ABSTRACT
Prolonged low-grade inflammation or smoldering inflammation is a hallmark of a cancer. Eosinophils are components of the immune microenvironment that modulates tumor initiation and progression. Although canonically associated with a detrimental role in allergic disorders, these cells can induce a protective immune response against helminthes, viral and bacterial pathogens. Eosinophils are a source of anti-tumorigenic (e.g., TNF-α, granzyme, cationic proteins, and IL-18) and protumorigenic molecules (e.g., pro-angiogenic factors) depending on the milieu. In several neoplasias (e.g., melanoma, gastric, colorectal, oral and prostate cancer) eosinophils play an anti-tumorigenic role, in others (e.g., Hodgkin's lymphoma, cervical carcinoma) have been linked to poor prognosis, whereas in yet others they are apparently innocent bystanders. These seemingly conflicting results suggest that the role of eosinophils and their mediators could be cancer-dependent. The microlocalization (e.g., peritumoral vs intratumoral) of eosinophils could be another important aspect in the initiation/progression of solid and hematological tumors. Increasing evidence in experimental models indicates that activation/recruitment of eosinophils could represent a new therapeutic strategy for certain tumors (e.g., melanoma). Many unanswered questions should be addressed before we understand whether eosinophils are an ally, adversary or neutral bystanders in different types of human cancers.
Introduction
Eosinophils, first identified in peripheral blood and named by Paul Ehrlich in the 1870 s, are present in all classes of vertebrates and it has been estimated that they have emerged, long before the development of adaptive immunity.Citation1 Human eosinophils derive from CD34+CD117+ pluripotent hematopoietic stem cells in the bone marrow, where they complete their maturation and subsequently enter the circulationCitation2 where they represent ≅ 1% of leukocytes. Upon activation, these cells synthesize and release a plethora of biologically active mediators that individually have potential positive or negative effects on various target cells.Citation3 Eosinophils act as sentinels of the surrounding environment, with the capacity to rapidly perceive tissue insults and initiate biochemical programs of inflammation or repair.Citation3–5
Eosinophils and their mediators have been canonically associated with a detrimental role in allergic diseases,Citation3,6 but these cells can induce a protective immune response of the host against helminthes,Citation7 viralCitation8,9 and microbial pathogens.Citation10–12 Interestingly, epidemiologicalCitation13,14 and experimental studiesCitation15 indicate an inverse association between IgE-mediated allergies and cancer, suggesting a tumor-protective effect of IgE.
The initiation and progression of cancer are the result of multi-step processes characterized by the accumulation of driver gene mutationsCitation16 and epigenetic alterations.Citation17,18 The immune system recognizes and eliminates mutant cells constantly generated.Citation19 However, immune-resistant cancer cells can slip through this system and proceed to develop tumors.Citation20
Normal microenvironment (innate and adaptive immune cells, fibroblasts, blood and lymphatic vessels, and extracellular matrix) maintains tissue homeostasis and is a barrier to cancer development.Citation21,22 Incorrect signals (chemokines, cytokines, reactive oxygen species, lipid mediators, etc.) from an aberrant microenvironment alter tissue homeostasis and initiate/promote tumor growth.Citation23 Smoldering inflammation is a hallmark of cancer.Citation24,25 Several innate and adaptive immune cells, such as macrophages, mast cells, lymphocytes, neutrophils, natural killer (NK) T cells and eosinophils, are stromal components of the inflammatory microenvironment that influence the development of experimental and human tumors.Citation26–29
Basic biology of eosinophils
Eosinophils represent a minority of peripheral blood leukocytes and have been erroneously neglected for decades. Immunologists and oncologists are now appreciating that these cells produce a plethora of mediators such as cationic proteins [major basic protein (MBP), eosinophil cationic protein (ECP), eosinophil peroxide (EPX), and eosinophil-derived neurotoxin (EDN)], cytokines, chemokines, angiogenicCitation30,31 and lipid mediators.Citation3 In addition, eosinophils can adhere to activated endothelial cells, leave the bloodstream and concentrate at inflammatory sites.Citation2,5 Resident eosinophils in the intestine,Citation32 thymus,Citation33 uterus,Citation34 lung,Citation35 and adipose tissueCitation36 are increasingly seen as homeostatic cells with critical roles in normal development and/or morphogenesis.Citation4,37–39 This function is thought to occur through secretion of cytokines and growth factors such as TGF-β, involved in tissue remodelling and immune homeostasis.Citation40 Moreover, human eosinophils play a major role in the modulation of a wide spectrum of innate and adaptive immune cells, including several subsets of lymphocytes, macrophages, mast cells, basophils, neutrophils, dendritic and plasma cells, epithelial and fat cells.Citation23,41,42
Interleukin-5 (IL-5) is the most important growth, differentiation, and activating factor for human eosinophils.Citation3,43 This cytokine acts on target cells by binding to the specific IL-5 receptor (IL-5R), which consists of an IL-5 Receptor α (IL-5Rα) subunit and common receptor β subunit (βc).Citation43 The βc subunit is a signal-transducing molecule shared with the receptors for IL-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF).Citation43 IL-5 is mainly produced by type-2 innate lymphoid cells (ILC2), Th2 cells, mast cells, invariant NKT cells, and eosinophils themselves.Citation44,45 IL-5, together with IL-3, and GM-CSF, is crucial for supporting the maturation of human eosinophils in the bone marrow and GATA-1 is a transcription factor critical for eosinophils development.Citation46 IL-5, along with IL-3 and GM-CSF, mediates eosinophil survival by NF-kB-induced Bcl-xL, which inhibits apoptosis. Increasing evidence indicates that IL-33 is required for basal eosinophil homeostasis.Citation47 This cytokine regulates eosinophils at multiple levels: during their maturation in the bone marrow, activation of mature cells, and probably development/activation of eosinophil progenitors within tissue.Citation2,48,49 In addition, IL-33 can directly activate eosinophils inducing up-regulation of the adhesion molecule CD11b and the activation marker CD69, expression of pro-inflammatory cytokines and chemokines, superoxide anion production and degranulation.Citation50–53
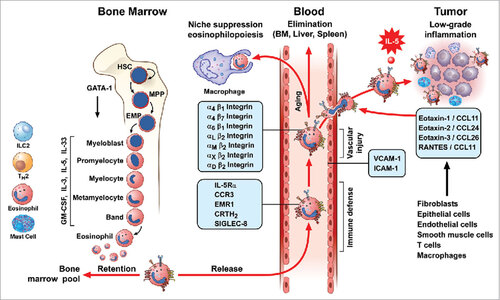
Eosinophils have the propensity to leave the bloodstream and migrate into inflamed tissues including tumor microenvironment (TME). Migration of eosinophils is mediated by the adhesion of several integrins expressed by activated eosinophils to endothelial cells.Citation54 The attraction of eosinophils into inflamed tissues is mediated by eotaxins (eotaxin-1/CCL11, eotaxin-2/CCL24, eotaxin-3/CCL26), and RANTES/CCL5 that activate the CCR3 receptor highly expressed on human eosinophils.Citation55–57 However, tissue accumulation of eosinophils not only relies on recruitment of from blood stream, but is also the result of in situ eosinophilopoiesis and self turnover in response to survival/differentiation factors produced by neighboring cells, including stem cells.Citation39 Thus, local production of IL-5, IL-33 and presumably other cytokines, can increase eosinophils survival and sustain regional eosinophilopoiesis.Citation2 schematically illustrates a life-cycle model of eosinophil maturation in the bone marrow, circulation in peripheral blood and migration into inflamed tissues including TME.
Figure 1. Life-cycle models of eosinophils. Eosinophils are produced in the bone marrow through a series of progenitors from the most immature stem cells (Hematopoietic Stem Cells: HSC), Multipotent Progenitor Cell: MPP) to committed eosinophil progenitors (Semper, Muehlberg et al.) that finally generate mature eosinophils. This process of proliferation and differentiation is driven by transcription factors (e.g. GATA-1). IL-5, together with IL-3 and GM-CSF, controls eosinophil development in the bone marrow. Recent evidence indicates that IL-33 precedes IL-5 in regulating eosinophilopoiesis via the activation of the IL-33R receptor, ST2. Circulating human eosinophils selectively express IL-5Rα, CCR3, EMR1, CRTH2, and Siglec-8. Eosinophil can leave the bloodstream and target the bone marrow, liver, and spleen for elimination. In some tissues, eosinophils can be phagocytosed by macrophages. Eosinophils express a wide spectrum of integrins (α and β) and can roll and adhere to VCAM-1 and ICAM-1 on activated endothelial cells. This interaction favors the chemotactic activity of several chemokines (eotaxin-1/CCL11, eotaxin-2/CCL24, eotaxin-3/CCL26, and RANTES) that activate the CCR3 receptor highly expressed on eosinophils. This explains the propensity of eosinophils to leave the bloodstream and migrate into inflamed tissues and certain tumors. Several cells (fibroblasts, epithelial and endothelial cells, smooth muscle cells, T cells, macrophages, and eosinophils itself) are a major source of these chemokines. IL-5, IL-33 and presumably other cytokines locally produced by both immune cells (eosinophils, ILC2, T cells, and mast cells) in tumor microenvironment and by tumor cells can prolong the life span of eosinophils at site of tumor growth.
Eosinophil recruitment at tumor sites
Eosinophils are present into the TME of several human solidCitation58–62 and hematological tumorsCitation63 and in experimental tumor models.Citation64 The precise mechanisms underlying eosinophils infiltration of tumors remain largely undefined; indeed, it is a complex process that depends on a combination of cytokines, adhesion molecules and chemokines. Eotaxin-1/CCL11, eotaxin-2/CCL24, and RANTES/CCL5, produced by human solidCitation65 and hematological tumorsCitation66,67 can activate CCR3 on eosinophils. Alarmins or damage-associated molecular patterns (DAMPs) potentially triggering eosinophils recruitment include the high-mobility group box 1 protein (HMGB1), IL-1α, and IL-33.Citation68 HMGB1 is a highly conserved and ubiquitously expressed protein that has both nuclear and extracellular functions in cancer.Citation69 HMGB1 can be released either passively by necrotic and damaged cells or by active mechanism triggered upon immune cell activation. Once released in the extracellular space, HMGB1 can mediate inflammation, cell migration, proliferation and differentiation. Extracellular HMGB1 acts as a chemoattractant for eosinophilsCitation70,71 through the activation of toll-like receptor (TLR)-2 and TLR-4, or the receptor for advanced glycation end products (RAGE).Citation72 IL-33, a member of IL-1 family, is mainly expressed by epithelial and endothelial cells and it is associated with allergic disorders, inflammation and infection. In the precursor form IL-33 is a transcriptional regulator factor but in the active form is released by stressed, damaged and necrotic cells in the extracellular space where it acts as an alarmin.Citation73 The minimal IL-33 receptor (IL-33R) complex consists of IL-1R4, also known as ST2, and IL-1R3, also known as IL-1RAcP.Citation74 The IL-33R complex is more sophisticated in mast cells.Citation75 IL-33 is expressed in several human cancersCitation76 and can attract eosinophils directly via the ST2 receptorCitation77 or indirectly through the activation of ILC2Citation78 and mast cellsCitation79,80 that in turn produce IL-5.Citation27 Lastly, extracellular adenosine triphosphate (ATP) is also recognized as a DAMP that is implicated in adaptive immune responses following immunogenic chemotherapy.Citation81 ATP can act as a potent chemoattractant for eosinophils in vitro and in vivo activating eosinophils effector responses through binding to P2Y purinergic receptors.Citation82,83
Lung cancer cells can release IL-5, explaining eosinophilia occasionally associated with this tumor.Citation84,85 Eosinophils can be recruited by vascular endothelial growth factors (VEGFs) and angiopoietin 1 (Ang1) produced by tumor and immune cellsCitation30,86,87 through the engagement of VEGF receptors (VEGFR-1/VEGFR-2) and Tie2, respectively expressed by human eosinophils.Citation88,89
Eosinophil infiltration of cancers can also be mediated by the production of chemotactic factors by tumor-infiltrating immune cells. Macrophages and mast cells can contribute to eosinophil recruitment through the production of VEGFs.Citation86,87 Adenosine, produced by tumor cellsCitation90 in TME,Citation91 potentiates the production of angiogenic factors from human macrophages and mast cells.Citation87,92 Moreover, histamine and PGD2 released by activated mast cellsCitation27 can mediate eosinophil migration through the activation of CRTH2Citation93 and H4 receptor,Citation94 respectively. IL-4 secreted by Th-2 cells may also promote indirectly, through induction of local production of CCL11, eosinophils recruitment to the tumor site.Citation95,96 In summary, a plethora of factors produced by cancer and immune cells can attract and/or activate eosinophils in TME.
