ABSTRACT
Interleukin (IL)-17 has been reported to play a controversial role in tumor immunity. Our previous studies showed that infiltration of IL-17-producing cells in esophageal squamous cell carcinoma (ESCC) induced tumor protective immunity by recruiting CD8+T lymphocytes, natural killer (NK) cells, and B lymphocytes into the tumor microenvironment. However, the mechanism of IL-17 regulation of tumor-associated neutrophils remains elusive in ESCC. In this study, we therefore evaluated the accumulation of myeloperoxidase (MPO)+ neutrophils and its association with IL-17-producing cells within ESCC tumor nests. We also investigated the effects of IL-17 on the recruitment and antitumor activity of neutrophils. MPO+ neutrophil infiltration was found to predict a favorable prognosis in ESCC patients and was positively correlated with IL-17+ cell density. IL-17 stimulated ESCC tumor cells to release more of the CXC chemokines CXCL2 and CXCL3, which are involved in neutrophil migration. Furthermore, IL-17 potentiates the direct killing capability of neutrophils by enhancing the production of cytotoxic molecules, including reactive oxygen species (ROS), MPO, TNF-related apoptosis-inducing ligand (TRAIL), and IFN-γ. Experiments in mice suggested that IL-17 alone might not affect tumor progression in the tumor-bearing host, but IL-17 can inhibit tumor growth by promoting beneficial neutrophil infiltration and activation at tumor sites. As emerging evidence indicates that targeting tumor-associated neutrophils is a strategy for antitumor therapy, our findings reveal a positive contribution of IL-17 to the modulation of neutrophil-mediated antitumor immunity in ESCC. This study provides further understanding of the mechanisms that selectively regulate functional activities of neutrophils, which may be critical for developing new tumor immunotherapy.
Abbreviations
| CRC | = | colorectal cancer |
| CTLs | = | cytotoxic T lymphocytes |
| ESCC | = | esophageal squamous cell carcinoma |
| H2O2 | = | hydrogen peroxide |
| HCC | = | hepatocellular carcinoma |
| HOCl | = | hypochlorous acid |
| IL-17 | = | interleukin-17 |
| MDSCs | = | myeloid-derived suppressor cells |
| MMP-9 | = | matrix metalloproteinase-9 |
| MPO | = | myeloperoxidase |
| NE | = | neutrophil elastase |
| ROS | = | reactive oxygen species |
| TRAIL | = | TNF-related apoptosis-inducing ligand |
Introduction
Esophageal carcinoma is one of the most common cancers, ranking 6th in mortality among all cancers worldwide.Citation1 Esophageal squamous cell carcinoma (ESCC), which represents the major histological type of esophageal carcinoma, is highly prevalent in China.Citation2,3 Despite recent advances in diagnosis and therapy, the prognosis of ESCC remains unsatisfactory—the 5-year survival rate is only 15% to 25%—due to late presentation and propensity for metastasis.Citation4 Currently, the standard treatment options for ESCC are still limited to surgery, endoscopic resection and chemoradiation. Therefore, to explore novel therapeutic strategies and improve the survival of ESCC patients, elucidation of the pathogenic mechanism underlying this disease is urgently needed.
It is now recognized that the tumor microenvironment plays an important role in controlling tumor development and progression.Citation5 Accumulating evidence has indicated that tumor cell-extrinsic factors, which include immune cells, fibroblasts, structural molecules, and inflammatory cytokines, are considered to be crucial features in ESCC tumorigenesis.Citation6-8 IL-17A (hereafter referred to as IL-17) is an inflammatory cytokine primarily secreted by Th17 helper cells.Citation9 Many studies have shown that IL-17 plays a critical role in autoimmune diseases, pathogenesis of inflammatory disorders, and immune responses against bacterial or fungal infections.Citation9,10 Recently, the function of IL-17 in the tumor microenvironment has been extensively investigated.Citation11,12 Although numerous studies suggest that IL-17 can mediate tumor immunity in various types of cancer including ESCC, its specific role and related mechanisms in modulating tumor immunology remain controversial.Citation13-15
As noted in various reports, IL-17 is significantly related to neutrophil accumulation and function in the tumor microenvironment. For example, IL-17 was found to induce migration of neutrophils into the peritumoral stroma of hepatocellular carcinoma tissues by up-regulating CXC chemokine expression in epithelial cells.Citation16 In addition, IL-17-producing cells can promote recruitment of beneficial neutrophils into colorectal cancer, and enhance myeloperoxidase (MPO) release by neutrophils.Citation15 However, the potential effects of IL-17 on neutrophil recruitment and activation in ESCC remain unknown.
In this study, we therefore evaluated the infiltration and prognostic significance of neutrophils as well as their association with IL-17-producing cells in ESCC tumor nests. Furthermore, we investigated the effects of IL-17 on the migration and antitumor activity of neutrophils to understand the novel mechanisms underlying the tumor immunity in ESCC.
Results
ESCC-infiltrating MPO+ neutrophils predict favorable prognosis in patients
MPO, a lysosomal enzyme involved in activation of neutrophils, is considered a cytoplasmic marker for the identification of neutrophils within human tumors.Citation17 MPO+ neutrophils have been found to be associated with clinical outcome in several types of cancer.Citation18 Thus, we evaluated the intratumoral MPO+ neutrophil infiltration using immunohistochemistry in ESCC tumor tissues. Representative photomicrographs of stained tissue are shown in and . The patients were assigned into high or low MPO+ neutrophil groups according to the median MPO+ cell count (12.5 cells/HPF, range: 0–46.4 cells/HPF). The association between MPO+ infiltrates and various clinicopathological parameters of ESCC patients is presented in . There was an inverse correlation between MPO+ cells and T stage (P = 0.001), N stage (P = 0.014) and TNM stage (P = 0.017). However, no association between MPO+ cells and tumor grade, tumor location, tumor size or distant metastasis was observed (). The impact of MPO+ cells on overall survival (OS) of ESCC patients was then analyzed. Strikingly, patients in the high MPO+ neutrophil group had a better OS than those in the low MPO+ neutrophil group (; Supplementary Figure 1A).
