ABSTRACT
Resident memory CD8+T cells (TRM) usually defined by the CD103 marker represent a new subset of long-lived memory T cells that remain in the tissues. We directly demonstrate their specific role in cancer vaccine-induced tumor regression. In human, they also seem to play a major role in tumor immunosurveillance.
Cancer immunotherapy based on immunomodulators (anti-CTLA-4, anti-PD-1/PD-L1) has recently demonstrated clinical efficacy for the treatment of cancer patients, but up until now cancer vaccines have failed to show any clinical impact in these same patients. Preclinical models have emphasized the role of CD8+T cells in tumor protection mediated by cancer vaccines. However, in humans, no clear correlation has been observed between the detection of anti-tumor CD8+T cells in the blood and their clinical benefit after cancer vaccine administration.Citation1
Resident memory CD8+T cells (TRM) usually defined by the CD103 marker represent a new subset of long-lived memory T cells that remain in the tissues, do not recirculate and therefore cannot be monitored by whole blood analysis. TRM appear as a specific T cell lineage as the transcription factor Hobit together with Blimp1 mediated the development of these cells from T cell precursorCitation2
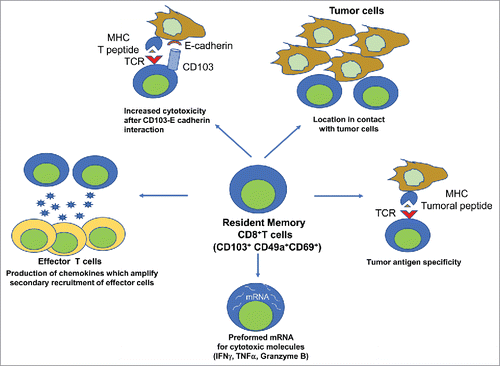
In a recent paper, we address the role of this population in the efficacy of cancer vaccines. After mucosal (intranasal) but not systemic (intramuscular) administration of a cancer vaccine targeting the E7 protein from HPV16, we induced anti-E7-TRM in the head and neck and lung tissue which correlated with the inhibition of the orthotopic tumor growth.Citation3 We then selected various strategies to specifically define the role of these cells, not biased by anti-E7 circulating CD8+T cells also induced by the vaccine: i) the use of FTY720, which blocks the recruitment of circulating T cells in the tissues; ii) co-administration of the vaccine with anti-TGFβ, which preferentially elicits circulating effector T cells over TRM; iii) parabiosis experiments, in which the blood circulations of 2 mice are connected, but not the non-circulating T cells (TRM). In all these experiments, we demonstrated that TRM are required for the efficacy of the cancer vaccine to inhibit tumor growth and that the presence of circulating CD8+T cells alone was not sufficient to control tumor growth.Citation3 In the past, the role of TRM has already been demonstrated after vaccination against pathogens.Citation1 In preclinical model of cancer, mucosal immunization was more efficient than the conventional systemic route (intramuscular, subcutaneous) to elicit TRM at the mucosal tumor site.Citation4 A correlation was observed between the ability to elicit these cells at the tumor site and control of tumor growth.Citation1,4-5 When dendritic cells were reprogrammed via treatment with the β-glucan, curdlan, a ligand of dectin-1, to induce mucosal antitumor CD8+T cells expressing CD103, these cells with a TRM phenotype were found to be more efficient than control CD8+T cells to inhibit tumor growth.Citation6 The location of TRM at the site of tumor, the enrichment of TRM in the tumor antigen specific fraction of CD8+T cells, their constitutive expression of deployment-ready mRNAs encoding pro-inflammatory cytokines and cytotoxic mediators and the potentiation of their lytic function after the CD103-E cadherin (expressed by epithelial tumor cells) interaction may explain the particular efficacy of TRM in tumor immunosurveillanceCitation7 (). In addition, they express multiple chemokines which could recruit and amplify secondary local recruitement of effector immune cells ().
Figure 1. Properties of resident memory CD8+T cells explaining their specific role in tumor immunosurveillance. TRM is located in the tumor nest via its specific interaction between CD103 and E-cadherin expressed by epithelial cells. This interaction and the preformed mRNA for cytotoxic molecules (IFNγ, TNF, Granzyme B) increase their cytotoxic activitiy toward tumor cells. They are enriched in the tumor specific fraction of CD8+T cells. They produce chemokines and IFNγ also favors chemokine production by epithelial cells, which results to a secondary recruitment of anti-tumor effector cells.
Mucosal priming seems to be required for the induction of TRM in lung tissue, as after nasal but not systemic vaccination, a co-culture of DC collected from the lung with CD8+T cells could promote a TRM phenotype .Citation8 In human, a lung-tissue-resident CD1c+DCs were able to drive CD103 expression on CD8+T cells.