Roles of eosinophils in cancer
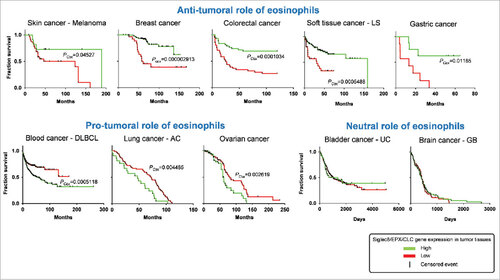
The heterogeneity of different subsets of immune cells (e.g. monocytes, macrophages, T helper cells, mast cells, neutrophils, NK, NKT cells),Citation97,98 their plasticityCitation26,99–101 and their reciprocal interactionsCitation28 have complicated the comprehension of the role of the TME in tumor initiation and development.Citation26 Despite eosinophils are readily identified by their specific morphology and staining characteristics, their phenotype, function and morphology can change upon induction of inflammation and aberrant microenvironment,Citation35,102,103 including the TME.Citation104 Several studies have attempted to identify the contributory functions of eosinophils in tumor growth control. In the majority of human and murine experimental studies, eosinophils appear to play an anti-tumorigenic role (). In clinical cancers, the presence of eosinophils at either tumor site or in peripheral blood is a favourable prognostic factor for most cancers, although evidence for a pro-tumorigenic role for eosinophils is reported (). Only few studies reported a non-contributing role of eosinophils in experimentalCitation105 and human tumors.Citation106,107 We performed a retrospective analysis exploiting public repository databases of microarray data from cancer patients biopsies (GEO, https://www.ncbi.nlm.nih.gov/geo/) in order to correlate the expression levels of Siglec-8, EPX and Charcot-Leyden crystal galectin (CLC/Galectin-10), three eosinophil-specific markersCitation108–110 in function of the survival rate in different types of cancer. To this aim, we used SurvExpress (http://bioinformatica.mty.itesm.mx/SurvExpress), an online biomarker validation tool to correlate multiple gene expression data to cancer patients survival rate.Citation111 These findings evidence an anti-tumoral role of eosinophils in most cancer types analyzed (skin melanoma, colorectal, breast and gastric), a pro-tumoral action in other tumors (blood, lung and ovarian) and no effect in brain and bladder cancer (, Supplementary ).
Table 1. Anti-tumorigenic activity of eosinophils in experimental tumors.
Table 2. Role of eosinophils in clinical cancers.
Figure 2. Role of eosinophils in survival rate of cancer patients. Kaplan-Meier plots depict the overall survival rate of all the indicated cancer types in function of the expression of indicated genes in cancer tissues. Survival events (vertical black lines) of patients were stratified based on the low (red color) or high expression (green color) of Siglec8 (Sialic Acid Binding Immunoglobulin-like Lectin 8, GenBank/Entrez ID: 27181), EPX (Eosinophil Peroxidase, GenBank/Entrez ID: 8288), and CLC (Charcot-Leyden crystal protein, GenBank/Entrez ID: 1178), three specific markers for human eosinophils. The survival rate data were extracted from patients in publicly available expression array datasets by the web-based tool SurvExpress (http://bioinformatica.mty.itesm.mx:8080/Biomatec/SurvivaX.jsp). Each graph depicts a distinct patient's cohort. LS, Liposarcoma; DLBCL, Diffuse Large B cell lymphoma; AC, Adenocarcinoma; UC: Urothelial carcinoma; GB, Glioblastoma. PCox, Log-Rank (Mentel-Cox) P value calculated by SurvExpress. P values below 0.05 were considered statistically significant.
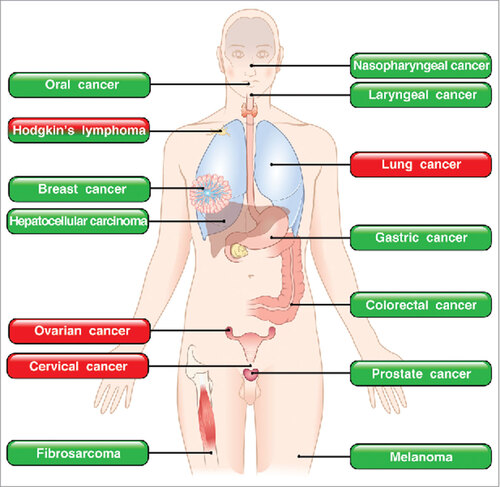
In several human tumors such as gastric,Citation112,113 colorectal,Citation60,62,114–116 nasopharyngeal,Citation117 oral,Citation118,119 laryngealCitation120 and breast cancerCitation121 eosinophils appear to be anti-tumorigenic. By contrast, in Hodgkin's lymphomaCitation122,123 and cervical cancer,Citation61,124 infiltration of eosinophils is associated with poor prognosis. In addition, blood eosinophilia has been associated with positive response to immunotherapy and is generally correlated with longer survival in advanced melanoma patients,Citation125–127 whereas it is an unfavorable prognostic factor in T-cell leukemia/lymphoma.Citation128 These apparently conflicting results are intriguing and suggest that the role of eosinophils and their mediators in human tumors could be cancer-specific.
Several initial mouse studies have implied eosinophils in anti-cancer responses, although a clear role for these cells was not directly demonstrated.Citation95,105,129 In experimental studies a protective role of eosinophils was found in several tumors such as colon carcinoma,Citation130,131 melanoma,Citation53,95,132,133 Hodgkin's lymphoma,Citation134 hepatocellular carcinoma,Citation135 prostate cancerCitation136 and fibrosarcoma.Citation137 Thus, studies addressing potential functions of eosinophils in experimental and human tumors have provided conflicting results.Citation138–140
Two recent studies have examined the role of eosinophils and their mediators in tumor initiation and rejection. Carretero and collaborators have elegantly demonstrated that eosinophils produce several chemokines (CCL5, CCL9, CXCL10) that are essential for the attraction of CD8+ T cells in TME in a model of melanoma.Citation132 Moreover, eosinophils favor macrophage M1 skewing through the production of IFN-γ and TNF-α. M1 macrophages amplify Th1 responses, providing a positive loop in the anti-tumor response.Citation141 In addition, M1 macrophages produce nitric oxide (NO), reactive oxygen species (ROS), IL-1α, and TNF-α, which are important components of the anti-tumor arsenal. Carretero et al. also demonstrated that eosinophils contribute to normalization of tumor vasculature, thus promoting tumor rejection. Lucarini and collaborators have investigated the role of IL-33/ST2 axis in a model of melanoma. IL-33 administration in mice-bearing melanoma resulted in tumor growth delay.Citation53 This effect was associated with intratumoral accumulation of eosinophils and CD8+ T cells and with local and systemic activation of NK and CD8+ T cells. Moreover, IL-33 caused ST2-dependent eosinophil recruitment in the lung that prevented pulmonary metastasis after intravenous injection of melanoma cells. In addition, depletion of eosinophils by treatment with an anti-Siglec-F antibody abolished the protective effects of IL-33 against both tumor growth and metastasis formation. Functional studies revealed that IL-33-activated eosinophils exert both an accessory role, promoting the recruitment of tumor-reactive CD8+ T cells at the tumor site and a direct cytotoxic effect against target melanoma cells, suggesting a dual mechanism for eosinophil-mediated anti-tumoral activity. Collectively, these two studies illuminate some of the immunologic mechanisms through which eosinophils play an anticancer role and open the way to the reconsideration of eosinophils in the development of new cancer immunotherapies. The roles of eosinophils in human and experimental tumors are summarized in .
Figure 3. Roles of eosinophils in human and experimental tumors. In green boxes are indicated those tumors in which eosinophils play a protective role. In red boxes those tumors in which eosinophils play a pro-tumorigenic role. In mixed red/green boxes are presented tumors in which eosinophils play both a pro- and anti-tumorigenic role in different studies.
Does the role of eosinophils in tumors vary according to their microlocalization?
In addition to quantitative and qualitative cell composition, the spatial distribution of immune cells at the tumor site may affect tumor outcome.Citation142 Moreover, different stages of tumors can be associated with qualitative and quantitative changes in different types of immune cells in the periphery and center of tumors.Citation143,144 For example, mast cells in perilesional stroma of melanoma play a protective role.Citation145 In NSCLC, mast cell infiltration of tumor islets confers a survival advantage independently of tumor stage.Citation146,147 By contrast, peritumoral mast cells were associated with a better prognosis only in stage I NSCLC.Citation148 In pancreatic ductal adenocarcinoma (PDAC) mast cell density in the intratumoral border zone, but not the peritumoral or the intratumoral center zone, was associated with a worse prognosis.Citation149 In patients with cutaneous lymphoma only the density of peripheral mast cells correlated with disease progression.Citation150 Collectively, these findings suggest that the microlocalization of immune cells is an important aspect in the initiation and progression of several tumors.
Tumor-associated eosinophils within the necrotic or perivascular areas of tumors have been reported.Citation64,95 The number of peritumoral eosinophils has a significant favourable impact on prognosis of colorectal cancer patients.Citation60 However, these studies did not examine differences between the periphery and the center of tumors. A novel technique allows a simultaneous single-cell analysis of the immune landscape of tumor microenvironment.Citation151 It has been found that lung adenocarcinoma and non-involved lung tissue were equally enriched in eosinophils and neutrophils whereas other immune cells (e.g. CD8+ T cells and macrophages) showed marked differences. Thus, this technique provides a powerful tool for the comparative identification of eosinophils in different compartments of TME.
