ABSTRACT
Background: Gliomas are aggressive tumors with various molecular and clinical characteristics and exhibit strongly resistance to radio-chemotherapy. Programmed cell death 1 ligand 2 (PD-L2) is a cell surface protein, which was reported in many cancers, modulating cancer-associated immune responses, while the role of PD-L2 in gliomas remained unclear. Herein, we aimed to investigate the biological behaviors and clinical prognostic values of PD-L2 in gliomas.
Methods: Totally, we enrolled RNA sequencing data of 325 glioma samples from Chinese Glioma Genome Atlas (CGGA) as training cohort and RNA expression data of 1032 samples from The Cancer Genome Atlas (TCGA) dataset as validation cohort in this research. Then, the clinical and molecular characteristics, and the prognostic value of PD-L2 were analyzed.
Results: We found that PD-L2 expression level was significantly upregulated in higher grade glioma and IDH wild-type glioma. Receiver Operating Characteristic (ROC) analysis revealed that PD-L2 was a potential indicator of mesenchymal subtype. PD-L2 exhibited tight relationship with immune response and immune-modulating process in glioma. Moreover, PD-L2 expression level could predict unfavorable prognoses of patients independent of age, grade, IDH status and 1p/19q status.
Conclusions: Our study revealed that PD-L2 was closely related with inflammation and immune response. Patients with lower PD-L2 expression level tended to experience improved survival. Targeting PD-L2 may become a valuable approach for the treatment of gliomas in clinical practice.
Introduction
Gliomas account for the majority of primary malignant brain tumor in adults and can be diagnosed based on histopathology and molecular features according to the 2016 WHO classification.Citation1,Citation2 In the past decades, despite of the improvement of surgical, radio- and chemo-therapies, the treatment of glioma remains a tremendous challenge.Citation3,Citation4 Recently, immunotherapies, which took advantage of body’s own natural defenses to fight cancer, dramatically changed treatment strategies of cancers.Citation5 Immune checkpoint blockade was one of well-known strategies which aimed to promote the robust anti-tumor T cell response. Fortunately, some immune checkpoint inhibitors have been approved by FDA to treat melanoma, lung cancer and bladder cancer.Citation6,Citation7
Programmed cell death 1 ligand 2 (PD-L2, also called CD273), a ligand of programmed death-1 receptor (PD-1, CD297), could be induced on a wide variety of immune cells, endothelium cell, and tumor cells (including renal cell carcinoma, melanoma, gliomas, etc.) depending on microenvironment stimulus.Citation8–Citation10 PD-L2 played a crucial role in modulation of T cell response, proliferation and might play a role in immune escape by human tumors, including non-small-cell lung cancer, esophageal cancer and B cell lymphoma.Citation11–Citation13 Previous studies investigating the relationship between PD-L2 and survival indicated that patients with upregulated PD-L2 expression had a significantly worse overall survival than those with downregulated PD-L2.Citation14,Citation15
However, little information was available concerning the PD-L2 expression in glioma in past decades. Therefore, in this manuscript, we conducted a comprehensive analysis to explore the molecular and clinical characteristics of PD-L2 in glioma. We employed CGGA RNA sequencing data as training cohort and then validated our findings in TCGA dataset successfully. We found that PD-L2 was upregulated in GBM and IDH wild-type glioma and was an unfavorable prognostic biomarker for patients with glioma.
This comprehensive and integrative analysis revealed the clinical and functional roles of PD-L2, which might provide evidence for potential anti-PD-L2 treatment in glioma.
Methods
Patients and samples
All RNA sequencing data of diffuse glioma patients from WHO II-IV were obtained from two independent databases: The CGGA dataset (n = 325) (http://www.cgga.org.cn) Citation16and TCGA dataset (n = 1032) (http://cancergenome.nih.gov/). This research was approved by the Ethics Committee of Capital Medical University and all patients written informed consent. Overall survival data was collected from clinics during patient visits and/or phone interviews. Patients clinical and molecular features were described in Table S1.
