, or triangle represents a mouse. The horizontal bar represents the mean value. * p < 0.05, ** p < 0.01.
MM cells exhibit a senescent phenotype after in vivo melphalan treatment
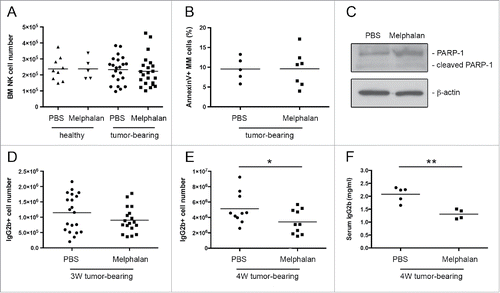
The upregulation of NKG2D and DNAM-1 ligands on MM cells in melphalan-treated tumor-bearing mice can be ascribed to cellular stress, and is likely sustained by the DDR activation, as shown by our previous in vitro studies.Citation28,41 Thus, we asked whether the administration of low doses of melphalan could induce a senescent phenotype on MM cells also in vivo. To address this question, we initially performed cell cycle analysis of tumor cells from the BM of melphalan- and PBS-treated tumor-bearing mice. CD138+ MM cells were isolated by fluorescence-activated cell sorting and the DNA content of propidium iodide-labeled cells was measured by flow cytometry. A significant increase of tumor cells in the G2/M phase was observed comparing melphalan- with PBS-treated tumor-bearing mice (). MM proliferation was also studied analyzing bromodeoxyuridine incorporation in IgG2b+ cells, revealing reduced levels in melphalan-treated tumor-bearing mice compared with PBS-treated tumor-bearing mice (). In order to determine if the G2/M phase increase was due to arrested cells undergoing cellular senescence, we carried out microscopy analysis to visualize the senescence-associated β-galactosidase (SA-β-Gal) activity. Indeed, senescent cells often exhibit an increase in the SA-β-Gal activity that is commonly used as senescence biomarker.Citation46 Nineteen percent of CD138+-sorted MM cells from melphalan-treated tumor-bearing mice displayed a clear blue staining (), confirming the induction of a senescent phenotype. Notably, the increased frequency of MM cells in the G2/M phase of the cell cycle was comparable with the frequency of cells positive for the SA-β-Gal staining. Accordingly to the senescent phenotype, phosphorylation of p53 and increased expression of p16 in melphalan-treated MM cells were shown by Western blot analysis of CD138+ sorted tumor cells (). The correspondence between cell cycle arrest and cellular senescence was further confirmed in in vitro melphalan-treated 5TGM1 MM cells. After 48 h of treatment, almost all cells were arrested in the G2/M phase of the cell cycle (Figs. S3A and B), and after further 24 h virtually every cell displayed SA-β-Gal staining and a flat, enlarged morphology, a feature of cellular senescence (Fig. S3C).
Figure 4. Analysis of the senescent phenotype of FACS-sorted CD138+ BM MM cells from melphalan- and PBS-treated tumor-bearing mice. (A) Representative histogram of MM cell cycle and (B) statistical analysis of three independent experiments. (C) Percentage of bromodeoxyuridine+ (BrdU+) cells among the IgG2b+ tumor cells after 16 h of BrdU in vivo labeling (three mice per group). Error bar represents SEM. * p < 0.05, ** p < 0.01. (D) SA-β-Gal microscopy analysis. Arrows indicate senescent cells identified as blue-stained. Magnification 400X. (E) Western blot analysis of p53 phosphorylation and p16 expression on FACS-sorted CD138+ BM MM cells. Numbers represent relative intensity of p16 versus PBS sample normalized to β-actin.
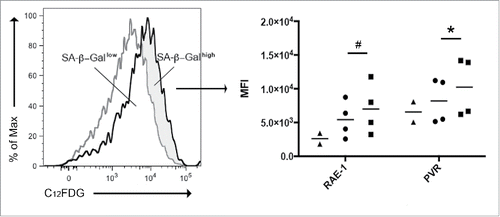
To further strengthen the relationship between the enhanced NKG2D and DNAM-1 ligand expression and the induction of a senescent phenotype, ligand expression on MM cells was assessed in association with their SA-β-Gal activity by using the fluorogenic substrate 5-dodecanoylaminofluorescein di-β-D-galactopyranoside (C12FDG) as previously reported.Citation41 Tumor cells from melphalan- and PBS-treated tumor-bearing mice were stained for C12FDG and MM cells were divided into SA-β-Gallow and SA-β-Galhigh. Immunostaining and flow cytometry on the two gated populations revealed that SA-β-Galhigh senescent tumor cells expressed higher levels of RAE-1 and PVR than SA-β-Gallow tumor cells ().