Eosinophils and tumor angiogenesis
Angiogenesis, the formation of new blood vessels, is an essential process for supplying essential nutrients and oxygen to growing malignant tissues.Citation152 Immune cells are an important source of pro-angiogenic factors.Citation86,87,153 Human eosinophils produce several pro-angiogenic factors such as VEGF-A,Citation31,154 fibroblast growth factor (FGF-2),Citation132,155 CXCL8/IL-8 Citation156 and osteopontin.Citation157 Several studies have highlighted the association in human tumors between increased eosinophil density and angiogenesis by evaluating the expression of VEGF-A.Citation158,159
The VEGF-A gene can be alternatively spliced to form the pro-angiogenic VEGF-A165 and the anti-angiogenic VEGF-A165b.Citation160 In certain tumors, the anti-angiogenic VEGF-A165b isoform is dominant.Citation161 In addition, human neutrophils can produce both pro- and anti-angiogenic isoforms of VEGF-A.Citation162 The results on VEGF-A in cancer should be confirmed differentiating between these two isoforms.Citation163 The different pro- and anti-angiogenic isoforms of VEGFs produced by eosinophils in cancer have not been investigated.
Lymphangiogenesis, the formation of new lymphatic vessels, is important for the development of metastasis.Citation164 Activated eosinophils play a relevant role in metastasis insurgence.Citation53 Eosinophils have been detected in metastatic lymph nodes of cancer patients and the production of lymphangiogenic factors (e.g. VEGF-C and VEGF-D) by eosinophils should be further addressed. Human eosinophils produce matrix metalloproteinases (e.g. MMP-9)Citation165–167 which regulate the digestion of extracellular matrix (ECM) favoring the invasive and metastatic behavior of cancer cells in TME.Citation167
Eosinophil extracellular traps and cancer
Several immune cells including human neutrophils,Citation168,169 mast cells,Citation170 and eosinophilsCitation10,171 can release granular and nuclear contents in the extracellular space in response to various stimuli (Extracellular Trap cell death, ETosis). Increasing evidence indicates that neutrophil ETosis (NET) plays a role in cancer.Citation168,169,172 NETs can accelerate the metastatic process by entrapping tumor cells that can travel through vessels and lead to seeding in other organs.Citation168 Degranulation and release of extracellular eosinophil secondary granule proteins in situ have been reported in both murineCitation64,95 and human cancers.Citation59,173 Functional in vitro assays have confirmed that eosinophils can mediate direct tumor cell killing via release of cytotoxic granules, including Granzymes.Citation53,95,131,174 It is reported that eosinophils exploit ETosis for their main activities during airway inflammation.Citation175 The role of eosinophil ETosis in cancer deserves further investigation.
Eosinophils as accessory cells in cancer
Besides exerting direct cytotoxic effects against cancer cells, eosinophils may participate to the anti-tumor response as accessory/immunomodulatory cells. In experimental melanoma models, tumor-associated eosinophils were shown to express chemokines (CCL5, CXCL9, CXCL10) promoting the recruitment of tumor-reactive CD8 T cells that mediate tumor rejection.Citation53,132 In addition, eosinophils may cooperate with other immune cells such as macrophages,Citation96 mast cells,Citation59 NK cellsCitation176 and dendritic cellsCitation177,178 to induce anti-tumor responses. Eosinophils may also affect local T cell responses by modulating the balance of Th1 and Th2-related cytokines.Citation53,132 Finally, eosinophils may serve as non-professional antigen presenting cells (APC). Resting eosinophils do not constitutively express MHC class-II or co-stimulatory markers. Upon activation by certain cytokines or other inflammatory stimuli, eosinophils can up-regulate these molecules and stimulate primed CD4 T cell responses in vitro and in vivo.Citation179,180 Whether eosinophils function as APC at the tumor site remains to be determined.
IgE, atopy and cancer
Eosinophils and their mediators play a pivotal role in several allergic disorders.Citation3,6,181 EpidemiologicalCitation182,183 and experimental studiesCitation184 have suggested an inverse association between allergic diseases or IgE and certain tumors. It has been suggested that one of the protective role of IgE/atopy in cancer could be mediated in part by hypereosinophilia associated with several allergic disorders.Citation185
Role of eosinophils in cancer immunotherapy
Initial attempts to stimulate the immune system in cancer patients with IL-2 showed blood eosinophiliaCitation186 caused by increased plasma concentration of IL-5. It has been suggested that tumor-associated eosinophils in IL-2-treated patients exert cytotoxic effects on cancer cells.Citation173,187 More recently, cancer immunotherapy with monoclonal antibodies targeting immune checkpoints (CTLA-4, PD-1 and PD-L1) has yielded clinical benefits, including durable responses, to a percentage of patients with different malignancies.Citation188–190 Interestingly, daily practice with ipilimumab (anti-CTLA-4) in patients with melanoma has shown that early increase in peripheral blood eosinophils is associated with improved survival.Citation191,192 Importantly, baseline peripheral blood eosinophilia is associated with a better clinical outcome in melanoma patients treated with ipilimumabCitation126,193 and pembrolizumab.Citation127
Outstanding questions and conclusions
Studies on eosinophils biology are routinely conducted under physiological pH and normoxia. By contrast, the metabolic phenotype of tumors is characterized by low pH and areas of either hypoxia or normoxia.Citation90 Tumor-associated macrophages (TAMs) in normoxic tumor tissues express M1 markers whereas those in hypoxic tissues express M2 markers.Citation194 These aspects caution against the over interpretation of results from in vitro studies of eosinophils at physiological conditions. It will be important to investigate how hypoxic conditions influence the proteomic and lipidomic phenotypes of eosinophils. Eosinophils can migrate to draining lymph nodes where they can act as APC.Citation195,196 An analysis of eosinophils in tumor-draining lymph nodes (TDLNs) and in tertiary lymphoid structures of tumors is missing. It has been reported basophils in TDLNs of patients with pancreatic ductal adenocarcinoma correlates with reduced survival.Citation197 The role of eosinophils in TDLNs, in tertiary lymphoid tissues and at metastatic sites of different tumors remains to be explored.
The anti- or pro-tumorigenic role of eosinophils in different human tumors appears to be cancer-specific. As shown for tumor-associated macrophages (M1 and M2)Citation99 and tumor-associated neutrophils (N1 and N2),Citation100,101 subpopulations of eosinophils are recently begun to emergeCitation198 and could play different, even opposite effects in various types of tumors. In this respect, it has been proposed that eosinophils may be distinguished into E1 or E2 based on the balance of Th1/Th2 cytokine expression patterns.Citation53,109,132 Alternatively, although eosinophils are relatively short lived, different TMEs could alter their phenotype. Indeed, eosinophils, like other immune cells, are endowed with phenotypic and functional plasticity depending on environmental factors which may vary in composition in the different cancer microenvironments.Citation104 Gene expression profiling has demonstrated that several individual human cancers (e.g. melanoma, gastric, lung and breast cancers) are heterogeneous with a spectrum of molecular changes.Citation199–203 The complex heterogeneity (spatial, temporal, intratumoral, intertumoral) of the TME adds an additional layer of complexity.Citation151,204,205 Simultaneous single-cell analysis of the immune landscape of TME of different subtypes of human cancers defined by genetic markers can greatly expand our knowledge of the role of eosinophils in tumor initiation and progression.
Tumor cells evade host immune attack by expressing several checkpoints such as programmed cell death-1 protein (PD-1) and its ligands (PD-L1 and PD-L2) which inhibit PD-1+ lymphocytes in TME.Citation206 Monoclonal antibodies targeting the PD-1/PD-L1 pathway unleash anti-tumor immunity and have revolutionized the management of a wide spectrum of malignancies. Certain cancer cells (e.g. melanoma) express also PD-1, in addition to PD-L1, providing an additional tumor intrinsic mechanism enhancing the protumorigenic effect of PD-1/PD-L1 axis.Citation207 Human eosinophils express PD-L1 and, to a lesser extent, PD-L2.Citation208 An important task will be to investigate the role of PD-1/PD-L1 axis of eosinophils in tumor microenvironment.
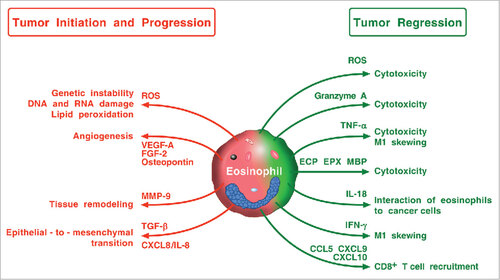
All the above implies that elucidation of the roles of eosinophils in different human tumors will demand studies of increasing complexity beyond those assessing merely eosinophil density and microlocalization. schematically illustrates some of the possible mechanisms by which eosinophils and their mediators may promote tumor regression or, vice versa, tumor initiation and progression in experimental and clinical tumors. These apparently controversial effects might reflect differences in stage, grade, and subtypes of tumors, different methods employed to identify eosinophils (e.g. Giemsa, cationic proteins), different microanatomical compartments analyzed (i.e. peritumoral vs intratumoral) in the various studies or perhaps subtypes of tumor-associated eosinophils. Therefore, many fundamental questions need to be addressed before we understand whether eosinophils are an ally, adversary or innocent bystander in human cancers.
Figure 4. Possible mechanisms by which eosinophils and their mediators play a protumorigenic or an antitumorigenic role. Eosinophils in TME can promote tumor initiation and progression through the release of ROS, angiogenic factors, metalloproteinase-9, and the induction of epithelial-to-mesenchymal transition. Eosinophils can exhibit antitumor activity through direct tumor cell cytotoxicity mediated by ROS, granzyme, TNF-α, eosinophil cationic proteins (ECP, EPX, MBP), and IL-18. IFN-γ produced by eosinophils favors M1 polarization of TAMs. Eosinophils can also exert antitumor activity indirectly through the attraction of CD8+ T cells via the production of CCL5, CXCL9, and CXCL10.
Author contributions
GV, MRG, and SL conceived and designed the review. All the authors contributed intellectually and to the writing of the submitted version of the manuscript.
2017ONCOIMM0665R-s02.docx
Download MS Word (17.2 KB)Acknowledgments
The authors apologize to the many authors who have contributed importantly to this field and whose work has not been cited due to space and citation restrictions. The authors thank Fabrizio Fiorbianco for preparing the figures and Gjada Criscuolo for excellent secretarial help.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Funding
This work was supported in part by grants from Regione Campania CISI-Lab Project, CRèME Project, and TIMING Project to GM, and AIRC IG 14297 to GS.
References
- Kay AB. The early history of the eosinophil. Clin Exp Allergy. 2015;45(3):575–582. https://doi.org/10.1111/cea.12480. PMID:25544991
- Johnston LK, Bryce PJ. Understanding Interleukin 33 and Its Roles in Eosinophil Development. Front Med (Lausanne). 2017;4(51); https://doi.org/10.3389/fmed.2017.00051.