To extrapolate these results to the role of TRM in human cancers, we set up an in situ multiparametric immunofluorescence study and clearly demonstrated the presence of TRM in human non-small cell lung tumor tissue. In multivariate analysis, the presence of TRM in a series of human lung cancers was correlated with better overall survival than other infiltrating immune cells. Interestingly, Ganesan et al recently confirmed these results by identifying the prognostic value of TRM in human lung cancer independently of the CD8+T cell density.Citation9
In melanoma patients vaccinated with a mixture of Melan-A peptide combined with CpG and Montanide, the ability to elicit anti-Melan A CD8+T cells expressing VLA1, a surrogate marker of TRM, was correlated with better survival of vaccinated patients.Citation10
Studies from our group and others therefore strongly argues in favor of the induction of TRM as a new goal to develop clinically successful therapeutic cancer vaccines. We also propose that the mucosal route of administration should be preferred to the systemic route (intramuscular, subcutaneous route) to preferentially elicit these cells. Our result could explain why monitoring of anti-tumor CD8+T cells often leads to controversial results in terms of correlation with the efficacy of cancer vaccines. TRM may constitute a new surrogate biomarker of spontaneous or vaccine-induced cancer immunosurveillance.
References
- Nizard M, Roussel H, Tartour E. Resident memory T cells as surrogate markers of the efficacy of cancer vaccines. Clin Cancer Res. 2016;22(3):530-2. doi:10.1158/1078-0432.CCR-15-2364. PMID:26542056
- Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C, Zaid A, Man K, Preston S, Freestone D, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 2016;352(6284):459-63. doi:10.1126/science.aad2035. PMID:27102484
- Nizard M, Roussel H, Diniz MO, Karaki S, Tran T, Voron T, Dransart E, Sandoval F, Riquet M, Rance B, et al. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat Commun. 2017;8:15221. doi:10.1038/ncomms15221. PMID:28537262
- Decrausaz L, Pythoud C, Domingos-Pereira S, Derre L, Jichlinski P, Nardelli-Haefliger D. Intravaginal live attenuated increase local antitumor vaccine-specific CD8 T cells. Oncoimmunology. 2013;2(1):e22944. doi:10.4161/onci.22944. PMID:23483225
- Sun YY, Peng S, Han L, Qiu J, Song L, Tsai Y, Yang B, Roden RB, Trimble CL, Hung CF et al. Local HPV recombinant vaccinia boost following priming with an HPV DNA vaccine enhances local HPV-Specific CD8+ T-cell-Mediated tumor control in the genital tract. Clin Cancer Res. 2016;22(3):657-69. doi:10.1158/1078-0432.CCR-15-0234. PMID:26420854
- Wu TC, Xu K, Banchereau R, Marches F, Yu CI, Martinek J, Anguiano E, Pedroza-Gonzalez A, Snipes GJ, O'Shaughnessy J, et al. Reprogramming tumor-infiltrating dendritic cells for CD103+ CD8+ mucosal T-cell differentiation and breast cancer rejection. Cancer Immunol Res. 2014;2(5):487-500. doi:10.1158/2326-6066.CIR-13-0217. PMID:24795361
- Hombrink P, Helbig C, Backer RA, Piet B, Oja AE, Stark R, Brasser G, Jongejan A, Jonkers RE, Nota B, et al. Programs for the persistence, vigilance and control of human CD8+ lung-resident memory T cells. Nat Immunol. 2016;17(12):1467-78. doi:10.1038/ni.3589. PMID:27776108
- Sandoval F, Terme M, Nizard M, Badoual C, Bureau MF, Freyburger L, Clement O, Marcheteau E, Gey A, Fraisse G, et al. Mucosal imprinting of vaccine-induced CD8+ T cells is crucial to inhibit the growth of mucosal tumors. Sci Transl Med. 2013;5(172):172ra20. doi:10.1126/scitranslmed.3004888. PMID:23408053
- Ganesan AP, Clarke J, Wood O, Garrido-Martin EM, Chee SJ, Mellows T, Samaniego-Castruita D, Singh D, Seumois G, Alzetani A, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol. 2017;18:940-50. doi:10.1038/ni.3775. PMID:28628092
- Murray T, Fuertes Marraco SA, Baumgaertner P, Bordry N, Cagnon L, Donda A, Romero P, Verdeil G, Speiser DE. Very late antigen-1 marks functional tumor-resident CD8 T cells and correlates with survival of melanoma patients. Front Immunol. 2016;7:573. doi:10.3389/fimmu.2016.00573. PMID:28018343