Figure 5. RAE-1 and PVR expression on MM cells in association with the SA-β-Gal cytofluorimetric assay. MM cells from melphalan-treated (black line) and PBS-treated (gray line) tumor-bearing mice were stained using the fluorogenic substrate C12FDG. MM cells from melphalan-treated tumor-bearing mice were divided into SA-β-Gallow and SA-β-Galhigh in respect to the staining of MM cells from PBS-treated tumor-bearing mice. RAE-1 and PVR expression was evaluated as median fluorescence intensity (MFI) by immunostaining and flow cytometry in the two-gated populations. Triangles represent MM cells from PBS-treated tumor-bearing mice, whereas circles and squares represent SA-β-Gallow and SA-β-Galhigh populations, respectively, from melphalan-treated tumor-bearing mice. The horizontal bar represents the mean value. * p < 0.05, # p = 0.0585.
Melphalan-treated MM cells more efficiently trigger NK cell activation
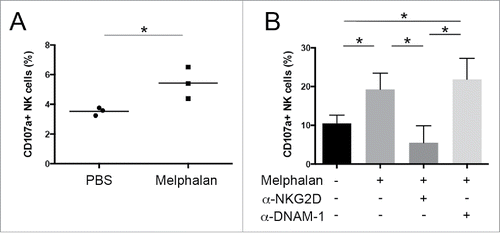
In order to investigate whether MM cells from melphalan-treated tumor-bearing mice were better recognized and lysed by NK cells because of their enhanced expression of NKG2D and DNAM-1 ligands, CD138+ MM cells were isolated from the BM of melphalan- and PBS-treated tumor-bearing mice by fluorescence-activated cell sorting and were assessed for their capacity of inducing NK cell degranulation. To this aim, the expression of the lysosomal marker CD107a, that correlates with NK cell cytotoxicity,Citation47 was evaluated by immunostaining and flow cytometry on IL-15 activated NK cells upon their interaction with sorted MM cells used as target. As shown in , tumor cells from melphalan-treated tumor-bearing mice more efficiently triggered NK cell degranulation. Similar results were obtained using 48 h in vitro melphalan-treated 5TGM1 MM cells, where the involvement of NKG2D and DNAM-1 in the NK cell degranulation triggered by drug-treated tumor cells was evaluated by the use of anti-NKG2D or anti-DNAM-1 blocking antibodies (). Anti-NKG2D antibody but not anti-DNAM-1 antibody was able to reduce NK cell degranulation to basal level. Thus NK cell degranulation capacity was largely dependent on NKG2D, with DNAM-1 having only an ancillary role.
Figure 6. Degranulation of NK cells against MM cells. Expression of the lysosomal marker CD107a was evaluated by immunostaining and flow cytometry on IL-15 activated NK cells upon their interaction with (A) FACS-sorted CD138+ MM cells from tumor-bearing mice or (B) 5TGM1 MM cells used as target at 1:1 cell ratio. To evaluate NKG2D and DNAM-1 contribution, degranulation assay was also performed by pre-incubating NK cells with anti-NKG2D or anti-DNAM-1 blocking antibodies. Each circle or square represents a mouse. The horizontal bar represents the mean value. Error bar represents SEM. * p < 0.05.
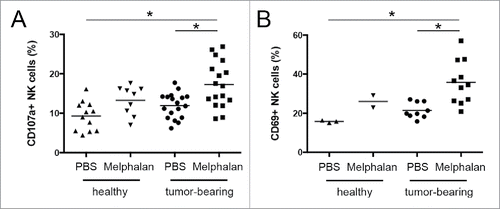
To further define NK cell activity in melphalan-treated tumor-bearing mice, we investigated the expression of CD107a as well as of the activation marker CD69Citation48,49 on freshly isolated BM NK cells by immunostaining and flow cytometry. We found an increased percentage of CD107a+ and CD69+ NK cells (), pointing out a stronger activation of BM NK cells in tumor-bearing mice after melphalan treatment. Interestingly, the increased NK cell activation and effector function were not observed in melphalan-treated healthy mice, indicating that they are linked to the enhanced recognition of melphalan-treated tumor cells.
Figure 7. CD107a and CD69 expression on BM NK cells. (A) CD107a and (B) CD69 surface expression was analyzed by flow cytometry. Each circle, square, or triangle represents a mouse. The horizontal bar represents the mean value. * p < 0.05.
Our previous findings using the same mouse model here presented have demonstrated the implication of NK cells during the immune response against MM.Citation44 Herein, we further confirmed in vivo the involvement of NK cells during tumor surveillance in melphalan-treated tumor-bearing mice by depleting NK1.1+ cells. Mice were intraperitoneally injected with anti-NK1.1 monoclonal antibody and tumor growth was shown to be higher in both PBS- and melphalan-treated NK1.1+ cell-depleted tumor-bearing mice as compared to non-depleted mice (Fig. S4).