Figure 1. Infiltration of MPO+ neutrophils into tumor nests and its association with survival in ESCC patients. The MPO+ neutrophils in ESCC surgical specimens were detected by immunohistochemistry. According to the median count of MPO+ cells, the patients were divided into a high MPO+ infiltration group (A) and a low MPO+ infiltration group (B). Upper panels, × 200 magnification; lower panels, × 400 magnification. (C) Survival analysis shows that overall survival (OS) was significantly improved in the high MPO+ infiltration group compared to the low MPO+ infiltration group.
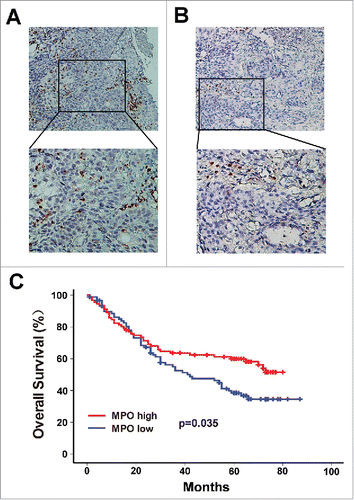
Table 1. Association of MPO+ neutrophile count and clinical characteristics in ESCC.
Tumor infiltration by MPO+ neutrophils is associated with the presence of IL-17-producing cells
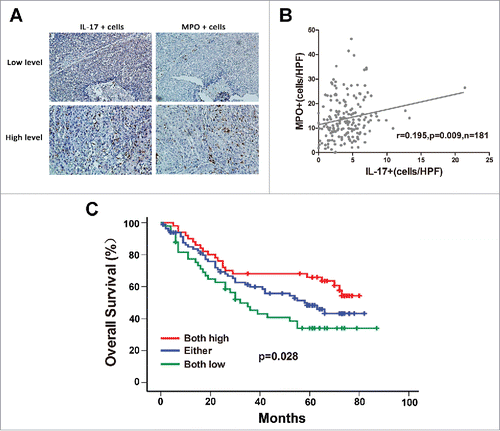
Since our previous studies indicated that IL-17-producing cells within tumor tissues were positively associated with accumulation of CD8+ CTLs and NK cells, which can mediate antitumor responses against ESCC,Citation19,20 we investigated whether IL-17+ cells were also related to the infiltration of MPO+ neutrophils in the same tumor microenvironment. Immunohistochemical analysis was performed to detect and quantify intratumoral infiltration of both cell types in serial tissue sections from the same tissue samples. As shown in , an increase in MPO+ infiltrates was seen in the tumor tissue with high IL-17+ cell counts, while decreased MPO+ cell density was associated with lower IL-17+ cell counts in the same tumor tissue. Linear regression analyses showed that the number of MPO+ neutrophils was positively correlated with the IL-17-producing cell density (n = 181, r = 0.195, P = 0.009, ). In addition, patients with high MPO+ neutrophil and high IL-17+ cell infiltration had a drastically improved OS than those with low infiltration of both MPO+ neutrophils and IL-17+ cells (; Supplementary Figure 1B).
Figure 2. Correlation between MPO+ neutrophil infiltration and IL-17-producing cells in intratumoral areas of ESCC. (A) Immunohistochemical staining of MPO+ cells and IL-17+ cells in the same tumor tissues (Upper panel: sample 1; lower panel: sample 2). Original magnification, × 200. (B) Linear regression analyses suggest that there is a significantly positive correlation between the densities of MPO+ cells and IL-17+ cells in ESCC tissues. (C) Increased infiltration of both MPO+ cells and IL-17+ cells indicated better overall survival in ESCC patients.
IL-17 favors neutrophil recruitment by triggering chemokine release from ESCC cells
We next investigated the molecular mechanism underlying the relationship between IL-17-producing cells and MPO+ neutrophils. Our previous study confirmed that ESCC-infiltrating IL-17+ cells were mainly CD4+ cells,Citation19 which ruled out the possibility of IL-17 production by neutrophils. Alternatively, we explored whether IL-17 may recruit neutrophils into tumor tissues either directly or by stimulating chemokine release by tumor cells. The effect of IL-17 on neutrophil migration was first examined with an in vitro chemotaxis system. As shown in and Supplementary Figure 2A, exposure to culture supernatants from IL-17-treated EC109 cells or KYSE30 cells significantly increased neutrophil migration compared to treatment with IL-17, supernatants from EC109 cells, KYSE30 cells alone, or a combination of the two. These data suggested that IL-17 may promote neutrophil recruitment by stimulating ESCC cells to produce more soluble mediators.
Figure 3. IL-17 recruits neutrophils by stimulating tumor cell-derived CXC chemokine production in ESCC. (A) The supernatants of IL-17-treated EC109 cells (IL17_ EC109) significantly increased the migration of neutrophils compared with IL-17 plus EC109 cell supernatant (IL17+ EC109), EC109 cell supernatant, IL-17, or medium alone. Data are presented from five separate experiments using one donor's neutrophils. (B) Fold changes in CXC chemokine mRNA levels in IL-17-treated EC109 cells compared to untreated EC109 cells were quantified by qRT-PCR. Data from three separate experiments are presented. (C) ELISA analysis showed that IL-17 stimulated EC109 cells to secrete more chemokines CXCL2 and CXCL3. Data from three separate experiments are presented. (D) Silencing of CXCL2 and CXCL3, but not of CXCL4 or CXCL16 in IL-17-treated EC109 cells attenuated the migration of neutrophils (siControl vs.siCXCL2, siCXCL3, siCXCL2+siCXCL3, siCXCL4, or siCXCL16). Data are presented from three separate experiments using one donor's neutrophils. (E and F) Immunohistochemical analysis of the association between CXCL2/ CXCL3 expression and the number of IL-17+ cells or MPO+ cells in 181 ESCC tumor samples. Representative examples of IL-17, MPO and CXCL2 or CXCL3 staining in the same tumor tissues are shown in (E). (F) Statistical analysis shows a significantly positive correlation between IL-17 and CXCL2 /CXCL3 expression, and between CXCL2 /CXCL3 expression and the number of MPO+ cells. (G) Overall survival was significantly enhanced in IL-17high MPOhigh CXCL2high or IL-17high MPOhigh CXCL3high patients compared with IL-17low MPOlow CXCL2low or IL-17low MPOlow CXCL3low patients. * P < 0.05, ** P < 0.01, *** P < 0.001.
