ABSTRACT
The programmed cell death 1 (PD-1)/PD-1 ligand 1 (PD-L1) pathway has emerged as a critical inhibitory pathway regulating T-cell response in non-small-cell lung cancer (NSCLC), and the development of PD-1/PD-L1 inhibitors has changed the landscape of NSCLC therapy. Nevertheless, the high degree of non-responders demonstrates that we are still far from completely understanding the events underlying tumor immune resistance. Although the expression of PD-L1 in tumor tissue has been correlated with clinical response to anti PD-1 inhibitors, the ability of this marker to discriminate the subgroup of patients who derive benefit from immunotherapy is suboptimal. Circulating tumor cells (CTCs), as an accessible source of tumor for biologic characterization that can be serially obtained with minimally invasive procedure, hold significant promise to facilitate treatment-specific biomarkers discovery. We recently demonstrated that the presence of PD-L1 on CTCs apparently predicts resistance to the anti-PD-1 Nivolumab in metastatic NSCLC patients and that PD-L1 positive CTCs usually have an elongated morphology that can be ascribed to epithelial–mesenchymal transition (EMT). We here demonstrate for the first time that PD-L1 positive CTCs isolated from NSCLC patients are characterized by partial EMT phenotype, and hypothesize that the co-expression of PD-L1 and EMT markers might represent for these cells a possible molecular background for immune escape.
Introduction
Immune checkpoints are inhibitory pathways that are crucial for maintaining self-tolerance and attenuating autoimmunity to prevent host tissue damage.Citation1 It is now well established that tumors co-opt these inhibitory pathways to evade the tumor-specific immune response. The programmed cell death 1 (PD-1)/PD-1 ligand (PD-L1) pathway has emerged as a critical inhibitory pathway, regulating T-cell response in non-small cell lung cancer (NSCLC) and the development of PD-1/PD-L1 inhibitors has changed the landscape of NSCLC therapy, since the approval from the US Food and Drug Administration of two PD-1 inhibitors for chemotherapy refractory patients.Citation2 Although the results for immune checkpoint inhibitors therapy in patients with lung cancer are encouraging, the high degree of non-responders prevents from a rational use of these agents and demonstrates that we are still far from completely understanding the events underlying tumor immune resistance. The expression levels of PD-L1 protein in tumor tissue has been correlated with clinical response to anti PD-1 inhibitors, even if the ability of this marker to discriminate the subgroup of patients who derive benefit from immunotherapy is suboptimal. Several reasons have been advocated to explain the poor predictive performance of PD-L1 expression in tumor tissue, mainly the multitude of PD-L1 antibodies and thresholds for positivity and the dynamic biology of PD-L1. Circulating tumor cells (CTCs), as an accessible source of tumor for biologic characterization that can be serially obtained with minimally invasive procedure, hold significant promise to facilitate treatment-specific biomarkers discovery. We have recently demonstrated that the persistence of PD-L1 positive CTCs at 6 mo from the beginning of treatment with the anti-PD-1 nivolumab is indicative for treatment resistance in metastatic chemo-refractory NSCLC patients.Citation3 Noteworthy, CTCs persistently positive for PD-L1 expression displayed an unusual elongated spindle-like morphology as compared with PD-L1 negative CTCs, which were mostly small and regularly rounded. It has been previously suggested that these elongated CTCs may represent a small population of partial epithelial-mesenchymal transition (EMT)-transformed cancer cells. The bidirectional crosstalk between PD-L1 expression and EMT is well described, although the molecular determinants of this association remain incompletely understood.Citation4 Although only limited data exist, preliminary research suggests that PD-L1 is expressed in CTCs with mesenchymal traits. Basing upon our previous observation that the presence of PD-L1 on CTCs apparently predicts resistance to the anti-PD-1 nivolumab in metastatic NSCLC patients, we further sought to investigate the co-expression of PD-L1 and EMT markers in these cells, as a possible molecular background of immune escape.