Discussion
Recognition of tumor cells by the immune system is a key step in cancer eradication and the use of chemotherapy to boost the antitumor immune response is now a promising approach for cancer treatment.Citation15,16,25,50 In this study, we investigated the ability of in vivo treatment with low doses of melphalan to modulate NK cell activity in a mouse model of MM, the growth of which is under the control of NK cells.Citation44
We found that drug administration results in enhanced surface expression of the stress-induced NKG2D ligands RAE-1 and MULT-1, and of the DNAM-1 ligand PVR (CD155) on MM cells, leading to better tumor cell recognition and killing by NK cells, as highlighted by increased NK cell degranulation upon interaction with melphalan-treated MM cells. Remarkably, BM NK cell number is not affected by melphalan at the selected dosage, in contrast to the impairing effects usually exerted by this drug at therapeutic doses.Citation42 Moreover, NK cells from tumor-bearing mice undergoing melphalan treatment, exhibit an increased expression of CD107a and CD69 markers, suggesting that they are functionally active in vivo. Furthermore, we observed that melphalan treatment in vivo promotes the establishment of a senescent tumor cell population, expressing enhanced levels of the NKG2D and DNAM-1 ligands. However, unlike in vitro-induced senescent cells that display a flat and enlarged morphology, the in vivo SA-β-Gal+ senescent population fails to exhibit such an enlarged morphology, questioning if this could be a feature of cellular senescence in vivo, where more constrains limit cell size, as already postulated.Citation13
Senescent cells are indefinitely viable in culture, but their fate in tissues has not been fully elucidated. Senescent cells have been observed in adult tissues, such as in naevi,Citation51 implying that, at least in some circumstances, they can persist during lifespan, and there is also evidence that senescent stem cells accumulate in tissues during aging.Citation52 On the other hand, senescent cells can participate in tissue homeostasis during both embryogenesis and in adult tissues,Citation53,54 being cleared by the immune system.Citation55,56 The mechanisms through which senescent cells are recognized by the immune system are still largely obscure. Different components of the immune system have been involved in the immune surveillance of senescent cells. Macrophages were first described to be implicated in the removal of senescent red blood cells and their role in patrolling senescent cells was further confirmed by a number of evidence showing their ability to eliminate senescent neutrophils, embryonic mesonephric cells, apical ectodermal ridge, and tumor cells.Citation8,9,56,57 More recently, also lymphoid cells, namely CD4+ T lymphocytesCitation55 and NK cells, have been involved in the immune surveillance of senescent cells, and a role for NK cells has been pointed out in liver fibrosis,Citation26,27 MM,Citation28 and hepatocellular carcinoma.Citation29,30
NK cells were found to preferentially kill senescent cells through a mechanism involving perforin- and granzyme-containing granule exocytosis, and to produce IFNγ following senescent cell interaction, leading to hypothesize that NK cell-mediated immune clearance of senescent cells not only relies on direct killing but also on cytokine production that in turn can lead to macrophage activation.Citation41 At present, whether NK cell-mediated clearance of senescent cells is the result of enhanced NK cell recognition is still matter of debate. Indeed, while senescent hepatic stellate cells and in vitro drug-induced MM senescent cells display increased levels of NKG2D and DNAM-1 NK cell activating ligands,Citation26,28 a similar finding was not reported for p53-induced senescent liver carcinoma cells. Indeed, in this in vivo context, clearance of senescent cells was ascribed to the senescence-associated secretory phenotype (SASP), resulting in the secretion of numerous pro-inflammatory cytokines and chemokines. In particular, CCL2 was reported to play a major role in the increased infiltration of senescent tumors by NK cells, thus promoting tumor cell elimination.Citation30
A possible explanation to reconcile the controversial results on the expression of NK cell activating receptor ligands may rely on the different in vivo models used. In the mouse model of liver carcinoma investigated by Iannello et al.Citation30 the senescent phenotype is achieved by p53 restoration, while in the present study, cellular senescence of tumor cells is due to anticancer drug administration. Stimulation of NKG2D and DNAM-1 ligand expression by chemotherapy and DDR activation has been reported to be p53-independent, whereas involving E2F1 transcription factor.Citation41,58,59
In this scenario, our results show, for the first time to our knowledge, in vivo upregulation of NKG2D and DNAM-1 ligands in drug-induced senescent tumor cells, and provide further insights on the mechanisms through which the immune system can patrol senescent cells. This finding highlights the biological relevance of our previous in vitro and ex vivo studies showing how genotoxic stress modulates the expression of NKG2D and DNAM-1 ligands on MM cells.Citation28,41,60 We propose that the engagement of the activating receptors is a key mechanism regulating NK cell killing of senescent cells, thus NK cells playing a pivotal role in the immune surveillance of senescent cells in vivo. Accordingly, a recent paper by Sagiv et al. has revealed the importance of NKG2D receptor–ligand interaction in preventing the accumulation of senescent activated stellate cells by NK cells in a mouse model of liver fibrosis.Citation61
Premature cell senescence is now under extensive investigation as it is thought to be a major barrier to tumor formation. Indeed, its anti-proliferative features as well as its immunological implications make cellular senescence an effective tumor suppressor mechanism. So far, in vivo induction of tumor cell senescence has been achieved by restoration of tumor suppressorsCitation56 and in response to oncogene induction.Citation62 The present study shows that senescence of cancer cells can be induced by a conventional anticancer drug and, along with other studies reporting chemotherapy-induced senescence,Citation3,63,64 supports the concept that drug-induced senescence may contribute to treatment outcome in vivo.
