?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Patients with metastatic melanoma were treated with tremelimumab and interferon-α (IFN) in a previously reported clinical trial [NCT00610857]. Responses were assessed by RECIST criteria as complete (CR) or partial (PR), stable disease (SD) or progressive disease (PD). In this study, T-cell receptor (TCR) beta-chain repertoire was immunosequenced in peripheral blood mononuclear cells (PBMC) specimens (N = 33) and tumor samples (N = 18) utilizing the immunoSEQ® Assay to determine repertoire clonality and T cell fractions at pre-treatment (tumor and PBMC), one month (PBMC) and 3 months (PBMC) time points and evaluate its association with clinical outcomes. In the pretreatment tumor microenvironment (TME), T cell clonality was significantly (p = .035) different and greater in patients who achieved disease control (CR, PR, SD) versus those with non-disease control (PD) as best response to treatment. Further, there was significantly (p = .001) increased TCR fraction in tissue of responders (CR, PR) versus non-responders (PD, SD). In examining T cell clonality in the circulation (PBMC), no significant associations were found in the pretreatment samples. However, early on-treatment (4 weeks) there was a significant decrease in T cell clonality that was associated with improved overall survival (p = .01) and progression-free survival (p = .04). In addition, analysis of temporal changes in tumor-infiltrating lymphocytes (TIL) and peripheral TCR repertoire revealed that responders had significantly higher clonal expansion of TIL in the circulation at 4 weeks than non-responders (p = .036). Our study provided interesting mechanistic data related to CTLA-4 Blockade and IFN and potential biomarkers of immunotherapeutic benefit.
Introduction
The treatment landscape of metastatic melanoma has improved drastically over the last decade with the identification of small molecule inhibitors of BRAF and MEK and immunotherapeutic antibodies directed at cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell-death protein 1 (PD-1).Citation1,Citation2 However, despite the success with these therapies, more than half of the patients do not experience long-term clinical remission and there is a critical need to investigate novel drug combinations in the salvage setting as well as mechanistic studies and predictive biomarkers of response to extend therapeutic benefits to the majority of patients.
In metastatic melanoma, interferon-α (IFN) has been shown to interrupt tumor immune tolerance, via shifting Th polarity towards Th1 in the tumor microenvironment (TME),Citation3 increasing dendritic cell antigen presentation to immune effectors,Citation4,Citation5 and potentiating natural killer cell-mediated immunity.Citation6 However, tumor tolerance has been shown to limit IFN clinical activity when used as monotherapy. Therefore, adding CTLA-4 blockers plays a critical role in reversing this effect and increasing T cell activation and proliferation.Citation7–Citation13 Based on this hypothesis of potential synergistic antitumor effects of CTLA-blockade and IFN combination treatment, patients with metastatic melanoma were treated with tremelimumab and IFN in a previously reported study.Citation14 The study demonstrated promising durable antitumor activity of the combination with acceptable toxicity.
Early studies have shown that increased T-cell infiltration within the tumor biopsy of primary melanoma is associated with improved clinical outcomes following immunotherapy.Citation15,Citation16 Additionally, increased density of tumor-infiltrating lymphocytes (TILs) is an independent prognostic factor for sentinel lymph node metastatic status and survival.Citation17 Since antitumor effects of anti-CTLA-4 are mediated in part by reprogramming of T-cells, a number of studies have investigated blood and tumor biopsy T-cell subpopulations and correlated immunologic changes with patient outcomes. It has been reported that during treatment with ipilimumab, survival was significantly associated with increased total CD4 and CD8 lymphocyte count.Citation18–Citation21 Other studies have explored the impact of CTLA-4 blockade on the circulating T-cell receptor (TCR) repertoire, as TCR repertoire is proposed to be a mirror of human adaptive immune responses and the presence of diverse T-cell clones may indicate biological effects of treatment. In one study, CTLA-4 blockade using tremelimumab in metastatic melanoma diversified the clonal T cell pool in the blood, without clear correlation with survival.Citation22 In another study, pre-treatment peripheral blood TCR diversity was correlated to clinical outcomes with ipilimumab treatment in a small group of melanoma patients.Citation23
The aim of the present study was to evaluate the effect of combined treatment of tremelimumab and IFN on the clonality of TCR repertoire in tumor and peripheral blood, and correlate it with clinical outcome. Utilizing biospecimens from our previously reported study,Citation14 immunosequencing of the TCR beta chain repertoire was performed in the TME and PBMCs utilizing Adaptive Biotechnologies’ immunoSEQ® Assay to determine repertoire clonality and T cell fraction.