- Varricchi G, Bagnasco D, Borriello F, Heffler E, Canonica GW. Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needs. Curr Opin Allergy Clin Immunol. 2016;16(2):186–200; https://doi.org/10.1097/aci.0000000000000251. PMID:26859368
- Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13(1):9–22; https://doi.org/10.1038/nri3341. PMID:23154224
- Furuta GT, Atkins FD, Lee NA, Lee JJ. Changing roles of eosinophils in health and disease. Ann Allergy Asthma Immunol. 2014;113(1):3–8; https://doi.org/10.1016/j.anai.2014.04.002. PMID:24795292
- Varricchi G, Senna G, Loffredo S, Bagnasco D, Ferrando M, Canonica GW. Reslizumab and Eosinophilic Asthma: One Step Closer to Precision Medicine? Front Immunol. 2017;8:242; https://doi.org/10.3389/fimmu.2017.00242. PMID:28344579
- Huang L, Appleton JA. Eosinophils in Helminth Infection: Defenders and Dupes. Trends Parasitol. 2016;32(10):798–807; https://doi.org/10.1016/j.pt.2016.05.004. PMID:27262918
- Wang Z, Lai Y, Bernard JJ, MacLeod DT, Cogen AL, Moss B, Di Nardo A. Skin Mast Cells Protect Mice against Vaccinia Virus by Triggering Mast Cell Receptor S1PR2 and Releasing Antimicrobial Peptides. J Immunol. 2012;188(1):345–357; https://doi.org/10.4049/jimmunol.1101703. PMID:22140255
- Samarasinghe AE, Melo RC, Duan S, LeMessurier KS, Liedmann S, Surman SL, Lee JJ, Hurwitz JL, Thomas PG, McCullers JA. Eosinophils Promote Antiviral Immunity in Mice Infected with Influenza A Virus. J Immunol. 2017;198(8):3214–3226; https://doi.org/10.4049/jimmunol.1600787. PMID:28283567
- Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14(9):949–953; https://doi.org/10.1038/nm.1855. PMID:18690244
- Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38(5):709–750; https://doi.org/10.1111/j.1365-2222.2008.02958.x. PMID:18384431
- Chan CY, St John AL, Abraham SN. Plasticity in mast cell responses during bacterial infections. Curr Opin Microbiol. 2012;15(1):78–84; https://doi.org/10.1016/j.mib.2011.10.007. PMID:22055570
- Josephs DH, Spicer JF, Corrigan CJ, Gould HJ, Karagiannis SN. Epidemiological associations of allergy, IgE and cancer. Clin Exp Allergy. 2013;43(10):1110–1123; https://doi.org/10.1111/cea.12178. PMID:24074329
- Jensen-Jarolim E, Bax HJ, Bianchini R, Capron M, Corrigan C, Castells M, Dombrowicz D, Daniels-Wells TR, Fazekas J, Fiebiger E, et al. AllergoOncology – the impact of allergy in oncology: EAACI position paper. Allergy. 2017;72(6):866–887; https://doi.org/10.1111/all.13119. PMID:28032353
- Platzer B, Elpek KG, Cremasco V, Baker K, Stout MM, Schultz C, Dehlink E, Shade KT, Anthony RM, Blumberg RS, et al. IgE/FcepsilonRI-Mediated Antigen Cross-Presentation by Dendritic Cells Enhances Anti-Tumor Immune Responses. Cell Rep. 2015;10(9):1487–1495; https://doi.org/10.1016/j.celrep.2015.02.015. PMID:25753415
- Tomasetti C, Li L, Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science. 2017;355(6331):1330–1334; https://doi.org/10.1126/science.aaf9011. PMID:28336671
- Dawson MA, Kouzarides T, Huntly BJ. Targeting epigenetic readers in cancer. N Engl J Med. 2012;367(7):647–657; https://doi.org/10.1056/NEJMra1112635. PMID:22894577
- Dawson MA. The cancer epigenome: Concepts, challenges, and therapeutic opportunities. Science. 2017;355(6330):1147–1152; https://doi.org/10.1126/science.aam7304. PMID:28302822
- Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118(6):1991–2001; https://doi.org/10.1172/JCI35180. PMID:18523649
- Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):74–88; https://doi.org/10.1016/j.immuni.2013.06.014. PMID:23890065
- Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17(3):320–329; https://doi.org/10.1038/nm.2328. PMID:21383745
- Galdiero MR, Varricchi G, Marone G. The immune network in thyroid cancer. Oncoimmunology. 2016;5(6):e1168556; https://doi.org/10.1080/2162402X.2016.1168556. PMID:27471646
- Varricchi G, Galdiero MR, Loffredo S, Marone G, Iannone R, Granata F. Are Mast Cells MASTers in Cancer? Front Immunol. 2017;8:424; https://doi.org/10.3389/fimmu.2017.00424. PMID:28446910
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322; https://doi.org/10.1016/j.ccr.2012.02.022. PMID:22439926
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444; https://doi.org/10.1038/nature07205. PMID:18650914
- Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2013;228(7):1404–1412; https://doi.org/10.1002/jcp.24260. PMID:23065796
- Marone G, Varricchi G, Loffredo S, Granata F. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur J Pharmacol. 2016;778:146–151; https://doi.org/10.1016/j.ejphar.2015.03.088. PMID:25941082
- Varricchi G, Galdiero MR, Marone G, Granata F, Borriello F. Controversial role of mast cells in skin cancers. Exp Dermatol. 2017;26(1):11–17; https://doi.org/10.1111/exd.13107. PMID:27305467
- Galdiero MR, Marone G, Mantovani A. Cancer Inflammation and Cytokines. Cold Spring Harb Perspect Biol. 2017;pii: a028662; in press. https://doi.org/10.1101/cshperspect.a028662. PMID:28778871
- Varricchi G, Granata F, Loffredo S, Genovese A, Marone G. Angiogenesis and lymphangiogenesis in inflammatory skin disorders. J Am Acad Dermatol. 2015;73(1):144–153; https://doi.org/10.1016/j.jaad.2015.03.041. PMID:25922287
- Nissim Ben Efraim AH, Levi-Schaffer F. Roles of eosinophils in the modulation of angiogenesis. Chem Immunol Allergy. 2014;99:138–154; https://doi.org/10.1159/000353251. PMID:24217607
- Jung Y, Wen T, Mingler MK, Caldwell JM, Wang YH, Chaplin DD, Lee EH, Jang MH, Woo SY, Seoh JY, et al. IL-1[beta] in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. 2015;8(4):930–942; https://doi.org/10.1038/mi.2014.123. PMID:25563499
- Throsby M, Herbelin A, Pléau J-M, Dardenne M. CD11 c+Eosinophils in the Murine Thymus: Developmental Regulation and Recruitment upon MHC Class I-Restricted Thymocyte Deletion. J Immunol. 2000;165(4):1965–1975; https://doi.org/10.4049/jimmunol.165.4.1965. PMID:10925279
- Gouon-Evans Vr, Pollard JW. Eotaxin Is Required for Eosinophil Homing into the Stroma of the Pubertal and Cycling Uterus. Endocrinology. 2001;142(10):4515–4521; https://doi.org/10.1210/endo.142.10.8459. PMID:11564717
- Mesnil C, Raulier Sf, Paulissen Gv, Xiao X, Birrell MA, Pirottin D, Janss T, Starkl P, Ramery E, Henket M, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126(9):3279–3295; https://doi.org/10.1172/jci85664. PMID:27548519
- Wu D, Molofsky AB, Liang H-E, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils Sustain Adipose Alternatively Activated Macrophages Associated with Glucose Homeostasis. Science. 2011;332(6026):243–247; https://doi.org/10.1126/science.1201475. PMID:21436399
- Rothenberg ME, Hogan SP. THE EOSINOPHIL. Ann Rev Immunol. 2006;24(1):147–174; https://doi.org/10.1146/annurev.immunol.24.021605.090720.
- Travers J, Rothenberg ME. Eosinophils in mucosal immune responses. Mucosal Immunol. 2015;8(3):464–475; https://doi.org/10.1038/mi.2015.2. PMID:25807184
- Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40(4):563–575; https://doi.org/10.1111/j.1365-2222.2010.03484.x. PMID:20447076
- Long H, Liao W, Wang L, Lu Q. A Player and Coordinator: The Versatile Roles of Eosinophils in the Immune System. Transfus Med Hemother. 2016;43(2):96–108; https://doi.org/10.1159/000445215. PMID:27226792
- Wen T, Rothenberg ME. The Regulatory Function of Eosinophils. Microbiol Spectr. 2016;4(5); https://doi.org/10.1128/microbiolspec.MCHD-0020-2015. PMID:27780017
- Galdiero MR, Varricchi G, Seaf M, Marone G, Levi-Schaffer F, Marone G. Bidirectional mast cell – eosinophil Interactions in inflammatory disorders and cancer Front Med (Lausanne). 2017;4:103; https://doi.org/10.3389/fmed.2017.00103.