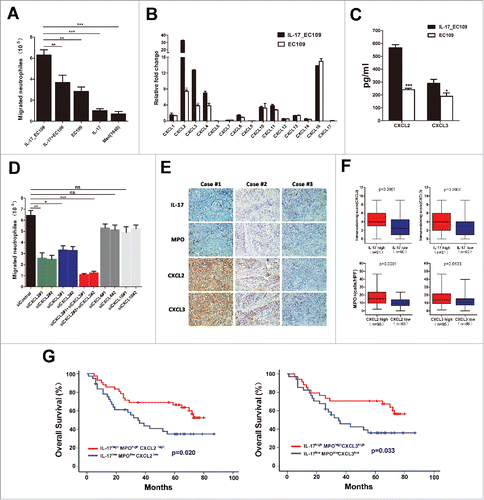
Neutrophil migration into tumor tissues is primarily induced by CXC chemokines that bind to and activate CXCR1 and/or CXCR2.Citation17 Thus, we performed real-time PCR to detect the CXC chemokine profiles of EC109 cells or IL-17-treated EC109 cells. The mRNA expression of the chemokines CXCL2 and CXCL3 was significantly up-regulated in IL-17-treated EC109 cells compared to control EC109 cells (). ELISA analysis showed that exposure to IL-17 also promoted CXCL2 and CXCL3 secretion from EC109 cells().Furthermore, neutrophil migration mediated by IL-17-treated EC109 cells was significantly attenuated by specific siRNA (Supplementary ) to inhibit CXCL2 or CXCL3 expression, or expression of both proteins (Supplementary and ).However, knockdown of CXCL4 or CXCL16 expression by specific siRNA(Supplementary ) did not significantly impair the migration of neutrophils (Supplementary Figure 3 and Figure 3D). These findings were validated when using KYSE30 cells to analyze the mechanism underlying the promotion of IL-17 on neutrophil migration (Supplementary and Supplementary Figure 3). Consistent with these in vitro data, immunohistochemical analysis from the same tissue slides showed that a high degree of IL-17+ cell infiltration was positively associated with CXCL2 expression, which was also positively correlated with the level of infiltrating MPO+ neutrophils in intratumoral tissues ( and ). Similar results were obtained when analyzing the relationship between CXCL3 expression and IL-17+ cells or MPO+ cells in the same tissues ( and ). In addition, we found that the IL-17high MPOhigh CXCL2high or the IL-17high MPOhigh CXCL3high patients exhibited a markedly improved survival compared with the IL-17low MPOlow CXCL2low or the IL-17low MPOlow CXCL3low patients (; Supplementary and ). These data indicated that IL-17 may promote beneficial neutrophil recruitment into ESCC through up-regulation of tumor cell-derived CXCL2 and CXCL3 chemokine levels.
IL-17 enhances the antitumor activity of neutrophils
As an innate immune cell population, neutrophils have been proposed to be able to kill tumor cells directly via production of several cytotoxic molecules, such as toxic oxidative metabolites, proteases, and cytokines such as tumor necrosis factor-α (TNF-α).Citation21 Hence, we tested the effect of IL-17 on the direct tumor cell killing capacity of neutrophils in vitro. Neutrophils were cultured alone or with IL-17 overnight and then co-cultured with EC109 cells at a 3:1, 10:1, or 30:1 neutrophil to tumor cell ratios. Interestingly, exposure of neutrophils to IL-17 significantly enhanced the cytotoxicity against ESCC tumor cells ().
Figure 4. IL-17 intensifies the antitumor activity of neutrophils against ESCC tumor cells by enhancing the expression of cytotoxic molecules. (A) LDH assays show that IL-17 promotes the cytotoxicity of neutrophils against EC109 cells. Data are presented from three separate experiments using one donor's neutrophils. (B) IL-17 increases ROS production in neutrophils determined by flow cytometric analysis. The flow cytometry histogram plot for depicting ROS generation is shown on the left. The corresponding mean fluorescence intensity (MFI) is presented on the right from three separate experiments using one donor's neutrophils. (C) ELISA analysis shows that IL-17 significantly enhances the release of TRAIL and IFN-γ but does not affect NE or TNF-α production from neutrophils. Data are presented from four separate experiments using one donor's neutrophils. (D-G) Flow cytometric analysis was conducted to evaluate the effect of IL-17 on the expression of MPO, CXCR1, and CXCR2 on neutrophils. Data from four separate healthy donors' neutrophils are presented. (D) The gating routine for CD16+ neutrophils from healthy donors. (E-G) The dot plots represent the MPO+ cell subset (E), the CXCR1+ cell subset (F) and the CXCR2+ cell subset (G) gating on the CD16+fraction, and the statistical analysis suggests that IL-17 can up-regulate MPO expression in neutrophils but does not affect CXCR1 or CXCR2 expression. * P < 0.05, ** P < 0.01, *** P < 0.001.
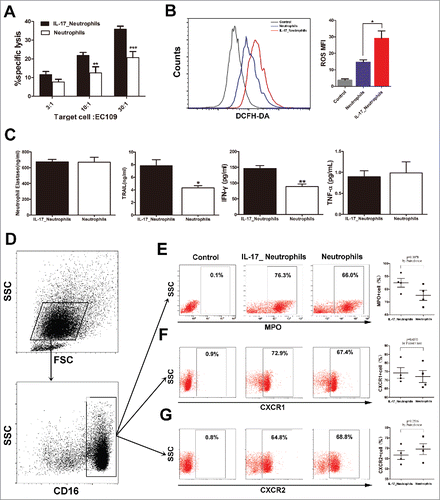
We next explored the mechanism underlying the promotive effect of IL-17 on the antitumor activity of neutrophils. The expression of cytolytic molecules was examined, including ROS, MPO, NE, and TRAIL, which are involved in inducing apoptosis and inhibition of tumor cell proliferation by neutrophils.Citation22 We observed that IL-17 significantly promoted the production of ROS, MPO and TRAIL in neutrophils (), but showed no significant direct effects on NE secretion (). Moreover, IL-17-stimulated neutrophils released higher levels of IFN-γ, though exposure of neutrophils to IL-17 did not significantly enhance TNF-α release (). Thus, IL-17 might directly stimulate neutrophils to produce more cytotoxic molecules to kill ESCC tumor cells.