Results
Patient characteristics, clinical efficacy, and safety
Patient demographics and baseline disease characteristics of the 37 enrolled patients have been summarized in . Clinical efficacy and toxicity data were reported previously.Citation14
Table 1. Patient demographics and baseline disease characteristics (N = 37 patients).
Association of T-cell receptor clonality in tumor tissue with response
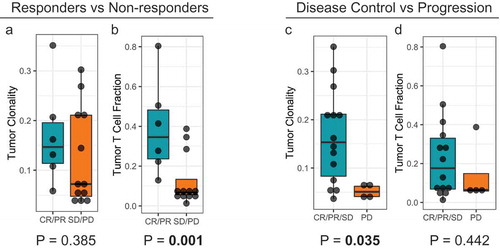
To examine whether pre-treatment TCR clonality was associated with clinical benefit, deep immunosequencing of the T-cell repertoire was done using pre-treatment tumor samples of patients (n = 18) ( and supplemental figure 1). It was observed that T cell clonality was significantly (p = .035) different and greater in patients who achieved disease control (CR, PR, SD) versus those with non-disease control (PD) ()). Further, TCR fraction in tumor tissues was compared in responders and non-responders and it was observed that there was a significant (p = .001) increase in TCR fraction in the tumor tissue in responders (CR, PR) versus non-responders (PD, SD) ()
Figure 1. Correlation of T-cell repertoire characteristics of pre-treatment tumor specimens with clinical outcomes. (a) and (b) T-cell clonality and T-cell fraction stratified by responders (CR/PR) and non-responders (SD/PD). (c) and (d) T-cell clonality and T-cell fraction stratified by disease control (CR/PR/SD) and disease progression (PD).
Association of T-cell receptor clonality in tumor tissue with survival
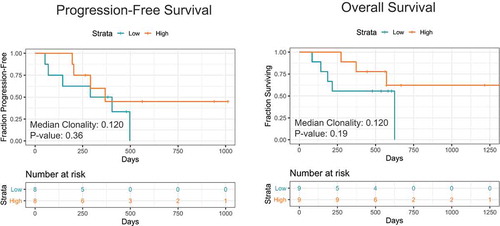
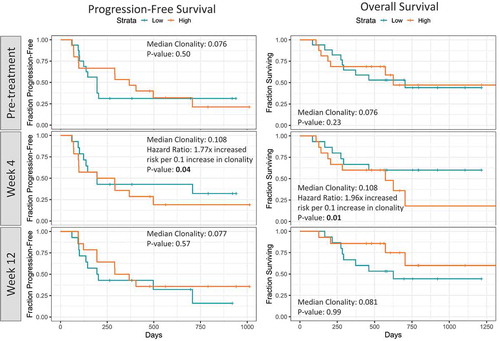
Further, we evaluated if pre-treatment TCR clonality correlated with PFS and OS outcomes. However, we observed no significant correlations. Interestingly, there was a separation of the survival curves where higher TCR clonality at baseline (> 0.12) potentially correlated with better PFS (p = .36) and OS (p = .19) (). The lack of significance in terms of survival associations may be a factor of the limited sample size of the study.