- Broughton SE, Nero TL, Dhagat U, Kan WL, Hercus TR, Tvorogov D, Lopez AF, Parker MW. The betac receptor family – Structural insights and their functional implications. Cytokine. 2015;74(2):247–258; https://doi.org/10.1016/j.cyto.2015.02.005. PMID:25982846
- Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502(7470):245–248; https://doi.org/10.1038/nature12526. PMID:24037376
- Dubucquoi S, Desreumaux P, Janin A, Klein O, Goldman M, Tavernier J, Capron A, Capron M. Interleukin 5 synthesis by eosinophils: association with granules and immunoglobulin-dependent secretion. J Exp Med 1994;179(2):703–708; https://doi.org/10.1084/jem.179.2.703. PMID:8294877
- Drissen R, Buza-Vidas N, Woll P, Thongjuea S, Gambardella A, Giustacchini A, Mancini E, Zriwil A, Lutteropp M, Grover A, et al. Distinct myeloid progenitor-differentiation pathways identified through single-cell RNA sequencing. Nat Immunol. 2016;17(6):666–676; https://doi.org/10.1038/ni.3412. PMID:27043410
- Johnston LK, Hsu CL, Krier-Burris RA, Chhiba KD, Chien KB, McKenzie A, Berdnikovs S, Bryce PJ. IL-33 Precedes IL-5 in Regulating Eosinophil Commitment and Is Required for Eosinophil Homeostasis. J Immunol. 2016;197(9):3445–3453; https://doi.org/10.4049/jimmunol.1600611. PMID:27683753
- Gabriele L, Schiavoni G, Mattei F, Sanchez M, Sestili P, Butteroni C, Businaro R, Mirchandani A, Niedbala W, Liew FY, et al. Novel allergic asthma model demonstrates ST2-dependent dendritic cell targeting by cypress pollen. J Allergy Clin Immunol. 2013;132(3):686–695; https://doi.org/10.1016/j.jaci.2013.02.037. PMID:23608732
- Anderson EL, Kobayashi T, Iijima K, Bartemes KR, Chen CC, Kita H. IL-33 Mediates reactive eosinophilopoiesis in response to airborne allergen exposure. Allergy. 2016;71(7):977−-988; https://doi.org/10.1111/all.12861. PMID:26864308
- Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121(6):1484–1490; https://doi.org/10.1016/j.jaci.2008.04.005. PMID:18539196
- Bouffi C, Rochman M, Zust CB, Stucke EM, Kartashov A, Fulkerson PC, Barski A, Rothenberg ME. IL-33 Markedly Activates Murine Eosinophils by an NF-κB-Dependent Mechanism Differentially Dependent upon an IL-4-Driven Autoinflammatory Loop. J Immunol. 2013;191(8):4317–4325; https://doi.org/10.4049/jimmunol.1301465. PMID:24043894
- Hashiguchi M, Kashiwakura Y, Kojima H, Kobayashi A, Kanno Y, Kobata T. IL-33 activates eosinophils of visceral adipose tissue both directly and via innate lymphoid cells. Eur J Immunol. 2015;45(3):876–885; https://doi.org/10.1002/eji.201444969. PMID:25504587
- Lucarini V, Ziccheddu G, Macchia I, La Sorsa V, Peschiaroli F, Buccione C, Sistigu A, Sanchez M, Andreone S, D'Urso MT, et al. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology. 2017;6(6):e1317420; https://doi.org/10.1080/2162402x.2017.1317420. PMID:28680750
- Bochner BS. Novel Therapies for Eosinophilic Disorders. Immunol Allergy Clin North Am. 2015;35(3):577–598; https://doi.org/10.1016/j.iac.2015.05.007. PMID:26209901
- Ponath PD, Qin S, Post TW, Wang J, Wu L, Gerard NP, Newman W, Gerard C, Mackay CR. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med 1996;183(6):2437–2448; https://doi.org/10.1084/jem.183.6.2437. PMID:8676064
- Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. RANTES and macrophage inflammatory protein 1 alpha induce the migration and activation of normal human eosinophil granulocytes. J Exp Med 1992;176(6):1489–1495; https://doi.org/10.1084/jem.176.6.1489 . PMID:1281207
- Shinkai A, Yoshisue H, Koike M, Shoji E, Nakagawa S, Saito A, Takeda T, Imabeppu S, Kato Y, Hanai N, et al. A novel human CC chemokine, eotaxin-3, which is expressed in IL-4-stimulated vascular endothelial cells, exhibits potent activity toward eosinophils. J Immunol 1999;163(3):1602–1610. PMID:10415065
- Looi LM. Tumor-associated tissue eosinophilia in nasopharyngeal carcinoma. A pathologic study of 422 primary and 138 metastatic tumors. Cancer 1987;59(3):466–470; https://doi.org/10.1002/1097-0142(19870201)59:3<466::AID-CNCR2820590319>3.0.CO;2-P.PMID:3791157
- Caruso RA, Parisi A, Quattrocchi E, Scardigno M, Branca G, Parisi C, Luciano R, Paparo D, Fedele F. Ultrastructural descriptions of heterotypic aggregation between eosinophils and tumor cells in human gastric carcinomas. Ultrastruct Pathol. 2011;35(4):145–149; https://doi.org/10.3109/01913123.2011.578233. PMID:21657821
- Harbaum L, Pollheimer MJ, Kornprat P, Lindtner RA, Bokemeyer C, Langner C. Peritumoral eosinophils predict recurrence in colorectal cancer. Mod Pathol. 2015; 28(3):403–413; https://doi.org/10.1038/modpathol.2014.104. PMID:25216222
- Xie F, Liu LB, Shang WQ, Chang KK, Meng YH, Mei J, Yu JJ, Li DJ, Li MQ. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. 2015;364(2):106–117; https://doi.org/10.1016/j.canlet.2015.04.029. PMID:25979231
- Prizment AE, Vierkant RA, Smyrk TC, Tillmans LS, Lee JJ, Sriramarao P, Nelson HH, Lynch CF, Thibodeau SN, Church TR, et al. Tumor eosinophil infiltration and improved survival of colorectal cancer patients: Iowa Women's Health Study. Mod Pathol. 2016;29(5):516–527; https://doi.org/10.1038/modpathol.2016.42. PMID:26916075
- Molin D, Glimelius B, Sundstrom C, Venge P, Enblad G. The serum levels of eosinophil cationic protein (ECP) are related to the infiltration of eosinophils in the tumours of patients with Hodgkin's disease. Leuk Lymphoma. 2001;42(3):457–465; https://doi.org/10.3109/10428190109064602. PMID:11699410
- Cormier SA, Taranova AG, Bedient C, Nguyen T, Protheroe C, Pero R, Dimina D, Ochkur SI, O'Neill K, Colbert D, et al. Pivotal Advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol. 2006;79(6):1131–1139; https://doi.org/10.1189/jlb.0106027. PMID:16617160
- Lorena SC, Oliveira DT, Dorta RG, Landman G, Kowalski LP. Eotaxin expression in oral squamous cell carcinomas with and without tumour associated tissue eosinophilia. Oral Dis. 2003;9(6):279–283; https://doi.org/10.1034/j.1601-0825.2003.00958.x. PMID:14629326
- Kay AB, McVie JM, Stuart AE, Krajewski A, Turnbull LW. Eosinophil chemotaxis of supernatants from cultured Hodgkin's lymph node cells. J Clin Pathol 1975;28(6):502–505; https://doi.org/10.1136/jcp.28.6.502. PMID:1141453
- Teruya-Feldstein J, Jaffe ES, Burd PR, Kingma DW, Setsuda JE, Tosato G. Differential chemokine expression in tissues involved by Hodgkin's disease: direct correlation of eotaxin expression and tissue eosinophilia. Blood 1999;93(8):2463–2470. PMID:10194423
- Bertheloot D, Latz E. HMGB1, IL-1alpha, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol. 2017;14(1):43–64; https://doi.org/10.1038/cmi.2016.34. PMID:27569562
- Nguyen AH, Detty SQ, Agrawal DK. Clinical Implications of High-mobility Group Box-1 (HMGB1) and the Receptor for Advanced Glycation End-products (RAGE) in Cutaneous Malignancy: A Systematic Review. Anticancer Res. 2017;37(1):1–7; https://doi.org/10.21873/anticanres.11282. PMID:28011467
- Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165(6):2950–2954; https://doi.org/10.4049/jimmunol.165.6.2950. PMID:10975801
- Lotfi R, Herzog GI, DeMarco RA, Beer-Stolz D, Lee JJ, Rubartelli A, Schrezenmeier H, Lotze MT. Eosinophils Oxidize Damage-Associated Molecular Pattern Molecules Derived from Stressed Cells. J Immunol. 2009;183(8):5023–5031; https://doi.org/10.4049/jimmunol.0900504. PMID:19794066
- Cebrian MJ, Bauden M, Andersson R, Holdenrieder S, Ansari D. Paradoxical Role of HMGB1 in Pancreatic Cancer: Tumor Suppressor or Tumor Promoter? Anticancer Res. 2016;36(9):4381–4389; https://doi.org/10.21873/anticanres.10981. PMID:27630273
- Martin NT, Martin MU. Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol. 2016;17(2):122–131; https://doi.org/10.1038/ni.3370. PMID:26784265
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–490; https://doi.org/10.1016/j.immuni.2005.09.015. PMID:16286016
- Drube S, Heink S, Walter S, Lohn T, Grusser M, Gerbaulet A, Berod L, Schons J, Dudeck A, Freitag J, et al. The receptor tyrosine kinase c-Kit controls IL-33 receptor signaling in mast cells. Blood. 2010;115(19):3899–3906; https://doi.org/10.1182/blood-2009-10-247411.. PMID:20200353
- Griesenauer B, Paczesny S. The ST2/IL-33 Axis in Immune Cells during Inflammatory Diseases. Front Immunol. 2017;8:475; https://doi.org/10.3389/fimmu.2017.00475. PMID:28484466
- Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113(7):1526–1534; https://doi.org/10.1182/blood-2008-05-157818. PMID:18955562
- Endo Y, Hirahara K, Iinuma T, Shinoda K, Tumes DJ, Asou HK, Matsugae N, Obata-Ninomiya K, Yamamoto H, Motohashi S, et al. The interleukin-33-p38 kinase axis confers memory T helper 2 cell pathogenicity in the airway. Immunity. 2015;42(2):294–308; https://doi.org/10.1016/j.immuni.2015.01.016. PMID:25692703
- Taracanova A, Alevizos M, Karagkouni A, Weng Z, Norwitz E, Conti P, Leeman SE, Theoharides TC. SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors. Proc Natl Acad Sci U S A. 2017;114(20):E4002−E4009; https://doi.org/10.1073/pnas.1524845114. PMID:28461492
- Morita H, Arae K, Unno H, Miyauchi K, Toyama S, Nambu A, Oboki K, Ohno T, Motomura K, Matsuda A, et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation by Promoting Regulatory T Cell Numbers. Immunity. 2015;43(1):175–186; https://doi.org/10.1016/j.immuni.2015.06.021. PMID:26200013
- Aymeric L, Apetoh L, Ghiringhelli Fo, Tesniere A, Martins I, Kroemer G, Smyth MJ, Zitvogel L. Tumor Cell Death and ATP Release Prime Dendritic Cells and Efficient Anticancer Immunity. Cancer Res. 2010;70(3):855–858; https://doi.org/10.1158/0008-5472.can-09-3566. PMID:20086177
- Müller T, Robaye B, Vieira RP, Ferrari D, Grimm M, Jakob T, Martin SF, Di Virgilio F, Boeynaems JM, Virchow JC, et al. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65(12):1545–1553; https://doi.org/10.1111/j.1398-9995.2010.02426.x. PMID:20880147
- Kobayashi T, Soma T, Noguchi T, Nakagome K, Nakamoto H, Kita H, Nagata M. ATP drives eosinophil effector responses through P2 purinergic receptors. Allergol Int. 2015;64, Suppl S30–S36; https://doi.org/10.1016/j.alit.2015.04.009. .