Chemotherapy is often able to reduce tumor burden, but fails to be always curative. This suggests that direct killing of tumor cells may not be sufficient to eradicate residual disease and thus more effective therapeutic strategies are needed. The boosting of the immune system to overcome tumor immune escape represents an innovative therapeutic approach, and may be the key for a long-lasting strategy. To this regard, the present study supports the concept of using chemotherapy to elicit immune responses. Adoptive immunotherapy of NK cells against MM is now under investigationCitation65 and our data show that melphalan, at doses that fail to have a direct killing effect on cancer cells, is able to render tumor cells more visible to NK cell activity. This provides a rationale for using melphalan in combination with adoptive immunotherapy. This observation is in line with a recent study of Lu and colleagues that reported the efficacy of melphalan to enhance adoptive immunotherapy using tumor-specific CD4+ T cells against advanced B cell lymphoma and colorectal carcinoma through the induction of immunogenic cell death.Citation66
In conclusion, our study investigates in vivo both cellular senescence and potentiation of antitumor innate immune response after chemotherapy, and provides novel evidence supporting the notion of immunogenic senescence, a mechanism by which drug-treated tumor cells may be controlled by innate lymphocytes, including NK cells.
Materials and methods
Mice and cells
Female C57BL/KaLwRij mice were purchased from Harlan Laboratories and housed in the animal facility of Istituto Superiore di Sanità (Rome, Italy) under specific pathogen-free conditions in accordance with national guidelines for animal care and use (D.lgs. 116/92 and D.lgs. 26/2014, Health Ministry authorization n° 229/2012 and 230/2012). 5TGM1 MM cells were kindly provided by Dr Yoneda (University of Texas, San Antonio, TX, USA) and maintained in RPMI 1640 medium supplemented with 10% foetal bovine serum, 2 mM glutamine, 55 μM β-mercaptoethanol, and antibiotics (complete medium). Five to six-week old C57BL/KaLwRij mice were intravenously injected with 2 × 106 5TGM1 cells suspended in 200 μL of PBS, and melphalan-treated mice were intraperitoneally injected with 50 μg of melphalan in 100 μL of PBS every 3 d until day 20 or day 26 beginning at day 11 after tumor cell injection. Sham animals were treated with PBS alone. NK cell in vivo depletion was obtained by intraperitoneal injection of anti-NK1.1 (clone PK136) mAb (100 μg/mouse) at day −2, 0, 2, 7, and 14 after 5TGM1 MM cell transplantation. Mice were killed by cervical dislocation at 3 or 4 weeks after tumor injection. BM cells were collected in complete medium by extensive flushing of femurs and tibias with syringe.
Antibodies and reagents
Antibodies directly conjugated to FITC, PE, PerCP-Cy5.5, APC fluorochromes or biotin and specific for the following antigens (clone name in parentheses) were used for immunostaining: CD138 (281-2), RAE-1 (186107), PVR (690912), MULT-1 (237104), CD3ϵ (145-2C11), NK1.1 (PK136), CD107a (1D4B), CD69 (H1.2F3), CD4 (RM4-5), CD8 (53-6.7), CD11b (M1/70), F4/80 (CI:A3-1), CD19 (6D5), and IgG2b (RMG2b-1). Antibodies were purchased from BD PharMingen, R&D System, eBioscience, and BioLegend. FITC-conjugated AnnexinV was purchased from BD PharMingen and staining was performed following manufacturer's instructions. Recombinant mouse IL-15 and human IL-2 were from R&D System and PeproTechEC, respectively. Melphalan was from Sigma-Aldrich (catalog number M2011) and was dissolved in acidified ethanol to a concentration of 10 mg/mL, stored at −20°C, and diluted in PBS prior to use. A concentration of 0.5 µg/mL was used for in vitro experiments.
Immunostaining and cytofluorimetric analysis
Cells were washed and suspended in staining buffer (PBS without Ca2+ MgCitation2+, 0.5% BSA, 2 mM EDTA, 0.025% NaN3). Anti-CD16/32 (clone 24G2) was added for 10 min to prevent non-specific and Fc-mediated binding. Then, cells were stained with the indicated antibodies for 20 min at 4°C. Samples were analyzed using a flow cytometer FACSCanto II (BD Biosciences) and data were elaborated using FlowJo 7.6 software (TreeStar).