CXCR1and CXCR2 are known to participate in neutrophil activation and migration into tumor nests.Citation17 IL-17 was found to promote neutrophil recruitment by stimulating tumor cell secretion of CXC chemokines, which bind to CXCR1 and/or CXCR2. We therefore investigated whether IL-17 can affect the expression of CXCR1and CXCR2 on neutrophils. Unexpectedly, no significant difference was observed in the CXCR1 or CXCR2 expression levels between neutrophils and IL-17-treated neutrophils ( and ).
IL-17 promotes neutrophil recruitment and antitumor function in vivo
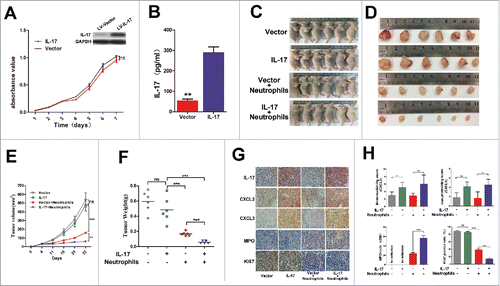
To test the effect of IL-17 on neutrophil migration and activation in vivo, we initially generated a stable IL-17-overexpressing EC109 cell line and then established a xenograft nude mouse model by subcutaneous injection of EC109 cells transfected with LV-IL-17 or LV-Vector. Before establishment of the tumor xenografts, IL-17 secretion from EC109/IL-17or EC109/Vector cells and the effect of IL-17 on EC109 cell viability in vitro was confirmed. We found that EC109/IL-17 cells can release much more IL-17 than EC109/Vector cells, but IL-17 did not significantly affect the ex vivo growth of EC109 cells ( and ). As expected, no significant difference was also observed in tumor growth between the mice injected with EC109/IL-17 cells and those injected with EC109/Vector cells (). However, there was a significant reduction in tumor size and tumor weight in neutrophil-treated mice compared with untreated controls, whereas tumors showed the most significant delays when neutrophils were infused into the mice injected with EC109/IL-17 cells (). At the end of the observation period, the tumor tissues were separated from the mice and used to evaluate CXCL2 and CXCL3 expression, beneficial neutrophil infiltration and Ki-67 expression via immunohistochemical analysis. Expectedly, CXCL2 and CXCL3 expression is much higher in the tumors with overexpression of IL-17 compared to those with Vector ( and ).In addition, expression of Ki-67 was remarkably reduced, and the level of human MPO+ cell infiltration was significantly increased in the combined IL-17-overexpression and neutrophil-treated group than in that of the other groups ( and ). These findings suggest that IL-17 itself might not regulate tumor progression in the tumor-bearing host, but it can stimulate CXCL2 and CXCL3 release to promote beneficial neutrophil infiltration and activation at tumor sites and thereby inhibit tumor growth.
Figure 5. IL-17 is involved in neutrophil recruitment and activation against ESCC tumor growth in vivo. (A) MTS assays indicated that overexpression of IL-17 does not significantly affect ex vivo EC109 cell proliferation. Data from three separate experiments are presented. (B) IL-17 secretion from EC109/IL-17 cells or EC109/Vector cells was detected by ELISA. Data from three separate experiments are presented. (C and D) Photographs of the EC109 cell xenograft model (C) and dissected tumors from nude mice (D) (n = 6). (E) The tumor growth curves for each group are presented. IL-17 overexpression markedly reduced the ESCC tumor growth rate when the nude mice were treated with human neutrophils via tail vein injection. (F) The weights of tumors from each group are shown. The final tumor weights were much lower in the group with overexpression of IL-17 plus human neutrophil infusions. (G and H) Immunohistochemical analysis for quantification of IL-17 expression, CXCL2 and CXCL3 expression, MPO+ cell infiltration, and the proliferation marker Ki-67 expression in the dissected tumors from nude mice. (G) Representative pictures of IL-17, CXCL2, CXCL3, MPO and Ki-67 staining at tumor sites for each group are shown. Original magnification, × 200. (H) The statistical analysis shows that CXCL2 and CXCL3 expression is much higher in the IL-17 overexpressing group. The tumors from the IL-17 overexpression plus human neutrophil infusion group have higher MPO+ cell infiltration and lower Ki-67 expression. Data from six separate experiments are presented. ** P < 0.01, *** P < 0.001; ns, no significance.
Discussion
Neutrophils represent the most abundant innate immune system cell population and play a critical role in immune response and host defense against microorganism invasion.Citation23 Recently, studies have begun to emerge showing the significant influence of neutrophils on the tumor microenvironment in a wide variety of human cancers.Citation24 Neutrophils are overwhelmingly considered to promote tumor progression by increasing metastasis, promoting tumor growth, immune suppression and enhancing angiogenesis.Citation25,26 However, neutrophils also have differential states of activation/differentiation, suggesting that they can convert to an anti-tumorigenesis (“N1-phenotype”) or a pro-tumorigenesis (“N2- phenotype”) state. A recent study by Fridlender and colleagues indicated that the antitumor N1 neutrophils generated in the absence of TGF-β produced more immuno-activating cytokines (e.g., TNF-a, MIP-1a, H2O2, and NO) and chemokines, lower levels of arginase, and had an enhanced ability to kill tumor cells in vitro and in vivo.Citation27
While a recent series of reports have shown that an increased neutrophil-to-lymphocyte ratio is associated with poor outcome in patients with esophageal cancer,Citation28 the study reported here, to our knowledge, is the first to identify tumor-infiltrating MPO+ neutrophils as a favorable prognostic factor in ESCC. Although the molecular mechanism underlying MPO+ cell infiltration unknown and the prognostic value in ESCC is unclear, MPO can catalyze the activity of the NADPH oxidase complex and promote the production of hypochlorous acid (HOCl) from hydrogen peroxide (H2O2), which are involved in inducing tumor cell death by neutrophils.Citation29 Thus, these data suggested that MPO might favor the neutrophil-mediated antitumor effect in ESCC. This notion is also supported by our results that the density of MPO+ neutrophils within the tumor tissues was inversely related to advanced disease stages in ESCC patients. Consistent with our present findings, several previous studies have shown that high MPO+ cell infiltration was independently associated with prolonged survival in human colorectal cancer (CRC).Citation15,18
Our previous studies found that IL-17-producing cells accumulate within the tumor tissues of ESCC patients,Citation19 where they mediate an immune response against the tumor by attracting and activating various immune cells to the tumor microenvironment, such as CD8+ T cells, NK cells, and CD1a+ DCs.Citation19,20 In this study, we further investigated whether neutrophils are also involved in IL-17-mediated antitumor immunity. Interestingly, IL-17-producing cells exhibited a positive correlation with MPO+ neutrophil infiltration in the same tumor microenvironment. Moreover, IL-17 was observed to induce neutrophil migration into ESCC by enhancing tumor cell-derived CXC chemokine levels, and subsequently, neutrophils were activated by IL-17 and exhibited a cytolytic effect against ESCC tumor cells though production of immuno-activating cytokines such as ROS, MPO, TRAIL and IFN-γ. These findings reveal that IL-17 might exert an antitumor effect by promoting a beneficial neutrophil-mediated immune response in ESCC tumor nests.