Immunohistochemical analysis
PD-L2 immunostains were done using formalin-fixed, paraffin-embedded tissues. Four-micrometer-thick sections were cut from each paraffin block, dewaxed in xylene, rinsed in graded ethanol, and rehydrated in double-distilled water. For antigen retrieval, slides were pretreated by steaming in sodium citrate buffer (10 mM sodium citrate, pH 6.0) for 15 min at 100°C. Anti-PD-L2 antibody (18251–1-AP, dilution:1:200, Proteintech) was used to detect PD-L2 protein expression. Each stained slide was individually reviewed and scored by two independent neuropathologists. Staining was scored using a four-point scale from 0–3: 0 = no staining or rare staining, 1 = 10% of cells positively stained, 2 = 10–30% of cells positively stained, 3 = > 30% of cells positively stained. Scores of 2 and 3 were defined as strong nuclear staining in at least 10% of the tumor cells. Scores of 0 and 1 were defined as positive staining in < 10% of cells. Negative controls without primary antibody and positive control tissues were included in all experiments to ensure the quality of the staining.
Isocitrate dehydrogenase (IDH1/2) mutations detection
In CGGA cohort, IDH1/2 mutations were detected by DNA pyro-sequencing as previous reported.Citation17 And the IDH1/2 mutations information were downloaded from TCGA website in TCGA cohort.
Statistical analysis
The overall survival difference was calculated by with the Kaplan–Meier method, and Cox regression analysis was performed by the survival package in R. Pearson correlation was used to determine significant differences. One-way ANOVA was used to test for differences among at least 3 groups. The Student’s t-test was used to determine differences in each 2-group comparison. All figures and statistical analysis were performed based on R language for Windows, version 3.4.2 (http://www.r-project.org). All differences were considered statistically significant at the level of p < 0.05.
Result
PD-L2 expression was upregulated in GBM and IDH wild-type glioma
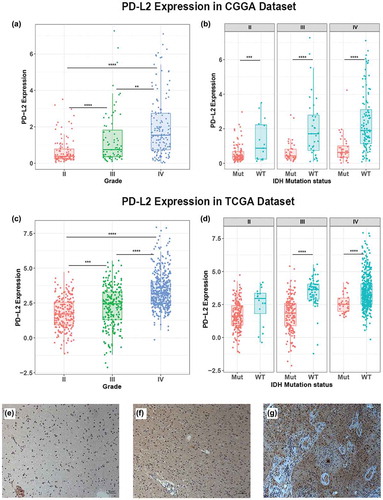
PD-L2 expression was analyzed according to the WHO grade system, histopathology and IDH mutation status in glioma. In CGGA cohort, PD-L2 expression was significantly increased with increasing grade of glioma and showed the highest expression level in GBM (p < 0.0001) (). This result was well validated in TCGA cohort () which indicated that PD-L2 expression was closely linked to the malignancy of glioma. IDH mutation status was a well-known clinically relevant molecular marker in glioma.Citation18 Here, we separated glioma patients into IDH mutation group and IDH wild-type group and investigated the association between PD-L2 expression level and IDH status. In CGGA cohort, across different grades, we found that PD-L2 expression was significantly higher in IDH wild-type group than IDH mutation group (). Similar result was shown in TCGA cohort () though no statistical significance was detected in grade II group. To further characterize the relationship of PD-L2 expression level and tumor specimens, we measured PD-L2 level in glioma specimens from 9 patients (3 with grade II glioma, 3 with grade III glioma, and 3 with GBM) using immunohistochemical analysis. Representative immune-histochemical staining of PD-L2 in gliomas is illustrated in . Immunostained PD-L2 expression levels were significantly lower in grade II than in grade III gliomas (p = 0.0114) and lower in grade III than in GBM specimens (p = 0.0004). Those results suggested that PD-L2 expression was more prevalent in aggressive glioma.
Figure 1. Comparison of PD-L2 expression level in CGGA and TCGA cohorts with different WHO grades (a, c) and IDH status (b, d). PD-L2 was significantly increased in GRADE IV and IDH-wildtype gliomas in CGGA and TCGA data set. Photographs of immunohistochemical staining of PD-L2 in different grades of gliomas. Positive cells are stained brown. (e) Diffuse astrocytoma (WHO grade II). (f) Anaplastic astrocytoma (WHO grade III). (g) Glioblastoma multiforme (WHO grade IV). Magnification, x200. *, **, ***, and **** indicate p < 0.05, p < 0.01, p < 0.001 and p < 0.0001, respectively.