Materials and methods
Blood sample collection and CTCs enrichment
Fifteen (15) patients with metastatic NSCLC progressing post-prior systemic treatment and included in the Expanded Access Program (EAP) with nivolumab were enrolled. 6 mL of blood was drawn from each patient before the beginning of treatment with nivolumab. Peripheral blood samples were collected in a K2-EDTA tube, kept at +4°C and processed within 3 h after sampling. To isolate fixed cells for cytological studies, the ScreenCell Cyto kit was used. For each patient, the blood filtration was performed in duplicate. At each round, 3 mL of blood sample were incubated with 4 mL of ScreenCell FC buffer, containing red blood cell lysis and fixation buffer, during 8 min at room temperature. Before using, FC buffer pH was checked and, when necessary, 30% NaOH was added until a pH of around 7. Seven mL of diluted blood were transferred into device tank and filtered under a pressure gradient using a vacutainer tube. After washing with 1.6 mL of PBS to remove red blood cells debris, the filter was released on absorbing paper dried at room temperature. Filtration was usually completed within 3 min. The filters were stored at −20°C until downstream analysis was performed. As control, 6 mL of blood was drawn from three normal subjects and processed as above described. The protocol was approved by the local Ethical Committee and all subjects signed an informed consent.
Light microscopy and immunofluorescence
For immunofluorescence, non-specific protein binding was blocked by 5% normal goat serum. Specimens were permeabilized in PBS-Tween 20 (PBS-T) for 20 min. Sections were incubated overnight at 4°C with primary antibodies against TTF-1 (mouse monoclonal, Leica Biosystem, code: NCL-L-TTF-1, dilution: 1:50) and Vimentin (rabbit polyclonal, Santa Cruz Biotechnology, code: sc-5565, dilution: 1:50), or with primary antibodies against Cytokeratin Wide Spectrum Screening (rabbit polyclonal, Dako, code: Z0622, dilution: 1:50) and N-Cadherin (mouse monoclonal, Santa Cruz Biotechnology, code: sc-59987, dilution: 1:50), or with primary antibodies against Cytokeratin Wide Spectrum Screening (rabbit polyclonal, Dako, code: Z0622, dilution: 1:50) and BCL-2 (mouse monoclonal, Dako, code: M0887, dilution: 1:50). All primary antibodies were diluted in 1% bovine serum albumin in PBS-T. The sections were then washed twice with PBS-T and incubated for 1 h with labeled isotype-specific secondary antibodies (dilution: 1:50): anti-mouse AlexaFluor-350, anti-mouse AlexaFluor-488, anti-rabbit Alexafluor-350, anti-rabbit AlexaFluor-488 (Alexa Fluor; Invitrogen Ltd, Paisley, UK). Then, sections were washed twice with PBS-T and incubated for 1 h at room temperature with a Phycoerythrin (PE)-conjugated primary antibody against B7-H1/PD-L1 (mouse monoclonal, R&D System, catalog number: FAB1561P, dilution: 1:20). Finally, specimens were counterstained with DRAQ5 (Cell Signaling technology, product code: #4084), a cell permeable far-red fluorescent DNA dye, for the visualization of cell nuclei. For all immunoreactions, negative controls consisted of the primary antibody being replaced with pre-immune serum.Citation5 The application use of Kwss, Vimentin, and B7-H1/PD-L1 in immunohistochemistry and immunofluorescence has been validated in specific positive human specimens (Fig. S1). Specimens were scanned by a digital scanner (Aperio Scanscope FL System, Aperio Technologies, Inc., Oxford, UK) and were also analyzed by Confocal Microscopy (Leica TCS-SP2). After fluorescence scanning, the coverslip and the mounting medium were removed using a wash of PBS, filters were stained with hematoxylin and eosin (H&E), and were examined in a coded fashion using the Leica Microsystems DM 4500 B Light and Fluorescence Microscopy (Wetzlar, Germany) equipped with a Jenoptik Prog Res C10 Plus Videocam (Jena, Germany). CTC clusters were defined as clusters of CTCs containing ≥ 3 distinct nuclei according to previous publications. The presence of CTC clusters or single CTCs was assessed by using a semi-quantitative score, as follows: 0 < no clusters/single cells; 1 = 1–3 clusters/single cells; 2 = 4–5 clusters/single cells; 3 = > 5 clusters/single cells. The number of TTF-1+ (or Kwss+) cells expressing mesenchymal markers and PD-L1 was counted in each specimen as the percentage of positive cells. The positivity was expressed by using a semi-quantitative score, as previously: 0 < 1%; 1 = 1–10%; 2 = 10–30%; 3 = 30–50%; 4 > 50%.Citation6 In representative images, different pseudo-colors were arbitrarily assigned, irrespectively to the actual fluorophore, to each of the four fluorescence channels and changed to optimize the visualization of the different signals. When displayed in the image, the nuclei were always showed in blue.