IL-17-producing cells have been investigated in various types of cancer, and IL-17 has been found to play a dual role in tumor immunity.Citation30 Kuang et al. reported that generation of IL-17-producing cells in peritumoral stroma was promoted by activated monocytes/macrophages in the same area, and their levels were associated with poor prognosis in HCC patients.Citation31 Conversely, in a study by Amicarella et al., the presence of intraepithelial but not stromal IL-17+ cells was positively associated with a favorable clinical outcome by driving beneficial neutrophils and highly cytotoxic CD8+ T cells into tumor nests through IL-8 secretion, and CCL5 and CCL20 release.Citation15 Our present and previous study showed that increased density of intratumoral IL-17-producing cell infiltration was positively correlated with improved survival in ESCC patients by recruiting and activating several antitumor immune cells including CD8+ T cells, NK cells, CD20+ B cells, and MPO+ neutrophils.Citation19,20,32 These discrepant findings suggested that the relationship between IL-17 and tumor immunopathology is highly reliant on context, including anatomic location, cellular source, and the associated tumor microenvironment.
Neutrophil infiltration has been found to be closely associated with tumor progression in diverse types of cancer.Citation24,33 Under different tumor contexts, neutrophils can be polarized into either a pro-tumoral (N2) or an anti-tumoral (N1) phenotype,Citation34 but the regulation mechanism of neutrophil plasticity in the tumor microenvironment remains largely unknown. Our current study shows that IL-17 may be an important mediator in neutrophil recruitment and polarization in ESCC based on the following findings. First, in addition to the significant correlation between the number of IL-17-producing cells and the MPO+ neutrophil density in tumor tissues, the intratumoral localization of both these two cell types was found to be associated with improved survival in ESCC patients. Additionally, exposure of ESCC tumor cells to IL-17 leads to higher production of several CXC chemokines that are known to be involved in neutrophil recruitment. This induction of neutrophil transmigration was inhibited by silencing the expression of CXC chemokines in ESCC tumor cells. Consistent with these in vitro findings, a high degree of IL-17+ cell infiltration was associated with up-regulation of CXC chemokine expression in ESCC tissues, and subsequently, more beneficial neutrophils infiltrated into the tumors. Moreover, IL-17 significantly enhanced the tumoricidal activity of neutrophil against ESCC tumor cells by stimulating the productions of cytolytic molecules, including ROS, MPO, TRAIL and IFN-γ. Finally, overexpression of IL-17 was shown to favor tumor infiltration by MPO+ neutrophils in the ESCC mouse model, ultimately mediating xenograft tumor eradication.
Our findings are supported by many other studies showing that IL-17 could recruit neutrophils by increasing epithelial cell or tumor cell-derived CXC chemokines including CXCL2 and CXCL3.Citation16,35 Although the mechanism underlying the promotion of IL-17 on CXCL2 and CXCL3 production is unclear, extensive studies have demonstrated that IL-17 exerts its effects on the expression of chemokines through NF-κB and MAPKs signaling pathways.Citation36,37 To explore whether IL-17 enhances CXCL2 and CXCL3 expression via NF-κB or MAPKs signaling pathways, we used specific siRNA to inhibit NF-κB (NF-κB1) or MAPK (p38) expression in the ESCC tumor cells (EC109 and KYSE30).However, we found that inhibition of NF-κB1 or p38 did not affect the increase of CXCL2 and CXCL3 production by IL-17 in EC109 and KYSE30 cells (data not shown). These indicates that other mechanism should be investigated underlying the impact of IL-7 on chemokines expression in ESCC.
In the report by Kuang et al.,Citation16 however, the peritumoral neutrophils that accumulated via IL-17 activity were found to secrete matrix metalloproteinase-9 (MMP-9), which in turn stimulated proangiogenic activity in hepatoma cells and ultimately fostered disease progression, whereas in our study IL-17 was shown to promote beneficial neutrophil migration into intratumoral areas and to enhance the antitumor activity of neutrophils, which ultimately induced tumor regression and predicted a favorable prognosis. These conflicting effects reported for IL-17 on neutrophils and tumor progression may be a consequence of complicated immunoregulation in different tumor microenvironments.Citation30,38 Indeed, IL-17 may contribute to protective human tumor immunity by inducing Th1-type chemokines and recruiting effector cells in ovarian cancer, whereas IL-17 induces IL-6 production by tumor cells or tumor-associated stromal cells and thus promotes tumor growth via an IL-6–Stat3 pathway in melanoma and bladder cancer.Citation39,40 Similarly, tumor-associated neutrophils are shown to have pro-tumorigenic or anti-tumorigenic functions, depending on the presence of TGF-β in the tumor microenvironment.Citation27 Therefore, based on the data in this study, we hypothesize that there may be a fine-tuned collaborative action between IL-17 and neutrophils in ESCC tumor microenvironments that reroutes the neutrophil-mediated immune response in an antitumor direction. Consistent with our findings, a recent report revealed a promotive effect of Th17 on neutrophil recruitment and activation in human colorectal cancer, which was associated with prolonged survival in patients.Citation15
Emerging evidence shows that targeting tumor-associated neutrophils may be a strategy for antitumor therapy. Exploring the mechanisms that selectively regulate the functional activities of neutrophils is critical for developing new neutrophil-targeted treatment. Indeed, the positive contribution of IL-17 to modulating neutrophil-mediated antitumor immune responses should not be disregarded when developing more effective tumor immunotherapy for ESCC.