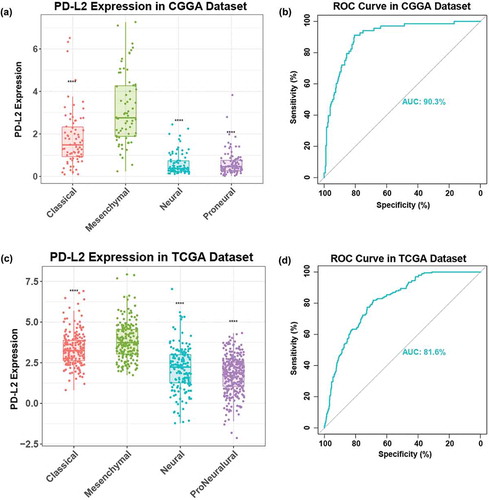
PD-L2 expression was tightly correlated with mesenchymal subtype
To explore the relationship between PD-L2 and TCGA-defined molecular subtypes, we investigated the distribution of PD-L2 expression in four TCGA molecular subtypes. PD-L2 expression level was raised significantly in mesenchymal subtype than other three subtypes in both CGGA and TCGA cohorts (, ). Then we used PD-L2 expression and mesenchymal subtype to generate ROC curves. In CGGA cohort, the area under the curve (AUC) was 0.903. At the optimal cutoff value (1.412), the sensitivity and specificity were 91.9%, 80.5%, respectively (). In TCGA cohort, AUC was 0.816. At the optimal cutoff value (2.87), the sensitivity and specificity were 81.9%,68.8%, respectively (). This result showed that PD-L2 expression was highly specified in mesenchymal subtype and might serve as a biomarker to predict mesenchymal subtype for glioma.
Figure 2. Comparison of PD-L2 expression level in CGGA and TCGA cohorts with different TCGA molecular subtypes (a, c), PD-L2 was significantly increased in mesenchymal subtype (p < 0.0001). ROC curves exhibited highly sensitivity in predicting mesenchymal subtype (b, d). Receiver operating characteristic (ROC) curve for mesenchymal subtype prediction in CGGA and TCGA datasets. ROC curve analysis showed that PD-L2 had highly sensitivity and specificity to predict mesenchymal subtype in CGGA and TCGA database. Area under curve(AUC) was 0.903 and 0.816, respectively.
Relationship between PD-L2 and immune response
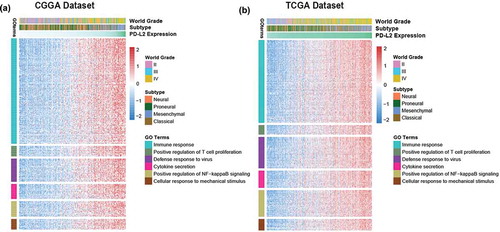
To identify the biological function of PD-L2 in glioma, we executed Gene Oncology analysis with DAVID Bioinformatics Resources 6.8 (https://david.ncifcrf.gov/). Firstly, we screened the genes strongly correlated with PD-L2 (correlation |R|> 0.5) in each cohort. Totally, 829 positively-correlated genes and 180 negatively-related genes in TCGA dataset and 688 positively-correlated genes and 70 negatively-correlated genes in CGGA dataset were screened out for GO analysis, individually (Table S2). We found that the PD-L2 positively-related genes mainly focused on immune response (FDR = 1.63E-21, Benjamini = 2.29E-21), positive regulation of T cell proliferation function (FDR = 2.01E-04, Benjamini = 1.88E-05), defense response to virus(FDR = 3.50E-08, Benjamini = 7.02E-09), cytokine secretion(FDR = 0.001234438, Benjamini = 9.63E-05), positively regulation of NF-kappaB signaling(FDR = 6.33E-05, Benjamini = 6.84E-06) and cellular response to mechanical stimulus(FDR = 0.001, Benjamini = 1.12E-04) (, ). While negatively genes were mainly involved in physiological function, such as synapse and postsynaptic protein. In consistence with other malignant tumors,Citation19,Citation20 these results indicated that PD-L2 mainly probably played an important role in immunologic biological processes of host immune system in glioma.