Association of T-cell receptor clonality in PBMCs with response
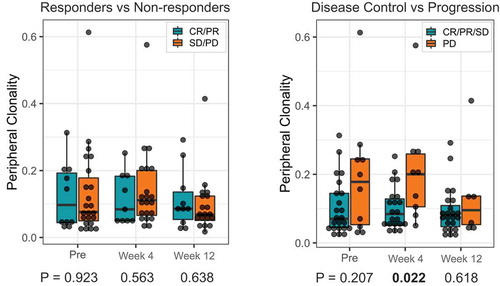
We further investigated whether TCR clonality in the PBMC specimens was associated with response to treatment by performing TCR sequencing at baseline and early on-treatment (n = 33 patients) ( and supplemental Figure 2 and 3). Unlike the tumor TCR clonality, no significant association was found between pre-treatment PBMC TCR clonality and response. However, there was a trend where patients with disease control tended to have lower clonality at baseline and at 4 weeks.
Association of T-cell receptor clonality in PBMC with survival
Next, we examined if peripheral T-cell repertoire features were associated with survival outcomes (). It was found that there was no statistical association of pre-treatment/baseline clonality with PFS and OS. However, early on-treatment (4 weeks), patients with lower peripheral clonality had longer PFS and OS intervals than those with higher peripheral clonality (p = .04 for PFS and p = .01 for OS).
Evolution of T cell repertoire in PBMCs during treatment
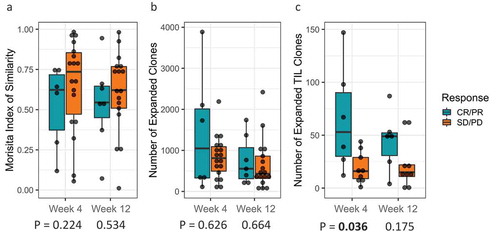
To investigate the dynamics of the peripheral T cell repertoire upon CTLA4 blockade and high-dose interferon-alfa (HDI) combination treatment, we compared the peripheral T cell repertoire over different time points (baseline, 4 weeks and 12 weeks). Repertoire turnover, as measured by the Morisita Index, showed a trend towards responders having greater turnover post-treatment than non-responders ( and supplementary figure 4A). This was evident as non-responders had higher values of Morisita index in comparison to responders, closer to 1, which indicates a more similar repertoire for different time points, while lower values of Morisita index indicate more dis-similarity, consistent with greater repertoire turnover. Similarly, the total number of clones expanding in the peripheral repertoire varied over time within an individual patient (p = .034) with responders showing a greater increase in the number of expanded clones than non-responders, though it was not statistically significant ( and supplementary figure 4B). When we restricted quantification to only expanded clones that were also found in the tumor repertoire, we observed that responders had significantly more TIL that expanded in the periphery at 4 weeks than non-responders (p = .036) ( and supplementary figure 4C).
Figure 5. (a) T-cell Repertoire turnover at week 4 and week 12 relative to baseline stratified by clinical response; (b) T-cell clonal expansion at week 4 and week 12 relative to baseline stratified by clinical response; (c) Tumor-infiltrating lymphocytes (TILs) clonal expansion at week 4 and week 12 stratified by clinical response.
Association of T-cell receptor clonality in PBMC with toxicity
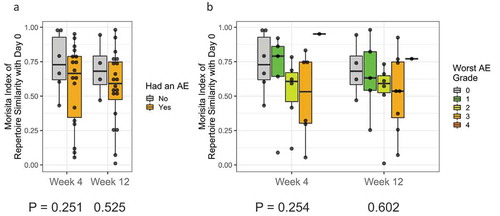
We hypothesized that a greater repertoire turnover (as measured by a lower Morisita Index) following immunotherapy treatment may also correlate with the development of immune-related adverse events due to the possible expansion of auto-reactive T cells. We tested PBMC samples from patients who experienced an adverse event and assessed the T-cell repertoire turnover relative to baseline at week 4 and week 12 stratified by presence or absence of adverse event and by adverse event severity. No significant associations were found as tested by linear mixed-effects model in terms of AE incidence (time point p-value = 0.302 and AE p-value = 0.263) (), or severity (time point p-value = 0.301 and AE p-value = 0.363) ().