SDS-PAGE and western blot analysis
Equal amount of FACS-sorted CD138+ MM cells were lysed in ice-cold lysis buffer containing 20 mM Tris-HCL pH 7.5, 150 mM NaCl, 1mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/mL leupeptin, and 1 mM PMSF. Centrifugation-cleared lysates were resolved by SDS-PAGE and transferred to nitrocellulose membrane (Amersham. GE Healthcare Life Sciences). After blocking with 5% nonfat dry milk, membrane was immunoblotted with antibodies anti PARP-1 (Santa Cruz Biotechnology), phospho-p53 (Ser15) (Cell Signaling Technology), p53 (Santa Cruz Biotechnology), p16 (Santa Cruz Biotechnology), and β-actin (Sigma-Aldrich). HRP-conjugated secondary antibodies and ECL kit (Amersham. GE Healthcare Life Sciences) were used to reveal immunochemiluminescence. Densitometric analysis was performed with the ImageJ Software.
ELISA assay
Detection of soluble IgG2b in serum was carried out as previously reported.Citation44 Briefly, sera from mice were obtained by incubating blood for 1 h at 37°C followed by centrifugation at 6000 rpm and supernatant collection. The assessment of IgG2b concentration was performed by coating over-night EIA/RIA plates with goat anti-mouse IgG2b antibody (ICL, catalog number GG2B-90A), blocking with PBS/BSA 1%, and then incubating with standards (ICL) or serum samples for 1 h at room temperature. HRP-conjugated goat anti-mouse IgG2b antibody (ICL, catalog number GG2B-90P) was used as detection antibody.
Cell cycle analysis
Cells were harvested and fixed over night at 4°C in cold 70% ethanol. Thereafter, cells were washed in PBS with 0.1% sodium azide, incubated for 30 min at room temperature with 100 µg/mL propidium iodide containing 40 U/mL RNAse, and immediately analyzed by flow cytometry.
SA-β-Gal staining
For microscopic assay cells were spotted on a poly-L-lysine-coated glass slide and cytospinned (700 rpm × 3 min). Cells were then fixed with 2% formaldehyde (3 min) and incubated 24 h at 37°C without CO2 with fresh SA-β-Gal staining solution: 1 mg/mL 5-bromo-4-chloro-3-indolyl β-D-galactopyranoside (X-Gal), 150 mM NaCl, 2 mM MgCl2, 40 mM citric acid/Na phosphate buffer (pH 6), 5 mM potassium ferrocyanide, and 5 mM potassium ferricyanide. Senescent cells were identified as blue-stained cells by standard light microscopy. Images were acquired using a Nikon Microphot-FXA microscope with magnification 400X. For cell count a total of 144 cells were counted among 11 different fields from two independent experiments. For flow cytometric assay, SA-β-Gal activity was measured using the fluorogenic substrate C12FDG (Invitrogen) as previously described.Citation67
Bromodeoxyuridine assay
In vivo bromodeoxyuridine (BrdU) labeling of MM cells was done as previously described.Citation68 Briefly, mice were intraperitoneally injected with BrdU (Sigma-Aldrich, 1 mg/200 μL of PBS per mouse) 16 h before killing. Cells were fixed with 30% methanol, 0.4% paraformaldehyde in PBS and permeabilized with 0.01% Tween 20, 1% paraformaldehyde. Cells were incubated with 250 μg/mL DNase (Sigma-Aldrich) for 15 min at 37°C and then stained with anti-BrdU-FITC antibody (BD Biosciences). MM cells were gated as IgG2b+ cells and non-specific binding was measured by staining cells from not BrdU-injected mice.
Degranulation assay
NK cells were isolated from the spleen of healthy mice using the “NK isolation kit II” from Miltenyi Biotec according to manufacturer's instructions. CD138+ tumor cells were isolated from the BM of melphalan- and PBS-treated tumor-bearing mice by fluorescence activated cell sorting. NK cells were stimulated over-night with 50 ng/mL IL-15 and co-incubated with target cells, CD138+ sorted tumor cells or in vitro cultured 5TGM1 cells, at 1:1 effector:target ratio for 4 h in complete medium at 37°C in the presence of 500 U/mL IL-2, 100 µM monensin (Sigma-Aldrich), and FITC-conjugated anti-CD107a or anti-IgG as previously reported.Citation69 Cells were than harvested and stained with fluorochrome-conjugated anti-CD3ϵ and anti-NK1-1 to identify NK cells. In some experiments, NK cells were treated for 20 min before target addition with 5 μg/mL anti-NKG2D (clone A10, eBioscience) and 5 μg/mL anti-DNAM-1 (clone 480.1, BioLegend) blocking antibodies.
Statistical analysis
Unpaired or paired Student's t test and one-way ANOVA followed by Tukey multiple-comparison test were used to analyze data. A value of p ≤ 0.05 was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_A_1218105_supplementary_data.zip
Download Zip (5.6 MB)Funding
This work was supported by grants from the Italian Association for Cancer Research (AIRC Investigator Grant and AIRC Special Program Molecular and Clinical Oncology—5 per Mille), the Italian Ministry of University and Research (PRIN/20103FMJEN), and the Sapienza University of Rome (Ateneo).