Figure 3. The biology function of PD-L2 positively related genes in CGGA and TCGA cohorts. The biological functions related with immune response, T cell proliferation, defense response to virus, cytokine secretion, NF-kappaB signaling and cellular response to mechanical stimulus were significantly positively correlated with PD-L2 expression (p < 0.05).
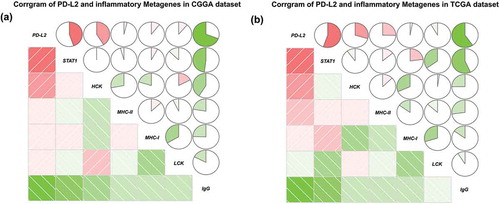
The strong association between PD-L2 and inflammation activities
To get a further comprehensive study of PD-L2-related immunologic biological processes in malignant tumor, we selected six inflammatory metagene clusters which were reported in our previous researchCitation21(Table S3). In both CGGA and TCGA datasets, we found that PD-L2 was significantly positively correlated with HCK, MHC-I and MHC-II and STAT1 while it exhibited a negative relationship with IgG (). The regulatory T cells (Tregs), formerly known as suppressor T cells, are a subpopulation of T cells that modulate the immune system. Then we investigated the relationship of Tregs signatures and PD-L2 expression. As shown in Fig S1, we found that PD-L2 expression was significantly positively correlation with Treg signatures expression. This result demonstrated that PD-L2 upregulated was associated with inflammation cells transduction signals activated in glioma and may play an important role in immunosuppressive functions.
Increased PD-L2 expression conferred a worse outcome
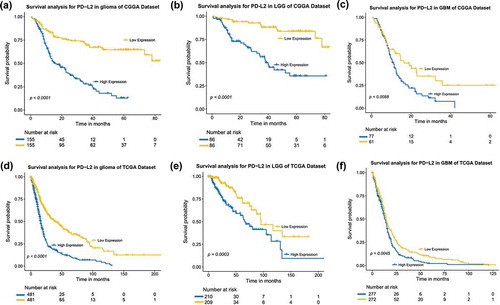
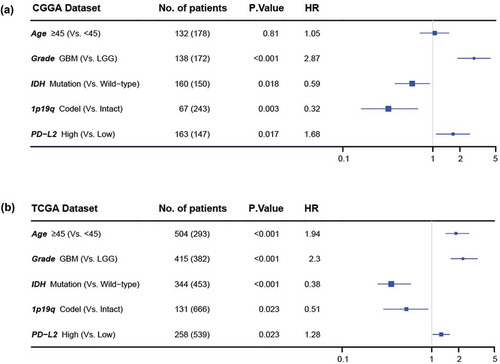
To further analyze the prognostic value of PD-L2, we divided glioma patients into PD-L2 low-expression group and high-expression group based on cut-off value (median PD-L2 expression level). As shown in , , in both CGGA and TCGA datasets, glioma patients of low expression group experienced significantly longer overall survival than their high expression counterparts. Similar Kaplan-Meier curves was significantly observed in lower grade gliomas (LGG) and GBM patients (, , and ). Consequently, we also explored the prognostic value of PD-L2 expression in patients of IDH mutant glioma and IDH wild-type glioma, respectively. As shown in Fig S2, patients who had a higher expression of PD-L2 had a shorter overall survival than their counterparts in both datasets. PD-L2 expression was a prognostic biomarker in gliomas independent of IDH mutation status. To further investigate the independent prognostic significance of PD-L2, we performed uni- and multi-variate cox regression analysis in CGGA and TCGA datasets. Age, grade, IDH status and 1p/19q status showed statistically significant prognostic value in univariate analysis in both two datasets as previously reported.Citation3,Citation4,Citation22 On multivariate analysis, after adjusting for the four clinicopathological factors mentioned above, PD-L2 expression remained an independent prognostic factor for glioma patients (, ). These findings indicated that PD-L2 conferred a poor prognosis in glioma patients.