Results and discussion
A 6 mL of blood was drawn from 15 patients. Samples from two patients were discarded because blood was coagulated. Out of the 13 evaluable samples, 10 were successfully filtered in duplicate, while the slow processing time (exceeding 3 min, according to manufacturer instructions) only allowed us to obtain one filter from the remaining three samples. The presence and phenotype of CTCs were evaluated in filters obtained by ScreenCell Cyto kit.
Isolation of CTCs from blood samples
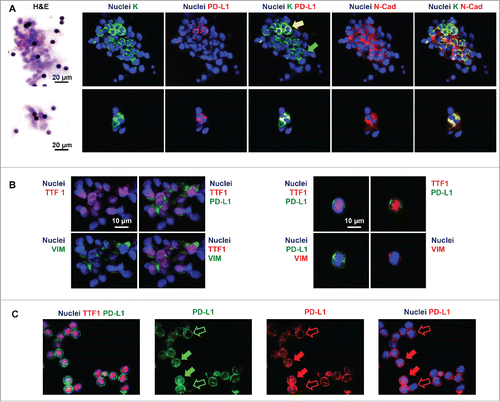
The presence of CTCs was investigated in filters stained with H&E and by immunofluorescence for markers specific of lung tumor cells such as wide spectrum screening cytokeratin (K) and TTF1. Large and small clusters of CTCs or single CTCs (, Panel A and B) were identified in 17/23 filters and an average of three clusters per filter was observed (semi-quantitative score= 1.7 ± 1.1). CTCs were variably round or elongated, presented an high nucleus to cytoplasm ratio, with scant cytoplasm and an oval nuclei.
Figure 1. In representative images, nuclei are displayed in blue. Pseudo-colors were changed for each of the four channels to optimize the visualization of the different signals. (Panel A) Phenotype of circulating tumor cells (CTCs) in filters obtained by ScreenCell Cyto kit. Hematoxylin and eosin (H&E) stains and immunofluorescence for wide spectrum screening cytokeratin (K), PD-L1, and N-Cadherin (N-Cad). Large (upper) or small (lower) clusters of K+ cells are shown. Subpopulations of K+ cells co-expressing PD-L1 and N-Cad (yellow arrow) and lacking PD-L1 expression (green arrow) are displayed. (Panel B) Immunofluorescence for thyroid transcription factor 1 (TTF1), PD-L1, and Vimentin (VIM) in filters. Clusters of TTF1+ cells (A) and single TTF1+ cells co-expressing PD-L1 and Vimentin. (Panel C) Immunofluorescence for thyroid transcription factor 1 (TTF1) and PD-L1. PD-L1 expression at cytoplasmic/membranous level (empty arrows) and at nuclear level (full arrows) in TTF1+ cells.
CTCs enumeration and immunophenotype in filters
All filters in which single or clustered CTCs were identified according to the presence of lung-cancer-specific markers (K+, TTF1+) were further evaluated by immunofluorescence for the co-expression of mesenchymal markers and PD-L1.