Materials and methods
Cell line and culture
The ESCC cell lines EC109 and KYSE30 was obtained from the Committee of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured at 37°C in 5% CO2 in RPMI 1640 medium (Invitrogen, Shanghai, China) supplemented with 10% fetal bovine serum (FBS; Gibco, NY, USA) and 1% penicillin-streptomycin. A total of 1 × 106 tumor cells were then harvested and placed in six-well plates and treated with or without 50 ng/ml human IL-17 (Proteintech, Chicago, USA) for 24 h.
Patients and tissue samples
Paraffin-embedded tissue samples were obtained from 181 ESCC patients who underwent surgical resection at the Sun Yat-sen University Cancer Center (SYSUCC) from October 21, 2002 to June 14, 2004. The follow-up data of the patients in this study were more than five years, with a median of44 months (range: 3–87 months) of follow-up time. These patients included 141 males and 40 females, with a median age of 56 years (range, 33–79 years). All of the diagnoses were histologically confirmed by experienced pathologists. All of the ESCC patients underwent curative surgical resection and none of them received chemotherapy or radiotherapy before the surgery. The clinical stage was determined according to the American Joint Committee on Cancer (AJCC) TNM staging system. OS, which was calculated from the date of surgery to the date of death or until the last follow-up, was used to determine the prognosis of patients. This study was approved by the Research Ethics Committee of Sun Yat-sen University Cancer Center. Written consent was obtained from each patient.
Immunohistochemical analysis and evaluation
Consecutive 3-µm-thick sections were cut from the tissue samples for immunohistochemical analysis according to standard protocols. In brief, the specimens were deparaffinized, rehydrated and washed followed by microwave antigen retrieval in Tris-EDTA (pH 8.0). After endogenous peroxidase was blocked with 0.3% H2O2, the sections were incubated with goat serum to block non-specific binding and then incubated with primary antibodies against human IL-17 (1:100, R&D Systems), MPO (1:400, R&D systems), CXCL2 (1:300, PeproTech Inc.), CXCL3 (1:300, PeproTech Inc.), or Ki-67 (1:400, Cell Signaling Technology) overnight at 4°C. The slides were then serially rinsed, incubated with HRP-conjugated secondary antibodies, visualized with 3, 3′-diaminobenzidine tetrahydrochloride and counterstained with hematoxylin followed by washing, dehydration and evaluation. For evaluating IL-17 and MPO staining, positively stained cells were manually counted in ten randomly selected fields under high power 400 × magnification (cells/HPF). The average number of positively stained cells per HPF was calculated as the density of IL-17+ cells or MPO+ cells. For Ki-67 evaluation, the percentage of immunoreactive cells among the total tumor cells was quantified. To assess CXCL2 or CXCL3 expression, the total immunostaining observed in each specimen was scored using a semiquantitative immunoreactivity score (IRS) as described previously.Citation41 Briefly, both staining intensity and percentage of positively stained tumor cells among the total tumor tissue were scored from 0–3. The intensity was classified as “0” (no staining), “1” (weakly stained), “2” (moderately stained) or “3” (well stained), and the percent positivity was scored as “0” ( < 5%), “1” (5%–25%), “2” (25%–50%), or “3” (≥50%). Multiplication of the intensity and percent positivity determined the final immunostaining score (ranging from 0 to 9). The protein expression levels of CXCL2 or CXCL3 were classified as high when the final immunostaining scores were ranged from 4 to 9; otherwise, the expression levels were evaluated as low (final immunostaining scores ranged from 0 to 3).
Neutrophil isolation and culture
Human peripheral blood from a total of 11 healthy donors were collected and subjected to Ficoll density-gradient centrifugation. Neutrophils were then isolated from the pale-red granulocyte and contaminated erythrocyte layers using a red blood cell lysis buffer (Sigma-Aldrich, Atlanta, GA) according to the manufacturer's protocol. After confirming the cell purity (>90%) by flow cytometry with human anti-CD16 antibody (BD Bioscience, San Diego, USA), neutrophils were resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated FBS and 100 ìg/ml streptomycin plus 100 U/ml penicillin. Thereafter, the cells were cultured with or without human IL-17 (50 ng/ml) for no more than 48 h at 37°C in 5% CO2 and used for subsequent experiments.
In Vitro neutrophil migration assay
A total of 2.5 × 106 neutrophils were resuspended in 100 ml of RPMI 1640 medium and added to the upper chamber of 24-well transwell chamber system (3.0 um pore size, Corning, NY). After incubation at 37°C for 4 h, the cells that had migrated into the lower chamber were harvested and counted using a hemocytometer. The migration assays were conducted in the presence of IL-17 or culture supernatant from ESCC cells or IL-17-treated ESCC cells in the lower chamber of the transwell chamber system. In some experiments, ESCC cells were transfected with negative control siRNA, CXCL2 and/or CXCL3 siRNA, CXCL4 siRNA, or CXCL16 siRNA before IL-17 stimulation, and the culture supernatants were harvested for migration assays.
Real-time quantitative PCR
The real-time quantitative PCR analysis was performed as previously described.Citation41 Briefly, total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) from ESCC cells that were treated with or without 50 ng/ml IL-17 for 24 h. The mRNA expression levels of CXC chemokines and GAPDH (an internal control) were measured using SYBR Green Master Mix (Invitrogen, USA) in an ABI Prism 7900HT Sequence Detection System (Life Technologies, Carlsbad, CA, USA). The specific primers used are presented in Supplementary Table 2. The relative expression levels of the target mRNA were calculated and normalized to the GAPDH expression level using the comparative threshold cycle (2-ÄÄCT) method.
Cytotoxicity assays
After culture with or without IL-17 for 24 h, the cytolytic ability of neutrophils against EC109 cells was assessed using a Cyto Tox 96 Lactate Dehydrogenase Assay Kit (Promega) according to the manufacturer's protocol. Effector neutrophils were added to the target at an effector cell to target cell (E:T) ratio of 3:1, 10:1, or 30:1, and the cells were co-cultured for 12 h.