Discussion
Gliomas are the most common and malignant intracranial tumor, which universally relapse and lethal in adults.Citation23 Despite the advances of standards surgical resection, alone with radiotherapy and chemotherapy, median survival of glioma patients still improved at a very limited speed. Therefore, new therapeutic approaches were urgently needed. Cancer immunotherapies are becoming one of the most promising approaches in treating cancers today. The key to most immunotherapeutic manners is to enhance cytotoxic effects of CD8+ T cells.Citation5,Citation6,Citation24 In tumor cells surface, the ligands of immune checkpoint, particularly PD-L1,Citation25 PD-L2Citation15 and TIM-3,Citation26 would bind with checkpoint receptors on T cell surface. In malignant tumors, these immune checkpoint ligands are upregulated to shut down T cell neoantigen recognition and cytotoxic attacks against tumor cells. PD-L1, which was commonly expressed on normal cells and immune cells, could suppress T-cell activity and facilitate cancer progression. PD-L1 expression was significantly upregulated in GBM and was an independent unfavorable prognostic biomarker for glioma patients.Citation27 PD-1/PD-L1 pathway is critical axis for inducing T-cell exhaustion of glioma.Citation28 Antagonizing or blocking PD-1/PD-L1 pathway to activate immune response and T cell function were well-recognized FDA-approved treatment approach. Patients suffering from non-clear-cell renal cell carcinoma, metastatic bladder cancer, melanoma and non-small cell lung carcinoma benefited from novel immunotherapeutic treatment.Citation29,Citation30 However, anti-cancer strategies exhibited limited efficacy in gliomas, raising the requirement to better understand of treatment failure. One mechanism may be due to the PD-1/PD-L2 axis which could halt or limit the development of T cell response.Citation10,Citation31
Compared to PD-L1, normally, PD-L2 mainly expressed on antigen-presenting cells and remained lower basal expression.Citation8,Citation13,Citation32 Nevertheless, PD-L2 would be significantly upregulated by a series of cytokines which were stimulated by tumor microenvironment, such as IL-15 and IL-7.Citation9,Citation13,Citation33,Citation34 In recent years, emerging data has shown that PD-L2 mainly focused on inducing the phase of T cell immunity and response in esophageal squamous cell carcinoma,Citation35 colorectal cancer,Citation36 and hepatocellular carcinoma.Citation37 However, the potential roles of PD-L2 in glioma were relatively less studied than PD-L1.
In this study, to further explore the role and the distribution of PD-L2 in whole grade glioma, we took advantage of CGGA and TCGA data set and totally 1357 samples were enrolled into the analysis. As expected, PD-L2 expression level was significantly upregulated with increasing grade. Furthermore, PD-L2 expression was significantly higher in glioma of IDH wild-type group and might be a potential predicting marker for mesenchymal subtype. Those results indicated that PD-L2 expression level was tightly correlated with malignant process. The risk of patients with higher PD-L2 expression level in tumor recurrence and progression was greater than others.
Then we took an in-depth exploration of the biological function analysis of PD-L2 in glioma. GO analysis revealed that PD-L2 was correlated with immune response, regulating T-cell function, cytokine secretion and cellar response to mechanical stimulus. And the six metagenes clusters found that PD-L2 was positively correlated with APCs function and activated STAT1 pathway. These results were in line with PD-L2 function in other tumors that PD-L2 upregulation could affect T cell either in the induction phase or in the effector phase in the tumor.Citation38,Citation39 The clinical significance of immune response is different in malignant tumors.Citation40 Melanoma is characterized by the expression of several TAAs, which can be recognized by T cells, resulting in a strong immunological response to the tumor. However, melanoma can escape the immune system and continue to grow and metastasize.Citation41 In pancreatic cancer, higher levels of immune related responses were significantly associated with shorter survival.Citation42 Meanwhile, an increased immune response had a poorer prognosis in pancreatic ductal adenocarcinoma, prostate carcinomaCitation43 and ovarian cancer.Citation44 Therefore, some immune response functions exhibited pro-tumorigenic functions in gliomas as well as in multiple malignancies. Hence, discovering the mechanism of PD-L2 expression in glioma may facilitate to vanquish this fatal disease.
Additionally, Kaplan-Meier survival curves results depicted that patients with higher PD-L2 expression level were correlated with a significantly impaired overall survival either in whole grade gliomas, LGG or GBM. And uni- and multi-variate cox regression analysis indicated that PD-L2 was an independent prognosticator for clinical outcome. These findings further verified that PD-L2 might be a crucial component for tumor inflammatory microenvironment that facilitated tumor growth.