- Slungaard A, Ascensao J, Zanjani E, Jacob HS. Pulmonary carcinoma with eosinophilia. Demonstration of a tumor-derived eosinophilopoietic factor. N Engl J Med 1983;309(13):778–781; https://doi.org/10.1056/NEJM198309293091307. PMID:6310397
- Pandit R, Scholnik A, Wulfekuhler L, Dimitrov N. Non-small-cell lung cancer associated with excessive eosinophilia and secretion of interleukin-5 as a paraneoplastic syndrome. Am J Hematol. 2007;82(3):234–237; https://doi.org/10.1002/ajh.20789. PMID:17160990
- Detoraki A, Staiano RI, Granata F, Giannattasio G, Prevete N, de Paulis A, Ribatti D, Genovese A, Triggiani M, Marone G. Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J Allergy Clin Immunol. 2009;123(5):1142–1149, 1149 e1141-1145; https://doi.org/10.1016/j.jaci.2009.01.044. PMID:19275959
- Granata F, Frattini A, Loffredo S, Staiano RI, Petraroli A, Ribatti D, Oslund R, Gelb MH, Lambeau G, Marone G, et al. Production of vascular endothelial growth factors from human lung macrophages induced by group IIA and group X secreted phospholipases A2. J Immunol. 2010;184(9):5232–5241; https://doi.org/10.4049/jimmunol.0902501. PMID:20357262
- Feistritzer C, Kaneider NC, Sturn DH, Mosheimer BA, Kahler CM, Wiedermann CJ. Expression and function of the vascular endothelial growth factor receptor FLT-1 in human eosinophils. Am J Respir Cell Mol Biol. 2004;30(5):729–735; https://doi.org/10.1165/rcmb.2003-0314OC. PMID:14607815
- Feistritzer C, Mosheimer BA, Sturn DH, Bijuklic K, Patsch JR, Wiedermann CJ. Expression and function of the angiopoietin receptor Tie-2 in human eosinophils. J Allergy Clin Immunol. 2004;114(5):1077–1084; https://doi.org/10.1016/j.jaci.2004.06.045. PMID:15536413
- Gottfried E, Kreutz M, Mackensen A. Tumor metabolism as modulator of immune response and tumor progression. Semin Cancer Biol. 2012;22(4):335–341; https://doi.org/10.1016/j.semcancer.2012.02.009. PMID:22414910
- Huang B, Lei Z, Zhang GM, Li D, Song C, Li B, Liu Y, Yuan Y, Unkeless J, Xiong H, et al. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112(4):1269–1279; https://doi.org/10.1182/blood-2008-03-147033. PMID:18524989
- Visciano C, Liotti F, Prevete N, Cali G, Franco R, Collina F, de Paulis A, Marone G, Santoro M, Melillo RM. Mast cells induce epithelial-to-mesenchymal transition and stem cell features in human thyroid cancer cells through an IL-8-Akt-Slug pathway. Oncogene. 2015;34(40):5175–5186; https://doi.org/10.1038/onc.2014.441. PMID:25619830
- Schratl P, Royer JF, Kostenis E, Ulven T, Sturm EM, Waldhoer M, Hoefler G, Schuligoi R, Lippe IT, Peskar BA, et al. The role of the prostaglandin D2 receptor, DP, in eosinophil trafficking. J Immunol. 2007;179(7):4792–4799; https://doi.org/10.4049/jimmunol.179.7.4792. PMID:17878378
- Capelo R, Lehmann C, Ahmad K, Snodgrass R, Diehl O, Ringleb J, Flamand N, Weigert A, Stark H, Steinhilber D, et al. Cellular analysis of the histamine H4 receptor in human myeloid cells. Biochem Pharmacol. 2016;103:74–84; https://doi.org/10.1016/j.bcp.2016.01.007. PMID:26774453
- Mattes J, Hulett M, Xie W, Hogan S, Rothenberg ME, Foster P, Parish C. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J Exp Med. 2003;197(3):387–393. PMID:12566422
- Tepper RI, Coffman RL, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science 1992;257(5069):548–551; https://doi.org/10.1126/science.1636093. PMID:1636093
- Chevrier S, Levine JH, Zanotelli VRT, Silina K, Schulz D, Bacac M, Ries CH, Ailles L, Jewett MAS, Moch H, et al. An Immune Atlas of Clear Cell Renal Cell Carcinoma. Cell. 2017;169(4):736–749 e718; https://doi.org/10.1016/j.cell.2017.04.016.
- Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng S, Lazo S, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356(6335):pii: eaah4573; https://doi.org/10.1126/science.aah4573. PMID:28428369
- Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–161; https://doi.org/10.1111/j.1600-065X.2008.00607.x. PMID:18364000
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–194; https://doi.org/10.1016/j.ccr.2009.06.017. PMID:19732719
- Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, Damti P, Lumbroso D, Polyansky L, Sionov RV, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10(4):562–573; https://doi.org/10.1016/j.celrep.2014.12.039. doi:10.1016/j.celrep.2014.12.039. PMID:25620698
- Abdala Valencia H, Loffredo LF, Misharin AV, Berdnikovs S. Phenotypic plasticity and targeting of Siglec-FhighCD11clow eosinophils to the airway in a murine model of asthma. Allergy. 2016;71(2):267–271; https://doi.org/10.1111/all.12776. PMID:26414117
- Percopo CM, Brenner TA, Ma M, Kraemer LS, Hakeem RMA, Lee JJ, Rosenberg HF. SiglecF+Gr1hi eosinophils are a distinct subpopulation within the lungs of allergen-challenged mice. J Leukoc Biol. 2017;101(1):321–328; https://doi.org/10.1189/jlb.3A0416-166R. PMID:27531929
- Reichman H, Karo-Atar D, Munitz A. Emerging Roles for Eosinophils in the Tumor Microenvironment. Trends Cancer. 2016;2(11):664–675; https://doi.org/10.1016/j.trecan.2016.10.002. PMID:28741505
- Noffz G, Qin Z, Kopf M, Blankenstein T. Neutrophils but not eosinophils are involved in growth suppression of IL-4-secreting tumors. J Immunol. 1998;160(1):345–350. PMID:9551990
- Ishibashi S, Ohashi Y, Suzuki T, Miyazaki S, Moriya T, Satomi S, Sasano H. Tumor-associated tissue eosinophilia in human esophageal squamous cell carcinoma. Anticancer Res. 2006;26(2B):1419–1424. PMID:16619553
- Keresztes K, Szollosi Z, Simon Z, Tarkanyi I, Nemes Z, Illes A. Retrospective analysis of the prognostic role of tissue eosinophil and mast cells in Hodgkin's lymphoma. Pathol Oncol Res. 2007;13(3):237–242; https://doi.org/PAOR.2007.13.3.0237. PMID:17922053
- Chua JC, Douglass JA, Gillman A, O'Hehir RE, Meeusen EN. Galectin-10, a Potential Biomarker of Eosinophilic Airway Inflammation. PLoS One. 2012;7(8):e42549. PMID:22880030
- Sakkal S, Miller S, Apostolopoulos V, Nurgali K. Eosinophils in Cancer: Favourable or Unfavourable? Curr Med Chem. 2016;23(7):650–666; https://doi.org/10.2174/0929867323666160119094313. PMID:26785997
- Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6 ′-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005;15(11):1125–1135; https://doi.org/10.1093/glycob/cwi097. PMID:15972893
- Aguirre-Gamboa R, Gomez-Rueda H, Martinez-Ledesma E, Martinez-Torteya A, Chacolla-Huaringa R, Rodriguez-Barrientos A, Tamez-Peña José G TV. SurvExpress: An Online Biomarker Validation Tool and Database for Cancer Gene Expression Data Using Survival Analysis. PLoS One. 2013;8(9):e74250; https://doi.org/10.1371/journal.pone.0074250. PMID:24066126
- Iwasaki K, Torisu M, Fujimura T. Malignant tumor and eosinophils. I. Prognostic significance in gastric cancer. Cancer 1986;58(6):1321–1327; https://doi.org/10.1002/1097-0142(19860915)58:6<1321::AID-CNCR2820580623>3.0.CO;2-O.
- Cuschieri A, Talbot IC, Weeden S. Influence of pathological tumour variables on long-term survival in resectable gastric cancer. Br J Cancer. 2002;86(5):674–679; https://doi.org/10.1038/sj.bjc.6600161. PMID:11875724
- Pretlow TP, Keith EF, Cryar AK, Bartolucci AA, Pitts AM, Pretlow TG, 2 nd, Kimball PM, Boohaker EA. Eosinophil infiltration of human colonic carcinomas as a prognostic indicator. Cancer Res 1983;43(6):2997–3000. PMID:6850611
- Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brunner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol 1999;189(4):487–495; https://doi.org/10.1002/(SICI)1096-9896(199912)189:4<487::AID-PATH484>3.0.CO;2-I. PMID:10629548
- Fernandez-Acenero MJ, Galindo-Gallego M, Sanz J, Aljama A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer. 2000;88(7):1544–1548; https://doi.org/10.1002/(SICI)1097-0142(20000401)88:7<1544::AID-CNCR7>3.0.CO;2-S. PMID:10738211
- Fujii M, Yamashita T, Ishiguro R, Tashiro M, Kameyama K. Significance of epidermal growth factor receptor and tumor associated tissue eosinophilia in the prognosis of patients with nasopharyngeal carcinoma. Auris Nasus Larynx. 2002;29(2):175–181; https://doi.org/10.1016/S0385-8146(01)00135-3. PMID:11893453
- Dorta RG, Landman G, Kowalski LP, Lauris JR, Latorre MR, Oliveira DT. Tumour-associated tissue eosinophilia as a prognostic factor in oral squamous cell carcinomas. Histopathology. 2002;41(2):152–157; https://doi.org/10.1046/j.1365-2559.2002.01437.x. PMID:12147093
- Jain M, Kasetty S, Sudheendra US, Tijare M, Khan S, Desai A. Assessment of tissue eosinophilia as a prognosticator in oral epithelial dysplasia and oral squamous cell carcinoma-an image analysis study. Patholog Res Int. 2014; 2014:507512; https://doi.org/10.1155/2014/507512. PMID:24693457
- Thompson AC, Bradley PJ, Griffin NR. Tumor-associated tissue eosinophilia and long-term prognosis for carcinoma of the larynx. Am J Surg 1994;168(5):469–471; https://doi.org/10.1016/S0002-9610(05)80102-3. PMID:7977976
- Ownby HE, Roi LD, Isenberg RR, Brennan MJ. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer. 1983;52(1):126–130; https://doi.org/10.1002/1097-0142(19830701)52:1<126::aid-cncr2820520123>3.0.co;2-y. PMID:6850535
- von Wasielewski R, Seth S, Franklin J, Fischer R, Hubner K, Hansmann ML, Diehl V, Georgii A. Tissue eosinophilia correlates strongly with poor prognosis in nodular sclerosing Hodgkin's disease, allowing for known prognostic factors. Blood. 2000;95(4):1207–1213. PMID:10666192
- Enblad G, Sundstrom C, Glimelius B. Infiltration of eosinophils in Hodgkin's disease involved lymph nodes predicts prognosis. Hematol Oncol 1993;11(4):187–193; https://doi.org/10.1002/hon.2900110404. PMID:8144133