Filters obtained from normal subjects were constantly devoid of K+ or TTF1+ cells; few white blood cells (polymorphonuclear and mononuclear cells) were present at the periphery of the filters; rarely, few white blood cells showed positivity for PD-L1.
CTCs were found to express markers of EMT such as N-cadherin or vimentin (semi-quantitative score= 2.0 ± 0.8), as well as PD-L1 (semi-quantitative score= 2.2 ± 1.3) (, Panel A and B). PD-L1 was found co-expressed with EMT markers in a percentage of cells that was ranging between 50% and 78% (percentage of PD-L1+ cells co-expressing EMT markers= 65.7 ± 11.9) (). PD-L1 expression pattern in K+/TTF1+ cells was membranous, cytoplasmic, and nuclear (, Panel C).
Table 1. Percentage of PD-L1 positive CTCs and of double PD-L1/EMT positive CTCs in patients with NSCLC.
Discussion
Cancer can be viewed as an evolving ecosystem, able to create favorable niches that allow it to preserve itself and to propagate.Citation7 During their migration to distant organs cancer cells face barriers, which include the hostile environmental conditions in the blood and the attack from the host immune system. Cancer cells overcome these hurdles through an extraordinary adaptive response, which consists in their ability to alter the environment and to accumulate advantageous alterations that increase cellular fitness.Citation8 Upon entry into the circulation, CTCs face a foreign and unfavorable environment and interact with immune cells, constantly circulating into the bloodstream. However, because of aberrant changes in their genetic makeup and cell biology, some of them can survive and establish at distant sites. Cell plasticity yields fitness across heterogeneous environmental conditions and, owing to its dynamic nature, leads to opportunistic adaptation.Citation9 Epithelial mesenchymal transition, a trans-differentiation process whereby epithelial cells exhibit different degrees of mesenchymal phenotype, is a widely reported strategy by which cancer cells gain plasticity. It is well recognized that cells that have undergone EMT survive in the circulation better than those with an epithelial phenotype and that this plasticity perpetuates dissemination under adverse environmental conditions. Phenotypic plasticity of cancer cells has serious implications in terms of immune escape, allowing tumors to evade immune destruction by becoming less immunogenic or more immunosuppressive.Citation10,Citation11 Activation of the EMT program in cancer cells induces antigenic changes that affect the immunogenicity of a tumor.Citation12 Besides, increasing evidence indicates that EMT-like processes can alter immune functions also through the induction of immunosuppression. Indeed, a mechanistic link has been recently demonstrated between EMT and immune evasion through the elevation of multiple immune checkpoints.Citation13,Citation14 A novel mechanism of tumor-intrinsic regulation of PD-L1 has been uncovered in lung cancer linking the EMT to cytotoxic T cells dysfunction and metastasis.15 Recent evidence indicates the elevation of multiple immune checkpoint molecules, including PD-L1, in lung adenocarcinoma displaying an EMT phenotype, thus suggesting that EMT mediates immunosuppressive tumor microenvironment changes.Citation16-18 Although the complex mechanisms connecting EMT and the activation of an immune escape program in lung cancer remain incompletely understood, it seems that a mutually regulatory loop exists between the two processes orchestrated by ZEB1,a transcriptional repressor of miR-200, able to activate the EMT program and to upregulate PD-L1 expression in tumor cells leading to CD8+ T cells immunosuppression.Citation15 From an ecological point of view, the co-expression of EMT markers and PD-L1 in CTCs, which we are here reporting, might represent a coordinated strategy that these cells adopt for surviving along the journey in the blood, while protecting themselves from the immune attack. This might provide