Nowadays, many pre-clinical and clinical trials were submitted for investigating the therapeutic effect on blocking PD-1/PD-L1 pathway. However, the clinical researches of PD-L2 blocking strategies were rarely studied. In one study, the Panc02 murine pancreatic tumor model significantly decrease tumor growth rate by blocking PD-L2.Citation45 Their inspiring discovery rendered a reliable support to our research. Since clinical data for antagonizing PD-L2 in human glioma are scarce, more studies are warranted to reveal the function of PD-L2. Finding checkpoint blockade inhibitors with synergistic effect is an important direction for immunotherapies in the future.
Conclusion
As far as we know, this was the first study to explore the molecular and clinical roles of PD-L2 in glioma. PD-L2 expression was significantly associated with immune response function and act as an unfavorable prognostic predictor in glioma patients. Targeting PD-L2 either alone or in combination with PD-L1 may improve the efficacy of therapies in restoring the function of immune cells.
Abbreviations
| WHO | = | World Health Organization |
| ROC | = | Receiver Operating Characteristic |
| AUC | = | the area under the curve |
| CGGA | = | Chinese Glioma Genome Atlas |
| TCGA | = | The Cancer Genome Atlas |
| IDH | = | Isocitrate dehydrogenase |
| FDR | = | False discovery rate |
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing interests.
Supplemental Material
Download Zip (1.5 MB)Supplementary material
Supplemental data can be accessed here.
Additional information
Funding
References
- Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19:v1–v88. doi: 10.1093/neuonc/nox158.
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1.
- Jiang T, Mao Y, Ma W, Mao Q, You Y, Yang X, Jiang C, Kang C, Li X, Chen L, et al. CGCG clinical practice guidelines for the management of adult diffuse gliomas. Cancer Lett. 2016;375:263–273. doi: 10.1016/j.canlet.2016.01.024.
- Yang P, Wang Y, Peng X, You G, Zhang W, Yan W, Bao Z, Wang Y, Qiu X, Jiang T. Management and survival rates in patients with glioma in China (2004–2010): a retrospective study from a single-institution. J Neurooncol. 2013;113:259–266. doi: 10.1007/s11060-013-1103-9.
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991.
- Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Reviews Clin Oncol. 2017. doi: 10.1038/nrclinonc.2017.101.
- Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005.
- Zhang Y, Chung Y, Bishop C, Daugherty B, Chute H, Holst P, Kurahara C, Lott F, Sun N, Welcher AA, et al. Regulation of T cell activation and tolerance by PDL2. Proc Natl Acad Sci U S A. 2006;103:11695–11700. doi: 10.1073/pnas.0601347103.
- Singh AK, Stock P, Akbari O. Role of PD-L1 and PD-L2 in allergic diseases and asthma. Allergy. 2011;66:155–162. doi: 10.1111/j.1398-9995.2010.02458.x.
- Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330.
- Wang H, Yao H, Li C, Liang L, Zhang Y, Shi H, Zhou C, Chen Y, Fang J-Y, Xu J. PD-L2 expression in colorectal cancer: independent prognostic effect and targetability by deglycosylation. Oncoimmunology. 2017;6:e1327494. doi: 10.1080/2162402X.2017.1327494.
- Howitt BE, Sun HH, Roemer MG, Kelley A, Chapuy B, Aviki E, Pak C, Connelly C, Gjini E, Shi Y, et al. Genetic basis for PD-L1 expression in squamous cell carcinomas of the cervix and vulva. JAMA oncol. 2016;2:518–522. doi: 10.1001/jamaoncol.2015.6326.
- Shi M, Roemer MG, Chapuy B, Liao X, Sun H, Pinkus GS, Shipp MA, Freeman GJ, Rodig SJ. Expression of programmed cell death 1 ligand 2 (PD-L2) is a distinguishing feature of primary mediastinal (thymic) large B-cell lymphoma and associated with PDCD1LG2 copy gain. Am J Surg Pathol. 2014;38:1715–1723. doi: 10.1097/PAS.0000000000000297.