Discussion
Over the past few years, application of immunosequencing has gained traction among cancer researchers as it provides a snapshot of the repertoire of immune cells at any given time and assists in tracking adaptive changes as a result of various immunotherapeutic perturbations. Several groups have attempted to study immune repertoire characteristics using immunosequencing in various solid tumors, in an effort to gain insights into the underlying mechanisms and identify novel biomarkers of response and toxicity.Citation24–Citation27 In this study, we have employed immunosequencing techniques to more deeply characterize both intratumoral and peripheral T-cell responses following treatment with combination therapy of tremelimumab and IFN. Our data showed that an increased TCR fraction and T cell clonality in the pre-treatment TME was associated with favorable clinical outcomes. In PBMCs, at 4 weeks post-treatment, a statistically significant association was noted between decrease in T cell clonality and improvement in PFS and OS.
Our findings in terms of the association of high T cell clonality and TCR fraction with immunotherapeutic response are consistent with previous studies that demonstrated increased baseline density and clonality of TIL to be associated with improved survival in solid tumors following treatment with immunotherapy.Citation24,Citation28,Citation29 In metastatic melanoma patients, Tumeh et al. showed that pre-treatment samples from responding patients treated with Pembrolizumab had higher CD8 T cell at the invasive tumor margin and inside tumors and were associated with a more diverse TIL repertoire.Citation24 However, another study involving sequential combination treatment with ipilimumab and nivolumab in melanoma patients did not show significant differences between increased baseline CD8 + T cell density, and clonality in responders vs non-responders.Citation30
TCR portion of T cell plays a critical role in antigen presentation, therefore an increased T cell fraction has been associated with increased antigenicity and tumor antigen presentation by dendritic cells.Citation31,Citation32 Further, increased T cell infiltration in tumor has been associated with a more robust inflammatory response, with marked increase in cytokines and interferon-γ secretion and regulatory ligands inhibitors expression, mitigating checkpoints inhibitors targets.Citation33 On the other hand, increased T cell clonality in responders may reflect a high tumor mutation rate, increased immunogenic neoantigen production, and easier immune cell recognition of the tumor as “foreign”.Citation34,Citation35 Thus, tumors with a high number of clonal neoantigens may be more likely to elicit effective immune responses following immunotherapy.Citation35 Therefore, defining the tumor repertoire at baseline provides a useful tool to determine general immunocompetence and possible response to immunotherapy. It was interesting to observe in our study, that patients with SD had a unique combination of high clonality (a feature associated with favorable response) and low TIL fraction (a feature associated with poor prognosis) (supplementary Figure 1). This is interesting when considering that patients with SD generally do not achieve durable clinical benefits and usually fall in the middle between responders and progressors in terms of clinical benefits. More work is needed to better understand this phenomenon immunologically.