a biologic explanation for our recently published data, which demonstrate that the persistence of PD-L1 positive CTCs in NSCLC patients after 6 months of treatment predicts resistance to the anti-PD-1 nivolumab.Citation3 We here report that PD-L1 expression pattern in CTCs with EMT-like traits is not only membranous/cytoplasmic, but also nuclear. In the pivotal study by Mazel et al., the frequent expression of PD-L1 was described for the first time in CTCs from hormone receptor-positive breast cancer patients.Citation19 In a very recent publication, Satelli et al. reported that PD-L1 is commonly expressed in the nucleus of vimentin-positive CTCs isolated from colorectal and prostate cancer patients and that nuclear expression of PD-L1 in CTCs predicts poor prognosis.Citation20 Inhibitory immune checkpoints apparently function through the binding of membrane-bound ligands and corresponding receptors on the surface of a diverse range of immune effector cells. Although the aberrant expression of PD-L1 on the surface of tumor cells is well recognized and known to provide direct tumor protection from T cell mediated antitumor immunity, its nuclear expression has been only recently revealed. Ghebeh et al.Citation21 for the first time demonstrated the presence of nuclear expression of PD-L1 in breast cancer cells and its upregulation after doxorubicin treatment. In their report, the authors offered the view that the nuclear localization of PD-L1 might suggest a function that extends beyond its role in inhibiting T lymphocytes. Specifically, authors speculate that the nuclear translocation of PD-L1 might allow its interaction with the apoptotic machinery resulting in the inhibition of apoptosis, as supported by the observation that the combination of PD-L1 knockdown and doxorubicin treatment significantly enhanced apoptosis. This previously unappreciated role of PD-L1 when expressed in the nucleus of tumor cells has been indeed elegantly detailed by Azuma et al. who further provided evidence that PD-L1 can act as a receptor to transmit anti-apoptotic signal to cancer cells, leading to resistance to cytolysis by cytotoxic T cells as well as drug-induced apoptosis.Citation22
Conclusions
We for the first time demonstrate that PD-L1 and EMT markers are co-expressed in CTCs from patients with NSCLC under treatment with the immune checkpoint inhibitor Nivolumab. The demonstration that PD-L1 positive CTCs are EMT transformed cells might reinforce our recently published hypothesis that the presence of PD-L1 on CTCs from NSCLC patients seems to identify a population of patients who are not responder to Nivolumab. This might open a new scenario in the identification of biomarkers to select patients candidate to immunotherapies as well as in the design of new combination strategies targeting EMT and PD-L1.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethics approval and consent to participate
All patients gave written informed consent, and the protocol was approved by the local Ethical Committee (protocol n. 668/09).
Availability of data and material
The data sets supporting the conclusions of this article are included within the article.
KONI_A_1315488_s02.pptx
Download MS Power Point (4.5 MB)Funding
This work was supported by A.R:Ger.On Onlus.
References
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:22437870; https://doi.org/10.1038/nrc3239
- Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 2015; 14:847-56; PMID:25695955; https://doi.org/10.1158/1535-7163.MCT-14-0983
- Nicolazzo C, Raimondi C, Mancini ML, Caponnetto S, Gradilone A, Gandini O, Mastromartino M, Del Bene G, Prete A, Longo F et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor nivolumab. Sci Rep 2016; 6:31726; PMID:27553175; https://doi.org/10.1038/srep31726
- Alsuliman A, Colak D, Al-Harazi O, Fitwi H, Tulbah A, Al-Tweigeri T, Al-Alwan M, Ghebeh H. Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: Significance in claudin-low breast cancer cells. Mol Cancer 2015; 14:149; PMID:26245467; https://doi.org/10.1186/s12943-015-0421-2