- Karunarathne DS, Horne-Debets JM, Huang JX, Faleiro R, Leow CY, Amante F, Watkins TS, Miles JJ, Dwyer PJ, Stacey KJ, et al. Programmed Death-1 Ligand 2-Mediated regulation of the PD-L1 to PD-1 axis is essential for establishing CD4(+) T cell immunity. Immunity. 2016;45:333–345. doi: 10.1016/j.immuni.2016.07.017.
- Liu X, Gao JX, Wen J, Yin L, Li O, Zuo T, Gajewski TF, Fu Y-X, Zheng P, Liu Y. B7DC/PDL2 promotes tumor immunity by a PD-1-independent mechanism. J Exp Med. 2003;197:1721–1730. doi: 10.1084/jem.20022089.
- Yan W, Zhang W, You G, Zhang J, Han L, Bao Z, Wang Y, Liu Y, Jiang C, Kang C, et al. Molecular classification of gliomas based on whole genome gene expression: a systematic report of 225 samples from the Chinese glioma cooperative group. Neuro Oncol. 2012;14:1432–1440. doi: 10.1093/neuonc/nos263.
- Yan W, Zhang W, You G, Bao Z, Wang Y, Liu Y, Kang C, You Y, Wang L, Jiang T, et al. Correlation of IDH1 mutation with clinicopathologic factors and prognosis in primary glioblastoma: a report of 118 patients from China. PloS one. 2012;7:e30339. doi: 10.1371/journal.pone.0030339.
- Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710.
- Yearley JH, Gibson C, Yu N, Moon C, Murphy E, Juco J, Lunceford J, Cheng J, Chow LQM, Seiwert TY, et al. PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer. Clin Cancer Res: an off J Am Assoc Cancer Res. 2017;23:3158–3167. doi: 10.1158/1078-0432.CCR-16-1761.
- Erlmeier F, Weichert W, Autenrieth M, Wiedemann M, Schrader AJ, Hartmann A, Ivanyi P, Steffens S. PD-L2: A prognostic marker in chromophobe renal cell carcinoma? Med oncol. 2017;34:71. doi: 10.1007/s12032-017-0926-1.
- Rody A, Holtrich U, Pusztai L, Liedtke C, Gaetje R, Ruckhaeberle E, Solbach C, Hanker L, Ahr A, Metzler D, et al. T-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Res: BCR. 2009;11:R15. doi: 10.1186/bcr2234.
- Zhao Z, Meng F, Wang W, Wang Z, Zhang C, Jiang T. Comprehensive RNA-seq transcriptomic profiling in the malignant progression of gliomas. Sci data. 2017;4:170024. doi: 10.1038/sdata.2017.24.
- Nikiforova MN, Hamilton RL. Molecular diagnostics of gliomas. Arch Pathol Lab Med. 2011;135:558–568.
- Goswami KK, Ghosh T, Ghosh S, Sarkar M, Bose A, Baral R. Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell Immunol. 2017;316:1–10. doi: 10.1016/j.cellimm.2017.04.005.
- Liu S, Wang Y, Fan X, Ma J, Ma W, Wang R, Jiang T, Szele F. Anatomical involvement of the subventricular zone predicts poor survival outcome in low-grade astrocytomas. PloS one. 2016;11:e0154539. doi: 10.1371/journal.pone.0154539.
- Li G, Wang Z, Zhang C, Liu X, Cai J, Wang Z, Hu H, Wu F, Bao Z, Liu Y, et al. Molecular and clinical characterization of TIM-3 in glioma through 1,024 samples. Oncoimmunology. 2017;6:e1328339. doi: 10.1080/2162402X.2017.1328339.
- Nduom EK, Wei J, Yaghi NK, Huang N, Kong L-Y, Gabrusiewicz K, Ling X, Zhou S, Ivan C, Chen JQ, et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016;18:195–205. doi: 10.1093/neuonc/nov172.
- Xue S, Hu M, Iyer V, Yu J. Blocking the PD-1/PD-L1 pathway in glioma: a potential new treatment strategy. J Hematol Oncol. 2017;10:81. doi: 10.1186/s13045-017-0455-6.
- Li Y, Li F, Jiang F, Lv X, Zhang R, Lu A, Zhang G. A mini-review for cancer immunotherapy: molecular understanding of PD-1/PD-L1 pathway & translational blockade of immune checkpoints. Int J Mol Sci. 2016;17. doi :10.3390/ijms17071151.
- Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3–potential mechanisms of action. Nat Reviews Immunol. 2015;15:45–56. doi: 10.1038/nri3790.
- He YF, Zhang GM, Wang XH, Zhang H, Yuan Y, Li D, Feng Z-H. Blocking programmed death-1 ligand-PD-1 interactions by local gene therapy results in enhancement of antitumor effect of secondary lymphoid tissue chemokine. J Immunol. 2004;173:4919–4928. doi: 10.4049/jimmunol.173.8.4919.
- Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, Connelly CF, Sun HH, Daadi SE, Freeman GJ, et al. PD-L1 and PD-L2 genetic alterations define classical hodgkin lymphoma and predict outcome. J Clin Oncol: off JAm Soc Clin Oncol. 2016;34:2690–2697. doi: 10.1200/JCO.2016.66.4482.
- Dong M, Wang HY, Zhao XX, Chen J-N, Zhang Y-W, Huang Y, Xue L, Li H-G, Du H, Wu X-Y, et al. Expression and prognostic roles of PIK3CA, JAK2, PD-L1, and PD-L2 in epstein-barr virus-associated gastric carcinoma. Hum Pathol. 2016;53:25–34. doi: 10.1016/j.humpath.2016.02.007.
- Nie X, Chen W, Zhu Y, Huang B, Yu W, Wu Z, Guo S, Zhu Y, Luo L, Wang S, et al. B7-DC (PD-L2) costimulation of CD4(+) T-helper 1 response via RGMb. Cell Mol Immunol. 2017. doi: 10.1038/cmi.2017.17.
- Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res: an off J Am Assoc Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469.
- Masugi Y, Nishihara R, Hamada T, Song M, Da Silva A, Kosumi K, Gu M, Shi Y, Li W, Liu L, et al. Tumor PDCD1LG2 (PD-L2) expression and the lymphocytic reaction to colorectal cancer. Cancer Immunol Res. 2017;5:1046–1055. doi: 10.1158/2326-6066.CIR-17-0122.
- Wang BJ, Bao JJ, Wang JZ, Wang Y, Jiang M, Xing M-Y, Zhang W-G, Qi J-Y, Roggendorf M, Lu M-J, et al. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J Gastroenterol. 2011;17:3322–3329. doi: 10.3748/wjg.v17.i28.3322.
- Takamura Y, Ikeda H, Kanaseki T, Toyota M, Tokino T, Imai K, Houkin K, Sato N. Regulation of MHC class II expression in glioma cells by class II transactivator (CIITA). Glia. 2004;45:392–405. doi: 10.1002/glia.10343.
- Chang H, Kim JS, Choi YJ, Cho J-G, Woo J-S, Kim A, Kim JS, Kang EJ. Overexpression of PD-L2 is associated with shorter relapse-free survival in patients with malignant salivary gland tumors. Onco Targets Ther. 2017;10:2983–2992. doi: 10.2147/OTT.S134589.
- Disis ML. Immune regulation of cancer. J Clin Oncol: off JAm Soc Clin Oncol. 2010;28:4531–4538. doi: 10.1200/JCO.2009.27.2146.
- Pandolfi F, Cianci R, Pagliari D, Casciano F, Bagalà C, Astone A, Landolfi R, Barone C. The immune response to tumors as a tool toward immunotherapy. Clin Dev Immunol. 2011;2011:894704. doi: 10.1155/2011/894704.
- Tang Y, Xu X, Guo S, Zhang C, Tang Y, Tian Y, Ni B, Lu B, Wang H, Shiku H. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PloS one. 2014;9:e91551. doi: 10.1371/journal.pone.0091551.
- Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G, Lanciotti M, Serni S, Chiarugi P. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene. 2014;33:2423–2431. doi: 10.1038/onc.2013.191.
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093.
- Okudaira K, Hokari R, Tsuzuki Y, Okada Y, Komoto S, Watanabe C, Kurihara C, Kawaguchi A, Nagao S, Azuma M, et al. Blockade of B7-H1 or B7-DC induces an anti-tumor effect in a mouse pancreatic cancer model. Int J Oncol. 2009;35:741–749.