Flow cytometry analysis
The intracellular production of reactive oxygen species (ROS) and MPO and the cell surface expression of CXCR1 and CXCR2 were measured with flow cytometry. To determine MPO expression, neutrophils cultured with or without IL-17 were stimulated by 50 ng/mL phorbol myristate acetate, 500 ng/mL ionomycin (Sigma-Aldrich) and 2 μM protein transport inhibitors (GolgiStop, BD Biosciences) for 4 h. The cells were then stained with human anti-MPO antibody (BD Bioscience, San Diego, USA) in reagent from a Fixation/Permeabilization Solution Kit (BD Pharmingen) according to the manufacturers' instructions and evaluated on a Cytomics FC500 Flow Cytometer (Beckman Coulter). To test CXCR1 and CXCR2 expression, neutrophils were stained with human anti-CXCR1and anti-CXCR2 antibodies (BD Bioscience) and then analyzed on the flow cytometer. ROS were measured using the oxidation-sensitive probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA) (Applygen, Beijing, China) according to the manufacturer's protocol. Briefly, neutrophils were treated with or without IL-17 overnight and then loaded with DCFH-DA in serum-free medium at 37 °C for 30 min. Then, the cells were washed with PBS, and DCFH fluorescence emission was measured by flow cytometry. The mean fluorescence intensity (MFI) data was quantified using FlowJo software.
Enzyme-linked immunosorbent (ELISA) assay
Secretion of the chemokines CXCL2 ,CXCL3 and the cytotoxic molecules neutrophil elastase (NE), TNF-related apoptosis-inducing ligand (TRAIL), tumor necrosis factor-α (TNF-α), or interferon-γ (IFN-γ) in the culture supernatants of neutrophils cultured with or without IL-17 was examined using a commercially available ELISA kit (4A Biotech Co., Ltd, Beijing, China) according to the manufacturer's protocol.
Generation of stable IL-17-overexpressing cell lines
Recombinant lentivirus carrying human IL-17 overexpression plasmid (designated as LV-IL-17) or empty control vector (designated as LV-Vector) was purchased from GenePharma (Shanghai, China). EC109 cells were cultured in 6-well plates and infected with the lentivirus in serum-free RPMI 1640 medium supplemented with 5 mg/ml polybrene (Sigma-Aldrich, USA). After 48 h, stable transformants were selected with 5 μg/ml puromycin and cultured for further analysis. The stable cell lines were designated EC109/IL-17 or EC109/Vector.
Proliferation assay
The proliferation rates of EC109/ IL-17 cells and EC109/Vector cells were measured with MTS assays. The cells were seeded in 96-well plates at a density of 1,000 cells/well, and the growth rate was assessed using an MTS cell proliferation kit (Promega, USA) according to the manufacturer's protocol.
Tumorigenicity assays in nude mice
Four-week-old female BALB/c nude mice were purchased from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and maintained under pathogen-free conditions in the animal experiment center of Sun Yat-sen University (Guangzhou, China). The mice were randomly divided into 4 groups, with six mice in each group, and subcutaneously injected with a total of 5 × 106 EC109/IL-17 cells (two groups) or EC109/Vector cells (two groups) suspended in 100 μl of PBS containing 30% Matrigel Basement Membrane Matrix. When tumors formed, one group of mice injected with EC109/IL-17 cells and one group injected with EC109/Vector cells were treated with 1 × 107 human neutrophils via tail vein injection twice per week. Tumor growth was estimated every 7 days by caliper measurement, and tumor volumes were calculated as previously described.Citation41 On day 32 after tumor cell implantation, all mice were sacrificed, and tumors were harvested, photographed, weighed, fixed in formalin overnight, dehydrated and embedded in paraffin. Tumor sections were then used to evaluate human IL-17 expression, CXCL2 and CXCL3 expression, Ki67-positive tumor cells and human MPO+ neutrophil infiltration through immunohistochemical analysis. All animal experiments in this study were performed according to the institutional ethical guidelines for animal experiments and the Guide for the Care and Use of Laboratory Animals (NIH publication Nos. 80-23, revised 1996).
Statistical analysis
All statistical analyses were conducted using SPSS version 20.0 or GraphPad Prism 5 software. The relationship between 2 groups was determined using Pearson correlation coefficient or linear regression analyses. Survival curves were calculated using the Kaplan-Meier method and compared using the log-rank test. Student's t-test or a Mann-Whitney U test was used to compare the differences between subgroups. The results are represented as the mean ± SD. A two-tailed P value ≤ 0.05 was considered statistically significant.
Disclosure of potential conflicts of interest
All authors have declared that they have no conflicts of interest regarding this work.
Authenticity of this article
Our study has been validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn) at Sun Yat-Sen UniversityCancer Center (approval number: RDDB2017000164).
supp_data.zip
Download Zip (3.2 MB)Acknowledgments
This work was supported by a grant from the National Natural Science Foundation for Young Scholar of China (81402560), the National Natural Science Foundation of China (31270964, 81572865 and 81472387), and the Guangdong Province Science and Technology Plan Project (2013B021800063).