- Carpino G, Cardinale V, Gentile R, Onori P, Semeraro R, Franchitto A, Wang Y, Bosco D, Iossa A, Napoletano C et al. Evidence for multipotent endodermal stem/progenitor cell populations in human gallbladder. J Hepatol 2014; 60:1194-202; PMID:24530598; https://doi.org/10.1016/j.jhep.2014.01.026
- Onori P, Wise C, Gaudio E, Franchitto A, Francis H, Carpino G, Lee V, Lam I, Miller T, Dostal DE et al. Secretin inhibits cholangiocarcinoma growth via dysregulation of the cAMP-dependent signaling mechanisms of secretin receptor. Int J Cancer 2010; 127:43-54; PMID:19904746; https://doi.org/10.1002/ijc.25028
- Yang KR, Mooney SM, Zarif JC, Coffey DS, Taichman RS, Pienta KJ. Niche inheritance: A cooperative pathway to enhance cancer cell fitness through ecosystem engineering. J Cell Biochem 2014; 115:1478-85; PMID:24700698; https://doi.org/10.1002/jcb.24813
- Casás-Selves M, Degregori J. How cancer shapes evolution, and how evolution shapes cancer. Evolution (N Y) 2011; 4:624-34; PMID:23705033; https://doi.org/10.1007/s12052-011-0373-y
- Ferrao PT, Behren A, Anderson RL, Thompson EW. Editorial: Cellular and phenotypic plasticity in cancer. Front Oncol 2015; 5:171; PMID:26301202; https://doi.org/10.3389/fonc.2015.00171
- Friedl P, Alexander S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell 2011; 147:992-1009; PMID:22118458; https://doi.org/10.1016/j.cell.2011.11.016
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565-70; PMID:21436444; https://doi.org/10.1126/science.1203486
- Terry S, Chouaib S. EMT in immuno-resistance. Oncoscience 2015; 2:841-2; PMID:26682272; https://doi.org/10.18632/oncoscience.226
- Chen L, Heymach JV, Qin FX, Gibbons DL. The mutually regulatory loop of epithelial-mesenchymal transition and immunosuppression in cancer progression. Oncoimmunology 2015; 4(5):e1002731; PMID:26155392; https://doi.org/10.1080/2162402X.2014.1002731
- Chouaib S, Janji B, Tittarelli A, Eggermont A, Thiery JP. Tumor plasticity interferes with anti-tumor immunity. Crit Rev Immunol 2014; 34:91-102; PMID:24940910; https://doi.org/10.1615/CritRevImmunol.2014010183
- Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 2014; 5:5241; PMID:25348003; https://doi.org/10.1038/ncomms6241
- Lou Y, Diao L, Cuentas ER, Denning WL, Chen L, Fan YH, Byers LA, Wang J, Papadimitrakopoulou VA, Behrens C et al. Epithelial-Mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clin Cancer Res 2016; 22:3630-42; PMID:26851185; https://doi.org/10.1158/1078-0432.CCR-15-1434
- Datar I, Schalper KA. Epithelial-Mesenchymal transition and immune evasion during lung cancer progression, the chicken or the egg? Clin Cancer Res 2016; 22:3422-4; PMID:27076625; https://doi.org/10.1158/1078-0432.CCR-16-0336
- Kim S, Koh J, Kim MY, Kwon D, Go H, Kim YA, Jeon YK, Chung DH. PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung. Hum Pathol 2016; 58:7-14; PMID:27473266; https://doi.org/10.1016/j.humpath.2016.07.007
- Mazel M, Jacot W, Pantel K, Bartkowiak K, Topart D, Cayrefourcq L, Rossille D, Maudelonde T, Fest T, Alix-Panabières C. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol 2015; 9:1773-82; PMID:26093818; https://doi.org/10.1016/j.molonc.2015.05.009
- Satelli A, Batth IS, Brownlee Z, Rojas C, Meng QH, Kopetz S, Li S. Potential role of nuclear PD-L1 expression in cell-surface vimentin positive circulating tumor cells as a prognostic marker in cancer patients. Sci Rep 2016; 6:28910; PMID:27363678; https://doi.org/10.1038/srep28910
- Ghebeh H, Lehe C, Barhoush E, Al-Romaih K, Tulbah A, Al-Alwan M, Hendrayani SF, Manogaran P, Alaiya A, Al-Tweigeri T et al. Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: Role of B7-H1 as an anti-apoptotic molecule. Breast Cancer Res 2010; 12(4):R48; PMID:20626886; https://doi.org/10.1186/bcr2605
- Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood 2008; 111:3635-43; PMID:18223165; https://doi.org/10.1182/blood-2007-11-123141