In examining T cell clonality within the circulation, no significant associations were found in the pretreatment samples and baseline TCR diversity was not associated with clinical outcomes. Similar to our findings, a study by Robert et al. found no association between baseline peripheral TCR repertoire diversity and response to CTLA-4 blockade with tremelimumab.Citation22 However, a pilot study by Postow and colleagues reported that higher response rate was observed in metastatic melanoma patients treated with ipilimumab, who presented with a more diverse peripheral TCR repertoire at baseline.Citation23 Similarly, in patients with pancreatic ductal adenocarcinoma receiving ipilimumab, a diverse baseline TCR repertoire was associated with longer median OS.Citation36
In our analysis of PBMC samples obtained early on-treatment (4 weeks), there was a significant association between a decrease in T cell clonality and OS and PFS. In our study, decrease in T cell clonality early on-treatment (4 weeks) and the improvement in OS and PFS can be a restoration of interferon-mediated effect on tumor-killing after using CTLA-4 blockers, increasing T cell activation and broadening the peripheral T cell repertoire compared with baseline.Citation11–Citation13 The addition of IFN interrupts immune tolerance, increases dendritic cell activation and survivalCitation4,Citation37 and increases Th1-mediated pro-inflammatory cytokines in peripheral circulation.Citation38
To address clone level changes in the peripheral TCR repertoire following treatment, we compared the peripheral T cell repertoire over 3 time points. In our analysis, no significant association was found between clonal expansion and clinical outcomes, though there was a trend towards greater clonal expansion in responding patients at 4 weeks and 12 weeks. In a previous study by Hopkins et al. long-term survivors of pancreatic cancer treated with ipilimumab had significantly more expanded clones in comparison to short-term survivors (p < .05).Citation36 Further, as individual T cell clones present in tumors can also be tracked in the peripheral blood during treatment, we restricted our clonal analysis to measure only expanded clones that are also found in the tumor repertoire. Interestingly, we observed that responders had significantly more TIL expanded in the periphery at 4 weeks than non-responders, consistent with clonal expansion of tumor antigen-specific T cells. Similar to our findings, Snyder et al. demonstrated that bladder cancer patients with substantial expansion of tumor-associated TCR clones in the peripheral blood 3 weeks after starting treatment with atezolizumab had more favorable outcomes.Citation39 The expansion of tumor-associated TCRs in the peripheral blood of responding patients may suggest that T cells shuttle back and forth between the tumor and the peripheral compartment where circulating immune cells somewhat reflect or overlap with the tumor’s TIL repertoire. Therefore, a greater number of tumor-specific TCR clones could limit the magnitude of tumor escape due to the presence of more specific T-cells in the circulation. With respect to biomarker development, our finding reinforces the potential value of pursuing noninvasive metrics such as peripheral TCR clonal expansion early on-treatment as a potential predictive biomarker for therapeutic decision-making.
Further, we also observed that T-cell repertoire turnover was not associated with both the development and severity of toxicities after receiving immunotherapy treatment. Prior studies have reported that increasing T cell repertoire turnover is associated with the development of autoimmune toxicities after receiving tremelimumabCitation22 and ipilimumab.Citation40,Citation41 These observations were explained by the fact that not all T cell activation following immunotherapy can be tumor antigen-specific and development of toxicity may be mechanistically linked to proliferation and mobilization of autoreactive T cell populations.
The major limitation of our study was the small number of patients who were enrolled. Our sample size might have not been adequate to show a statistically significant difference in a number of critical analyses. Nevertheless, our study provides further evidence to support the utility of TCRβ sequencing to elucidate the effects of immunotherapy on TIL and PBMC as well as the assessment of response and immune-related adverse events of immunotherapies.
Conclusion
Higher T cell clonality and TIL fraction in the pretreatment TME were found to be associated with immunotherapeutic response. In the circulation, lower peripheral clonality early on-treatment was significantly associated with improved survival. Further, higher TIL expansion in the peripheral repertoire early on-treatment was associated with response to therapy. These findings warrant further investigation and exploration in relation to other immunotherapeutics.
Patient and methods
Patients
Patients with inoperable metastatic melanoma were enrolled in the study.Citation14 Patients were treated with tremelimumab 15 mg/kg intravenously every 12 weeks. HDI was administered concurrently, including intravenous induction at 20 MU/m2/d for 5 days per week for 4 weeks, followed by maintenance at 10 MU/m2/day subcutaneously three times a week for 8 weeks per cycle of tremelimumab. From cycle 2 onward, HDI was administered subcutaneously. Patients without evidence of disease progression or limiting toxicities were offered additional cycles of therapy up to a maximum of four cycles. The institutional review board at the University of Pittsburgh approved the study and a written informed consent was obtained from all the patients.
Response and toxicity assessment
Response to treatment was evaluated by assessing target lesions with Response Evaluation Criteria in Solid Tumors [RECIST] version 1 using imaging studies, carried out at the end of each cycle or earlier if clinically indicated.Citation42 Patients were classified as having a complete response (CR), partial response (PR), stable disease (SD), or Progressive disease (PD). Descriptions and grading scales found in the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0) were used for grading and reporting of adverse events (AEs). In addition, overall survival (OS) and progression-free survival (PFS) data were obtained for all patients.