References
- Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res 2003; 63:2705-15; PMID:12782571
- Roninson IB, Broude EV, Chang BD. If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist Updat 2001; 4:303-13; PMID:11991684; http://dx.doi.org/10.1054/drup.2001.0213
- te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res 2002; 62:1876-83; PMID:11912168
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 2007; 8:729-40; PMID:17667954; http://dx.doi.org/10.1038/nrm2233
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res 1961; 25:585-621; PMID:13905658; http://dx.doi.org/10.1016/0014-4827(61)90192-6
- Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev 2010; 24:2463-79; PMID:21078816; http://dx.doi.org/10.1101/gad.1971610
- Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 2014; 15:482-96; PMID:24954210; http://dx.doi.org/10.1038/nrm3823
- Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, Rodriguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M et al. Programmed cell senescence during mammalian embryonic development. Cell 2013; 155:1104-18; PMID:24238962; http://dx.doi.org/10.1016/j.cell.2013.10.019
- Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 2013; 155:1119-30; PMID:24238961; http://dx.doi.org/10.1016/j.cell.2013.10.041
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 2014; 31:722-33; PMID:25499914; http://dx.doi.org/10.1016/j.devcel.2014.11.012
- Serrano M. Senescence helps regeneration. Dev Cell 2014; 31:671-2; PMID:25535913; http://dx.doi.org/10.1016/j.devcel.2014.12.007
- Hoenicke L, Zender L. Immune surveillance of senescent cells–biological significance in cancer- and non-cancer pathologies. Carcinogenesis 2012; 33:1123-6; PMID:22470164; http://dx.doi.org/10.1093/carcin/bgs124
- Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer 2010; 10:51-7; PMID:20029423; http://dx.doi.org/10.1038/nrc2772
- Kahlem P, Dorken B, Schmitt CA. Cellular senescence in cancer treatment: friend or foe? J Clin Invest 2004; 113:169-74; PMID:14722606; http://dx.doi.org/10.1172/JCI20784
- Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest 2008; 118:1991-2001; PMID:18523649; http://dx.doi.org/10.1172/JCI35180
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol 2008; 8:59-73; PMID:18097448; http://dx.doi.org/10.1038/nri2216
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331:44-9; PMID:21212348; http://dx.doi.org/10.1126/science.1198687
- Coudert JD, Held W. The role of the NKG2D receptor for tumor immunity. Semin Cancer Biol 2006; 16:333-43; PMID:16914326; http://dx.doi.org/10.1016/j.semcancer.2006.07.008
- Gilfillan S, Chan CJ, Cella M, Haynes NM, Rapaport AS, Boles KS, Andrews DM, Smyth MJ, Colonna M. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J Exp Med 2008; 205:2965-73; PMID:19029380; http://dx.doi.org/10.1084/jem.20081752
- Lanier LL. NK cell recognition. Annu Rev Immunol 2005; 23:225-74; PMID:15771571; http://dx.doi.org/10.1146/annurev.immunol.23.021704.115526
- Moretta L, Bottino C, Pende D, Castriconi R, Mingari MC, Moretta A. Surface NK receptors and their ligands on tumor cells. Semin Immunol 2006; 18:151-8; PMID:16730454; http://dx.doi.org/10.1016/j.smim.2006.03.002
- Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol 2003; 3:781-90; PMID:14523385; http://dx.doi.org/10.1038/nri1199
- Iannello A, Raulet DH. Immune surveillance of unhealthy cells by natural killer cells. Cold Spring Harb Symp Quant Biol 2013; 78:249-57; PMID:24135717; http://dx.doi.org/10.1101/sqb.2013.78.020255
- Raulet DH, Guerra N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat Rev Immunol 2009; 9:568-80; PMID:19629084; http://dx.doi.org/10.1038/nri2604
- Soriani A, Fionda C, Ricci B, Iannitto ML, Cippitelli M, Santoni A. Chemotherapy-elicited upregulation of NKG2D and DNAM-1 ligands as a therapeutic target in multiple myeloma. Oncoimmunology 2013; 2:e26663; PMID:24498552; http://dx.doi.org/10.4161/onci.26663
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell 2008; 134:657-67; PMID:18724938; http://dx.doi.org/10.1016/j.cell.2008.06.049
- Sagiv A, Biran A, Yon M, Simon J, Lowe SW, Krizhanovsky V. Granule exocytosis mediates immune surveillance of senescent cells. Oncogene 2013; 32:1971-7; PMID:22751116; http://dx.doi.org/10.1038/onc.2012.206
- Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A et al. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood 2009; 113:3503-11; PMID:19098271; http://dx.doi.org/10.1182/blood-2008-08-173914