References
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-86.[https://doi.org/10.1002/ijc.29210]. PMID:25220842
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-32.[https://doi.org/10.3322/caac.21338]. PMID:26808342
- Roshandel G, Nourouzi A, Pourshams A, Semnani S, Merat S, Khoshnia M. Endoscopic screening for esophageal squamous cell carcinoma. Arch Iran Med. 2013;16:351-7. PMID:23725069
- Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400-12.[https://doi.org/10.1016/S0140-6736(12)60643-6]. PMID:23374478
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646-74.[https://doi.org/10.1016/j.cell.2011.02.013]. PMID:21376230
- Lin EW, Karakasheva TA, Hicks PD, Bass AJ, Rustgi AK. The tumor microenvironment in esophageal cancer. Oncogene. 2016;35:5337-49.[https://doi.org/10.1038/onc.2016.34]. PMID:26923327
- Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947-53.[https://doi.org/10.1158/1078-0432.CCR-04-1469]. PMID:15837746
- Xu B, Chen L, Li J, Zheng X, Shi L, Wu C, Jiang J. Prognostic value of tumor infiltrating NK cells and macrophages in stage II+III esophageal cancer patients. Oncotarget. 2016;7:74904-16. PMID:27736796
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485-517.[https://doi.org/10.1146/annurev.immunol.021908.132710]. PMID:19132915
- Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148:32-46.[https://doi.org/10.1111/j.1365-2249.2007.03356.x]. PMID:17328715
- Marshall EA, Ng KW, Kung SH, Conway EM, Martinez VD, Halvorsen EC, Rowbotham DA, Vucic EA, Plumb AW, Becker-Santos DD, et al. Emerging roles of T helper 17 and regulatory T cells in lung cancer progression and metastasis. Mol Cancer. 2016;15:67.[https://doi.org/10.1186/s12943-016-0551-1]. PMID:27784305
- Qian X, Chen H, Wu X, Hu L, Huang Q, Jin Y. Interleukin-17 acts as doubleedged sword in anti-tumor immunity and tumorigenesis. Cytokine. 2017;89:34-44.[https://doi.org/10.1016/j.cyto.2015.09.011]. PMID:26883678
- Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, Vernes JM, Jiang Z, Meng YG, Peale FV, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013;19:1114-23.[https://doi.org/10.1038/nm.3291]. PMID:23913124
- Honorati MC, Neri S, Cattini L, Facchini A. IL-17 enhances the susceptibility of U-2 OS osteosarcoma cells to NK cell lysis. Clin Exp Immunol. 2003;133:344-9.[https://doi.org/10.1046/j.1365-2249.2003.02234.x]. PMID:12930359
- Amicarella F, Muraro MG, Hirt C, Cremonesi E, Padovan E, Mele V, Governa V, Han J, Huber X, Droeser RA, et al. Dual role of tumour-infiltrating T helper 17 cells in human colorectal cancer. Gut. 2017;66:692-704.[https://doi.org/10.1136/gutjnl-2015-310016]. PMID:26719303
- Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin XY, Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54:948-55.[https://doi.org/10.1016/j.jhep.2010.08.041]. PMID:21145847
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618-31.[https://doi.org/10.1038/nrc2444]. PMID:18633355
- Droeser RA, Hirt C, Eppenberger-Castori S, Zlobec I, Viehl CT, Frey DM, Nebiker CA, Rosso R, Zuber M, Amicarella F, et al. High myeloperoxidase positive cell infiltration in colorectal cancer is an independent favorable prognostic factor. PLoS One. 2013;8:e64814.[https://doi.org/10.1371/journal.pone.0064814]. PMID:23734221
- Lv L, Pan K, Li XD, She KL, Zhao JJ, Wang W, Chen JG, Chen YB, Yun JP, Xia JC. The accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinoma. PLoS One. 2011;6:e18219. PMID:21483813
- Lu L, Pan K, Zheng HX, Li JJ, Qiu HJ, Zhao JJ, Weng DS, Pan QZ, Wang DD, Jiang SS, et al. IL-17A promotes immune cell recruitment in human esophageal cancers and the infiltrating dendritic cells represent a positive prognostic marker for patient survival. J Immunother. 2013;36:451-8. PMID:23994890
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: From mechanisms to disease. Annu Rev Immunol. 2012;30:459-89. PMID:22224774
- Lavazza C, Carlo-Stella C, Giacomini A, Cleris L, Righi M, Sia D, Di Nicola M, Magni M, Longoni P, Milanesi M, et al. Human CD34+ cells engineered to express membrane-bound tumor necrosis factor-related apoptosis-inducing ligand target both tumor cells and tumor vasculature. Blood. 2010;115:2231-40. PMID:20075160
- Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657-70. PMID:21094463
- Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: Neutral no more. Nat Rev Cancer. 2016;16:431-46. PMID:27282249
- Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013; 123:3446-3458. PMID:23863628
- Tazzyman S, Niaz H, Murdoch C. Neutrophil-mediated tumour angiogenesis: Subversion of immune responses to promote tumour growth. Semin Cancer Biol. 2013;23:149-58. PMID:23410638
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16:183-94. PMID:19732719
- Yodying H, Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Yamada M, Uchida E. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: A systematic review and meta-analysis. Ann Surg Oncol. 2016;23:646-54. PMID:26416715
- Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20:300-14. PMID:21907922
- Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248-56. PMID:20336152
- Kuang DM, Peng C, Zhao Q, Wu Y, Chen MS, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology. 2010;51:154-64. PMID:19902483
- Lu L, Weng C, Mao H, Fang X, Liu X, Wu Y, Cao X, Li B, Chen X, Gan Q, et al. IL-17A promotes migration and tumor killing capability of B cells in esophageal squamous cell carcinoma. Oncotarget. 2016;7:21853-64. PMID:26942702
- Finisguerra V, Di Conza G, Di Matteo M, Serneels J, Costa S, Thompson AA, Wauters E, Walmsley S, Prenen H, Granot Z, et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature. 2015;522:349-53. PMID:25985180
- Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, Damti P, Lumbroso D, Polyansky L, Sionov RV, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10:562-73. PMID:25620698
- Gelderblom M, Weymar A, Bernreuther C, Velden J, Arunachalam P, Steinbach K, Orthey E, Arumugam TV, Leypoldt F, Simova O, et al. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood. 2012;120:3793-802. PMID:22976954
- Song X, Qian Y. The activation and regulation of IL-17 receptor mediated signaling. Cytokine. 2013;62:175-82. PMID:23557798
- Wu L, Chen X, Zhao J, Martin B, Zepp JA, Ko JS, Gu C, Cai G, Ouyang W, Sen G, et al. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J Exp Med. 2015;212:1571-87. PMID:26347473
- Fridlender ZG, Albelda SM. Tumor-associated neutrophils: Friend or foe? Carcinogenesis. 2012;33:949-55. PMID:22425643
- Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141-9. PMID:19470694
- Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457-64. PMID:19564351
- Chen CL, Wang Y, Pan QZ, Tang Y, Wang QJ, Pan K, Huang LX, He J, Zhao JJ, Jiang SS, et al. Bromodomain-containing protein 7 (BRD7) as a potential tumor suppressor in hepatocellular carcinoma. Oncotarget. 2016;7:16248-61. PMID:26919247