- van Driel WJ, Hogendoorn PC, Jansen FW, Zwinderman AH, Trimbos JB, Fleuren GJ. Tumor-associated eosinophilic infiltrate of cervical cancer is indicative for a less effective immune response. Hum Pathol 1996;27(9):904–911; https://doi.org/10.1016/S0046-8177(96)90216-6. PMID:8816884
- Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, Holland-Letz T, Umansky L, Beckhove P, Sucker A, et al. Myeloid Cells and Related Chronic Inflammatory Factors as Novel Predictive Markers in Melanoma Treatment with Ipilimumab. Clin Cancer Res. 2015; 21(24):5453–5459; https://doi.org/10.1158/1078-0432.CCR-15-0676. PMID:26289067
- Moreira A, Leisgang W, Schuler G, Heinzerling L. Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy. Immunotherapy. 2017;9(2):115–121; https://doi.org/10.2217/imt-2016-0138. PMID:28128709
- Weide B, Martens A, Hassel JC, Berking C, Postow MA, Bisschop K, Simeone E, Mangana J, Schilling B, Di Giacomo AM, et al. Baseline Biomarkers for Outcome of Melanoma Patients Treated with Pembrolizumab. Clin Cancer Res. 2016;22(22):5487–5496; https://doi.org/10.1158/1078-0432.ccr-16-0127. PMID:27185375
- Utsunomiya A, Ishida T, Inagaki A, Ishii T, Yano H, Komatsu H, Iida S, Yonekura K, Takeuchi S, Takatsuka Y, et al. Clinical significance of a blood eosinophilia in adult T-cell leukemia/lymphoma: a blood eosinophilia is a significant unfavorable prognostic factor. Leuk Res. 2007;31(7):915–920; https://doi.org/10.1016/j.leukres.2006.10.017. PMID:17123603
- Kruger-Krasagakes S, Li W, Richter G, Diamantstein T, Blankenstein T. Eosinophils infiltrating interleukin-5 gene-transfected tumors do not suppress tumor growth. Eur J Immunol 1993;23(4):992–995; https://doi.org/10.1002/eji.1830230438. PMID:8458388
- Gatault S, Delbeke M, Driss V, Sarazin A, Dendooven A, Kahn JE, Lefevre G, Capron M. IL-18 Is Involved in Eosinophil-Mediated Tumoricidal Activity against a Colon Carcinoma Cell Line by Upregulating LFA-1 and ICAM-1. J Immunol. 2015;195(5):2483–2492; https://doi.org/10.4049/jimmunol.1402914. . PMID:26216891
- Legrand F, Driss V, Delbeke M, Loiseau S, Hermann E, Dombrowicz D, Capron M. Human eosinophils exert TNF-alpha and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J Immunol. 2010;185(12):7443–7451; https://doi.org/10.4049/jimmunol.1000446. PMID:21068403
- Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16(6):609–617; https://doi.org/10.1038/ni.3159. PMID:25915731
- Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, Kouro T, Itakura A, Nagai Y, Takaki S, et al. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J Immunol. 2012;188(2):703–713; https://doi.org/10.4049/jimmunol.1101270. . PMID:22174445
- Glimelius I, Rubin J, Fischer M, Molin D, Amini RM, Venge P, Enblad G. Effect of eosinophil cationic protein (ECP) on Hodgkin lymphoma cell lines. Exp Hematol. 2011;39(8):850–858; https://doi.org/10.1016/j.exphem.2011.05.006. PMID:21679745
- Kataoka S, Konishi Y, Nishio Y, Fujikawa-Adachi K, Tominaga A. Antitumor activity of eosinophils activated by IL-5 and eotaxin against hepatocellular carcinoma. DNA Cell Biol 2004;23(9):549–560; https://doi.org/10.1089/dna.2004.23.549. . PMID:15383175
- Furbert-Harris P, Parish-Gause D, Laniyan I, Hunter KA, Okomo-Awich J, Vaughn TR, Forrest KC, Howland C, Abdelnaby A, Oredipe OA. Inhibition of prostate cancer cell growth by activated eosinophils. Prostate. 2003;57(2):165–175; https://doi.org/10.1002/pros.10286. PMID:12949941
- Simson L, Ellyard JI, Dent LA, Matthaei KI, Rothenberg ME, Foster PS, Smyth MJ, Parish CR. Regulation of Carcinogenesis by IL-5 and CCL11: A Potential Role for Eosinophils in Tumor Immune Surveillance. J Immunol. 2007;178(7):4222–4229; https://doi.org/10.4049/jimmunol.178.7.4222. PMID:17371978
- Lotfi R, Lee JJ, Lotze MT. Eosinophilic Granulocytes and Damage-associated Molecular Pattern Molecules (DAMPs): Role in the Inflammatory Response Within Tumors. J Immunother. 2007;30(1):16–28; https://doi.org/10.1097/01.cji.0000211324.53396.f6. PMID:17198080
- Davis BP, Rothenberg ME. Eosinophils and cancer. Cancer Immunol Res. 2014;2(1):1–8; https://doi.org/10.1158/2326-6066.CIR-13-0196. PMID:24778159
- Gatault S, Legrand F, Delbeke M, Loiseau S, Capron M. Involvement of eosinophils in the anti-tumor response. Cancer Immunol Immunother. 2012;61(9):1527–1534; https://doi.org/10.1007/s00262-012-1288-3. PMID:22706380
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–896; https://doi.org/10.1038/ni.1937. PMID:20856220
- Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189(7):832–844; https://doi.org/10.1164/rccm.201309-1611OC. PMID:24484236
- Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, Akimova T, Vachani A, Litzky L, Hancock WW, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124(12):5466–5480; https://doi.org/10.1172/JCI77053. . PMID:25384214
- Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016;375(9):819–829; https://doi.org/10.1056/NEJMoa1604958. PMID:27433843
- Siiskonen H, Poukka M, Bykachev A, Tyynela-Korhonen K, Sironen R, Pasonen-Seppanen S, Harvima IT. Low numbers of tryptase+ and chymase+ mast cells associated with reduced survival and advanced tumor stage in melanoma. Melanoma Res. 2015;25(6):479–485; https://doi.org/10.1097/CMR.0000000000000192. PMID:26317168
- Welsh TJ, Green RH, Richardson D, Waller DA, O'Byrne KJ, Bradding P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol. 2005;23(35):8959–8967; https://doi.org/10.1200/JCO.2005.01.4910. PMID:16219934
- Shikotra A, Ohri CM, Green RH, Waller DA, Bradding P. Mast cell phenotype, TNFalpha expression and degranulation status in non-small cell lung cancer. Sci Rep. 2016;6:38352; https://doi.org/10.1038/srep38352. PMID:27922077
- Carlini MJ, Dalurzo MC, Lastiri JM, Smith DE, Vasallo BC, Puricelli LI, Lauria de Cidre LS. Mast cell phenotypes and microvessels in non-small cell lung cancer and its prognostic significance. Hum Pathol. 2010;41(5):697–705; https://doi.org/10.1016/j.humpath.2009.04.029. PMID:20040391
- Cai SW, Yang SZ, Gao J, Pan K, Chen JY, Wang YL, Wei LX, Dong JH. Prognostic significance of mast cell count following curative resection for pancreatic ductal adenocarcinoma. Surgery. 2011;149(4):576–584; https://doi.org/10.1016/j.surg.2010.10.009. PMID:21167541
- Rabenhorst A, Schlaak M, Heukamp LC, Forster A, Theurich S, von Bergwelt-Baildon M, Buttner R, Kurschat P, Mauch C, Roers A, et al. Mast cells play a protumorigenic role in primary cutaneous lymphoma. Blood. 2012;120(10):2042–2054; https://doi.org/10.1182/blood-2012-03-415638. PMID:22837530
- Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, Remark R, Sweeney R, Becker CD, Levine JH, et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell. 2017;169(4):750–765 e717; https://doi.org/10.1016/j.cell.2017.04.014.
- Marone G, Granata F, eds. Angiogenesis, Lymphangiogenesis and Clinical Implications. Chem Immunol Allergy. Basel: Karger. 2014.
- Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267(16):10931–10934. PMID:1375931
- Horiuchi T, Weller PF. Expression of vascular endothelial growth factor by human eosinophils: upregulation by granulocyte macrophage colony-stimulating factor and interleukin-5. Am J Respir Cell Mol Biol 1997;17(1):70–77; https://doi.org/10.1165/ajrcmb.17.1.2796. PMID:9224211
- Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol. 2001;107(2):295–301; https://doi.org/10.1067/mai.2001.111928. PMID:11174196
- Yousefi S, Hemmann S, Weber M, Holzer C, Hartung K, Blaser K, Simon HU. IL-8 is expressed by human peripheral blood eosinophils. Evidence for increased secretion in asthma. J Immunol 1995;154(10):5481–5490.
- Puxeddu I, Berkman N, Ribatti D, Bader R, Haitchi HM, Davies DE, Howarth PH, Levi-Schaffer F. Osteopontin is expressed and functional in human eosinophils. Allergy. 2010;65(2):168–174; https://doi.org/10.1111/j.1398-9995.2009.02148.x. PMID:19804447
- Esposito I, Menicagli M, Funel N, Bergmann F, Boggi U, Mosca F, Bevilacqua G, Campani D. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J Clin Pathol. 2004;57(6):630–636; https://doi.org/10.1136/jcp.2003.014498. PMID:15166270
- Tataroglu C, Kargi A, Ozkal S, Esrefoglu N, Akkoclu A. Association of macrophages, mast cells and eosinophil leukocytes with angiogenesis and tumor stage in non-small cell lung carcinomas (NSCLC). Lung Cancer. 2004;43(1):47–54; https://doi.org/10.1016/j.lungcan.2003.08.013. PMID:14698536
- Varey AH, Rennel ES, Qiu Y, Bevan HS, Perrin RM, Raffy S, Dixon AR, Paraskeva C, Zaccheo O, Hassan AB, et al. VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer. 2008;98(8):1366–1379; https://doi.org/10.1038/sj.bjc.6604308. PMID:18349829
- Bates DO, Mavrou A, Qiu Y, Carter JG, Hamdollah-Zadeh M, Barratt S, Gammons MV, Millar AB, Salmon AH, Oltean S, et al. Detection of VEGF-A(xxx)b isoforms in human tissues. PLoS One. 2013;8(7):e68399; https://doi.org/10.1371/journal.pone.0068399. PMID:23935865
- Loffredo S, Borriello F, Iannone R, Ferrara AL, Galdiero MR, Gigantino V, Esposito P, Varricchi G, Lambeau G, Cassatella MA, et al. Group V Secreted Phospholipase A2 Induces the Release of Proangiogenic and Antiangiogenic Factors by Human Neutrophils. Front Immunol. 2017;8:443; https://doi.org/10.3389/fimmu.2017.00443. PMID:28458672
- Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8(11):880–887; https://doi.org/10.1038/nrc2505.
- Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14(3):159–172; https://doi.org/10.1038/nrc3677. PMID:24561443
- Baram D, Vaday GG, Salamon P, Drucker I, Hershkoviz R, Mekori YA. Human mast cells release metalloproteinase-9 on contact with activated T cells: juxtacrine regulation by TNF-alpha. J Immunol. 2001;167(7):4008–4016; https://doi.org/10.4049/jimmunol.167.7.4008. PMID:11564820
- Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM. Migration of eosinophils through basement membrane components in vitro: role of matrix metalloproteinase-9. Am J Respir Cell Mol Biol 1997;17(4):519–528; https://doi.org/10.1165/ajrcmb.17.4.2877. PMID:9376127
- Schwingshackl A, Duszyk M, Brown N, Moqbel R. Human eosinophils release matrix metalloproteinase-9 on stimulation with TNF-alpha. J Allergy Clin Immunol 1999;104(5):983–989; https://doi.org/10.1016/S0091-6749(99)70079-5. PMID:10550743
- Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123(8):3446–3458; https://doi.org/10.1172/JCI67484. PMID:23863628
- Cedervall J, Zhang Y, Huang H, Zhang L, Femel J, Dimberg A, Olsson AK. Neutrophil Extracellular Traps Accumulate in Peripheral Blood Vessels and Compromise Organ Function in Tumor-Bearing Animals. Cancer Res. 2015;75(13):2653–2662; https://doi.org/10.1158/0008-5472.CAN-14-3299. PMID:26071254
- Mollerherm H, von Kockritz-Blickwede M, Branitzki-Heinemann K. Antimicrobial Activity of Mast Cells: Role and Relevance of Extracellular DNA Traps. Front Immunol. 2016;7:265; https://doi.org/10.3389/fimmu.2016.00265. PMID:27486458
- Ueki S, Konno Y, Takeda M, Moritoki Y, Hirokawa M, Matsuwaki Y, Honda K, Ohta N, Yamamoto S, Takagi Y, et al. Eosinophil extracellular trap cell death-derived DNA traps: Their presence in secretions and functional attributes. J Allergy Clin Immunol. 2016;137(1):258–267; https://doi.org/10.1016/j.jaci.2015.04.041. PMID:26070883
- Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017;23(3):279–287; https://doi.org/10.1038/nm.4294. PMID:28267716
- Huland E, Huland H. Tumor-associated eosinophilia in interleukin-2-treated patients: evidence of toxic eosinophil degranulation on bladder cancer cells. J Cancer Res Clin Oncol 1992;118(6):463–467; https://doi.org/10.1007/BF01629431. PMID:1618895
- Costain DJ, Guha AK, Liwski RS, Lee TDG. Murine hypodense eosinophils induce tumour cell apoptosis by a granzyme B-dependent mechanism. Cancer Immunol Immunother. 2001;50(6):293–299; https://doi.org/10.1007/pl00006690. PMID:11570582
- Ueki S, Tokunaga T, Fujieda S, Honda K, Hirokawa M, Spencer LA, Weller PF. Eosinophil ETosis and DNA Traps: a New Look at Eosinophilic Inflammation. Curr Allergy Asthma Rep. 2016;16(8):54; https://doi.org/10.1007/s11882-016-0634-5. PMID:27393701
- O'Flaherty SM, Sutummaporn K, Häggtoft WL, Worrall AP, Rizzo M, Braniste V, Höglund P, Kadri N, Chambers BJ. TLR-Stimulated Eosinophils Mediate Recruitment and Activation of NK Cells In Vivo. Scand J Immunol. 2017;85(6):417–424; https://doi.org/10.1111/sji.12554. PMID:28426135
- Lotfi R, Lotze MT. Eosinophils induce DC maturation, regulating immunity. J Leukoc Biol. 2008;83(3):456–460; https://doi.org/10.1189/jlb.0607366. PMID:17991762
- Capobianco A, Manfredi AA, Monno A, Rovere-Querini P, Rugarli C. Melanoma and Lymphoma Rejection Associated With Eosinophil Infiltration Upon Intratumoral Injection of Dendritic and NK/LAK Cells. J Immunother. 2008;31(5):458–465; https://doi.org/10.1097/CJI.0b013e318174a512. PMID:18463539
- Shi H-Z. Eosinophils function as antigen-presenting cells. J Leukoc Biol. 2004;76(3):520–527; https://doi.org/10.1189/jlb.0404228. PMID:15218055
- Farhan RK, Vickers MA, Ghaemmaghami AM, Hall AM, Barker RN, Walsh GM. Effective antigen presentation to helper T cells by human eosinophils. Immunology. 2016;149(4):413–422; https://doi.org/10.1111/imm.12658. PMID:27502559
- Bagnasco D, Ferrando M, Caminati M, Bragantini A, Puggioni F, Varricchi G, Passalacqua G, Canonica GW. Targeting Interleukin-5 or Interleukin-5Ralpha: Safety Considerations. Drug Saf. 2017;40(7):559–570; https://doi.org/10.1007/s40264-017-0522-5. PMID:28321782
- Schlehofer B, Siegmund B, Linseisen J, Schuz J, Rohrmann S, Becker S, Michaud D, Melin B, Bas Bueno-de-Mesquita H, Peeters PH, et al. Primary brain tumours and specific serum immunoglobulin E: a case-control study nested in the European Prospective Investigation into Cancer and Nutrition cohort. Allergy. 2011;66(11):1434–1441; https://doi.org/10.1111/j.1398-9995.2011.02670.x. PMID:21726235
- Vajdic CM, Falster MO, de Sanjose S, Martinez-Maza O, Becker N, Bracci PM, Melbye M, Smedby KE, Engels EA, Turner J, et al. Atopic disease and risk of non-Hodgkin lymphoma: an InterLymph pooled analysis. Cancer Res. 2009;69(16):6482–6489; https://doi.org/10.1158/0008-5472.CAN-08-4372. PMID:19654312
- Nigro EA, Brini AT, Soprana E, Ambrosi A, Dombrowicz D, Siccardi AG, Vangelista L. Antitumor IgE adjuvanticity: key role of Fc epsilon RI. J Immunol. 2009;183(7):4530–4536; https://doi.org/10.4049/jimmunol.0900842. PMID:19748979
- Jensen-Jarolim E, Achatz G, Turner MC, Karagiannis S, Legrand F, Capron M, Penichet ML, Rodriguez JA, Siccardi AG, Vangelista L, et al. AllergoOncology: the role of IgE-mediated allergy in cancer. Allergy. 2008;63(10):1255–1266; https://doi.org/10.1111/j.1398-9995.2008.01768.x. PMID:18671772
- West WH, Tauer KW, Yannelli JR, Marshall GD, Orr DW, Thurman GB, Oldham RK. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med 1987;316(15):898–905; https://doi.org/10.1056/NEJM198704093161502. PMID:3493433
- Rivoltini L, Colombo MP, Parmiani G, Viggiano V, Spinazzè S, Santoro A, Takatsu K. In vitro anti-tumor activity of eosinophils from cancer patients treated with subcutaneous administration of interleukin 2. Role of interleukin 5. International Journal of Cancer 1993;54(1):8–15; https://doi.org/10.1002/ijc.2910540103. PMID:8386711
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723; https://doi.org/10.1056/NEJMoa1003466. PMID:20525992
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465; https://doi.org/10.1056/NEJMoa1200694. PMID:22658128
- Peters S, Gettinger S, Johnson ML, Janne PA, Garassino MC, Christoph D, Toh CK, Rizvi NA, Chaft JE, Carcereny Costa E, et al. Phase II Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1-Selected Advanced Non-Small-Cell Lung Cancer (BIRCH). J Clin Oncol. 2017;35(24):2781–2789; https://doi.org/1410 JCO2016719476. PMID:28609226
- Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N, Roy S, Eggermont AM, Routier E, Robert C. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol. 2013;24(6):1697–703; https://doi.org/10.1093/annonc/mdt027. PMID:23439861
- Umansky V, Utikal J, Gebhardt C. Predictive immune markers in advanced melanoma patients treated with ipilimumab. Oncoimmunology. 2016;5(6):e1158901; https://doi.org/10.1080/2162402X.2016.1158901. PMID:27471626
- Martens A, Wistuba-Hamprecht K, Geukes Foppen MH, Yuan J, Postow MA, Wong P, Romano E, Khammari A, Dreno B, Capone M, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. 2016; https://doi.org/10.1158/1078-0432.CCR-15-2412.
- Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In't Veld P, De Baetselier P, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6 C(high) monocytes. Cancer Res. 2010;70(14):5728–5739; https://doi.org/10.1158/0008-5472.CAN-09-4672. PMID:20570887
- Wang HB, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J Immunol. 2007;179(11):7585–7592; https://doi.org/10.4049/jimmunol.179.11.7585. PMID:18025204
- Jacobsen EA, Zellner KR, Colbert D, Lee NA, Lee JJ. Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. J Immunol. 2011;187(11):6059–6068; https://doi.org/10.4049/jimmunol.1102299. PMID:22048766
- De Monte L, Wormann S, Brunetto E, Heltai S, Magliacane G, Reni M, Paganoni AM, Recalde H, Mondino A, Falconi M, et al. Basophil Recruitment into Tumor-Draining Lymph Nodes Correlates with Th2 Inflammation and Reduced Survival in Pancreatic Cancer Patients. Cancer Res. 2016;76(7):1792–1803; https://doi.org/10.1158/0008-5472.CAN-15-1801-T. PMID:26873846
- Lingblom C, Andersson J, Andersson K, Wenneras C. Regulatory Eosinophils Suppress T Cells Partly through Galectin-10. J Immunol. 2017;198(12):4672–4681; https://doi.org/10.4049/jimmunol.1601005. PMID:28515279
- Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360(8):790–800; https://doi.org/10.1056/NEJMra0801289. PMID:19228622
- Popper HH, Ryska A, Timar J, Olszewski W. Molecular testing in lung cancer in the era of precision medicine. Transl Lung Cancer Res. 2014;3(5):291–300; https://doi.org/10.3978/j.issn.2218-6751.2014.10.01. PMID:25806314
- Kim SY, Kim SN, Hahn HJ, Lee YW, Choe YB, Ahn KJ. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J Am Acad Dermatol. 2015;72(6):1036–1046 e1032; https://doi.org/10.1016/j.jaad.2015.02.1113.
- Riquelme I, Saavedra K, Espinoza JA, Weber H, Garcia P, Nervi B, Garrido M, Corvalan AH, Roa JC, Bizama C. Molecular classification of gastric cancer: Towards a pathway-driven targeted therapy. Oncotarget. 2015;6(28):24750–24779; https://doi.org/10.18632/oncotarget.4990. PMID:26267324
- Holzel M, Landsberg J, Glodde N, Bald T, Rogava M, Riesenberg S, Becker A, Jonsson G, Tuting T. A Preclinical Model of Malignant Peripheral Nerve Sheath Tumor-like Melanoma Is Characterized by Infiltrating Mast Cells. Cancer Res. 2016;76(2):251–263; https://doi.org/10.1158/0008-5472.CAN-15-1090. PMID:26511633
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339(6117):286–291; https://doi.org/10.1126/science.1232227.
- Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. 2013;501(7467):355–364; https://doi.org/10.1038/nature12627. PMID:24048068
- Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–214; https://doi.org/10.1016/j.cell.2015.03.030. . PMID:25860605
- Kleffel S, Posch C, Barthel SR, Mueller H, Schlapbach C, Guenova E, Elco CP, Lee N, Juneja VR, Zhan Q, et al. Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth. Cell. 2015;162(6):1242–1256; https://doi.org/10.1016/j.cell.2015.08.052. PMID:26359984
- Pesce S, Thoren FB, Cantoni C, Prato C, Moretta L, Moretta A, Marcenaro E. The Innate Immune Cross Talk between NK Cells and Eosinophils Is Regulated by the Interaction of Natural Cytotoxicity Receptors with Eosinophil Surface Ligands. Front Immunol. 2017;8:510; https://doi.org/10.3389/fimmu.2017.00510.