- Iannello A, Raulet DH. Immunosurveillance of senescent cancer cells by natural killer cells. Oncoimmunology 2014; 3:e27616; PMID:24800169; http://dx.doi.org/10.4161/onci.27616
- Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med 2013; 210:2057-69; PMID:24043758; http://dx.doi.org/10.1084/jem.20130783
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011; 364:1046-60; PMID:21410373; http://dx.doi.org/10.1056/NEJMra1011442
- Carbone E, Neri P, Mesuraca M, Fulciniti MT, Otsuki T, Pende D, Groh V, Spies T, Pollio G, Cosman D et al. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood 2005; 105:251-8; PMID:15328155; http://dx.doi.org/10.1182/blood-2004-04-1422
- El-Sherbiny YM, Meade JL, Holmes TD, McGonagle D, Mackie SL, Morgan AW, Cook G, Feyler S, Richards SJ, Davies FE et al. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res 2007; 67:8444-9; PMID:17875681; http://dx.doi.org/10.1158/0008-5472.CAN-06-4230
- Garcia-Sanz R, Gonzalez M, Orfao A, Moro MJ, Hernandez JM, Borrego D, Carnero M, Casanova F, Barez A, Jimenez R et al. Analysis of natural killer-associated antigens in peripheral blood and bone marrow of multiple myeloma patients and prognostic implications. Br J Haematol 1996; 93:81-8; PMID:8611480; http://dx.doi.org/10.1046/j.1365-2141.1996.4651006.x
- Jinushi M, Vanneman M, Munshi NC, Tai YT, Prabhala RH, Ritz J, Neuberg D, Anderson KC, Carrasco DR, Dranoff G. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc Natl Acad Sci U S A 2008; 105:1285-90; PMID:18202175; http://dx.doi.org/10.1073/pnas.0711293105
- Jurisic V, Srdic T, Konjevic G, Markovic O, Colovic M. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med Oncol 2007; 24:312-7; PMID:17873307; http://dx.doi.org/10.1007/s12032-007-0007-y
- Osterborg A, Nilsson B, Bjorkholm M, Holm G, Mellstedt H. Natural killer cell activity in monoclonal gammopathies: relation to disease activity. Eur J Haematol 1990; 45:153-7; PMID:1699786; http://dx.doi.org/10.1111/j.1600-0609.1990.tb00443.x
- Fionda C, Abruzzese MP, Zingoni A, Soriani A, Ricci B, Molfetta R, Paolini R, Santoni A, Cippitelli M. Nitric oxide donors increase PVR/CD155 DNAM-1 ligand expression in multiple myeloma cells: role of DNA damage response activation. BMC Cancer 2015; 15:17; PMID:25609078; http://dx.doi.org/10.1186/s12885-015-1023-5
- Fionda C, Malgarini G, Soriani A, Zingoni A, Cecere F, Iannitto ML, Ricciardi MR, Federico V, Petrucci MT, Santoni A et al. Inhibition of glycogen synthase kinase-3 increases NKG2D ligand MICA expression and sensitivity to NK cell-mediated cytotoxicity in multiple myeloma cells: role of STAT3. J Immunol 2013; 190:6662-72; PMID:23686482; http://dx.doi.org/10.4049/jimmunol.1201426
- Fionda C, Soriani A, Zingoni A, Santoni A, Cippitelli M. NKG2D and DNAM-1 Ligands: Molecular Targets for NK Cell-Mediated Immunotherapeutic Intervention in Multiple Myeloma. Biomed Res Int 2015; 2015:178698; PMID:26161387; http://dx.doi.org/10.1155/2015/178698
- Soriani A, Iannitto ML, Ricci B, Fionda C, Malgarini G, Morrone S, Peruzzi G, Ricciardi MR, Petrucci MT, Cippitelli M et al. Reactive oxygen species- and DNA damage response-dependent NK cell activating ligand upregulation occurs at transcriptional levels and requires the transcriptional factor E2F1. J Immunol 2014; 193:950-60; PMID:24913980; http://dx.doi.org/10.4049/jimmunol.1400271
- Falco P, Bringhen S, Avonto I, Gay F, Morabito F, Boccadoro M, Palumbo A. Melphalan and its role in the management of patients with multiple myeloma. Expert Rev Anticancer Ther 2007; 7:945-57; PMID:17627453; http://dx.doi.org/10.1586/14737140.7.7.945
- Garrett IR, Dallas S, Radl J, Mundy GR. A murine model of human myeloma bone disease. Bone 1997; 20:515-20; PMID:9177864; http://dx.doi.org/10.1016/S8756-3282(97)00056-2
- Ponzetta A, Benigni G, Antonangeli F, Sciume G, Sanseviero E, Zingoni A, Ricciardi MR, Petrucci MT, Santoni A, Bernardini G. Multiple myeloma impairs bone marrow localization of effector Natural Killer cells by altering the chemokine microenvironment. Cancer Res 2015; PMID:26438594; http://dx.doi.org/10.1158/0008-5472.CAN-15-1320
- Yang Y, Yaccoby S, Liu W, Langford JK, Pumphrey CY, Theus A, Epstein J, Sanderson RD. Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood 2002; 100:610-7; PMID:12091355; http://dx.doi.org/10.1182/blood.V100.2.610
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 1995; 92:9363-7; PMID:7568133; http://dx.doi.org/10.1073/pnas.92.20.9363
- Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med 2005; 202:1001-12; PMID:16203869; http://dx.doi.org/10.1084/jem.20051143
- Testi R, D'Ambrosio D, De Maria R, Santoni A. The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol Today 1994; 15:479-83; PMID:7945773; http://dx.doi.org/10.1016/0167-5699(94)90193-7
- Ziegler SF, Ramsdell F, Alderson MR. The activation antigen CD69. Stem Cells 1994; 12:456-65; PMID:7804122; http://dx.doi.org/10.1002/stem.5530120502
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480:480-9; PMID:22193102; http://dx.doi.org/10.1038/nature10673
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 2005; 436:720-4; PMID:16079850; http://dx.doi.org/10.1038/nature03890
- Pelicci PG. Do tumor-suppressive mechanisms contribute to organism aging by inducing stem cell senescence? J Clin Invest 2004; 113:4-7; PMID:14702099; http://dx.doi.org/10.1172/JCI200420750
- Banito A, Lowe SW. A new development in senescence. Cell 2013; 155:977-8; PMID:24267881; http://dx.doi.org/10.1016/j.cell.2013.10.050
- Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol 2013; 75:685-705; PMID:23140366; http://dx.doi.org/10.1146/annurev-physiol-030212-183653
- Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011; 479:547-51; PMID:22080947; http://dx.doi.org/10.1038/nature10599
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007; 445:656-60; PMID:17251933; http://dx.doi.org/10.1038/nature05529
- de Back DZ, Kostova EB, van Kraaij M, van den Berg TK, van Bruggen R. Of macrophages and red blood cells; a complex love story. Front Physiol 2014; 5:9; PMID:24523696; http://dx.doi.org/10.3389/fphys.2014.00009
- Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005; 436:1186-90; PMID:15995699; http://dx.doi.org/10.1038/nature03884
- Jung H, Hsiung B, Pestal K, Procyk E, Raulet DH. RAE-1 ligands for the NKG2D receptor are regulated by E2F transcription factors, which control cell cycle entry. J Exp Med 2012; 209:2409-22; PMID:23166357; http://dx.doi.org/10.1084/jem.20120565
- Zingoni A, Cecere F, Vulpis E, Fionda C, Molfetta R, Soriani A, Petrucci MT, Ricciardi MR, Fuerst D, Amendola MG et al. Genotoxic Stress Induces Senescence-Associated ADAM10-Dependent Release of NKG2D MIC Ligands in Multiple Myeloma Cells. J Immunol 2015; 195:736-48; PMID:26071561; http://dx.doi.org/10.4049/jimmunol.1402643
- Sagiv A, Burton DG, Moshayev Z, Vadai E, Wensveen F, Ben-Dor S, Golani O, Polic B, Krizhanovsky V. NKG2D ligands mediate immunosurveillance of senescent cells. Aging (Albany NY) 2016. Feb; 8(2):328-44; PMID:26878797; http://dx.doi.org/10.18632/aging.100897
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997; 88:593-602; PMID:9054499; http://dx.doi.org/10.1016/S0092-8674(00)81902-9
- Roberson RS, Kussick SJ, Vallieres E, Chen SY, Wu DY. Escape from therapy-induced accelerated cellular senescence in p53-null lung cancer cells and in human lung cancers. Cancer Res 2005; 65:2795-803; PMID:15805280; http://dx.doi.org/10.1158/0008-5472.CAN-04-1270
- Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM, Lowe SW. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 2002; 109:335-46; PMID:12015983; http://dx.doi.org/10.1016/S0092-8674(02)00734-1
- Garg TK, Szmania SM, Khan JA, Hoering A, Malbrough PA, Moreno-Bost A, Greenway AD, Lingo JD, Li X, Yaccoby S et al. Highly activated and expanded natural killer cells for multiple myeloma immunotherapy. Haematologica 2012; 97:1348-56; PMID:22419581; http://dx.doi.org/10.3324/haematol.2011.056747
- Lu X, Ding ZC, Cao Y, Liu C, Habtetsion T, Yu M, Lemos H, Salman H, Xu H, Mellor AL et al. Alkylating agent melphalan augments the efficacy of adoptive immunotherapy using tumor-specific CD4+ T cells. J Immunol 2015; 194:2011-21; PMID:25560408; http://dx.doi.org/10.4049/jimmunol.1401894
- Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc 2009; 4:1798-806; PMID:20010931; http://dx.doi.org/10.1038/nprot.2009.191
- Parretta E, Cassese G, Barba P, Santoni A, Guardiola J, Di Rosa F. CD8 cell division maintaining cytotoxic memory occurs predominantly in the bone marrow. J Immunol 2005; 174:7654-64; PMID:15944266; http://dx.doi.org/10.4049/jimmunol.174.12.7654
- Ponzetta A, Sciume G, Benigni G, Antonangeli F, Morrone S, Santoni A, Bernardini G. CX3CR1 regulates the maintenance of KLRG1+ NK cells into the bone marrow by promoting their entry into circulation. J Immunol 2013; 191:5684-94; PMID:24184559; http://dx.doi.org/10.4049/jimmunol.1300090