Analysis of TCRβ repertoire
The TCR repertoire of PBMC specimens (N = 33 patients) and TME (N = 18) was immunosequenced at pre-treatment (TME and PBMC), one month post-treatment (PBMC), and three months post-treatment (PBMC). Immunosequencing of the CDR3 regions of human TCRβ chains was performed using the immunoSEQ® Assay (Adaptive Biotechnologies, Seattle, WA). Extracted genomic DNA was amplified in a bias-controlled multiplex polymerase chain reaction, followed by high-throughput sequencing. Sequences were collapsed and filtered in order to identify and quantitate the absolute abundance of each unique TCRβ CDR3 region for further analysis as previously described.Citation43–Citation45
Clonality and T-cell fraction were calculated for each immunosequenced sample. Clonality is defined as (1 – Peilou’s Evenness) and was calculated on productive rearrangements by Equation 1,
where is the proportional abundance of rearrangement
and
is the total number of rearrangements in a sample. Clonality values range from 0 to 1 and describe the shape of the frequency distribution: clonality values approaching 0 indicate a very even distribution of frequencies, whereas values approaching 1 indicate an increasingly asymmetric distribution in which a few clones are present at high frequencies.Citation46 T-cell fraction was defined as the proportion of T cells to all nucleated cells in a sample.
The Morisita Index is a measure of repertoire similarity, as defined by Equation 2,
where and
are the T cell counts of clone
in samples A and B,
and
are the total number of T cells in samples A and B, and
is the total number of unique clones in the union of samples A and B. Like clonality, the Morisita Index ranges from 0 to 1, with values closer to 1 indicating high similarity and less repertoire turnover.Citation47 In this study, post-treatment PBMC repertoires were compared to pre-treatment for each patient.
Clonal expansion also compared post-treatment PBMC repertoires to pre-treatment for each patient. As previously described, clones that increased in frequency above our statistical threshold (multiple testing corrected p-value 0.01) in the post-treatment repertoire (relative to pre-treatment) were identified as expanded.Citation48
Statistical methods
Two-tailed, non-parametric statistical tests were used to compare patient response (Wilcoxon Rank Sum or Kruskal-Wallis Tests) and time points (Wilcoxon Signed Rank Test). Multivariate analyses were performed with linear mixed-effects models. Likelihood Ratio Tests were applied to Cox Proportional Hazard models to determine associations between continuous variables and PFS/OS. Unless otherwise specified, p-values 0.05 were considered statistically significant. Statistical analyses were performed in R version 3.5.1. Given the limited sample size, our attempts for adjusting the p-values for multiple testing led to the loss of statistical significance using a p-value of 0.05 (except for the correlation of T-fraction in pre-treatment tumor specimens with an objective response as summarized in ).
Disclosure of interest
Arjun Khunger report no conflict of interest
Julie A. Rytlewski, Paul Fields and Erik C. Yusko have equity and employment with Adaptive Biotechnologies.
Ahmad A. Tarhini declared consultant role with Bristol Myers Squibb, Merck, Novartis, Genentech-Roche, Array Biopharma, HUYA, Immunocore, NewLink Genetics.
Disclaimers
immunoSEQ and their associated designs are trademarks of Adaptive Biotechnologies. immunoSEQ Assays are for research use only and not for use in diagnostic procedures.
List of where and when study presented elsewhere: Partly presented at the 2017 and 2019 ASCO annual meeting.
Supplemental Material
Download MS Power Point (834.8 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi:10.1038/nature00766.
- Pasquali S, Hadjinicolaou, AV, Sileni, VC, Rossi, CR, & Mocellin, S Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev. 2018;2.
- Parlato S, Santini SM, Lapenta C, Di Pucchio T, Logozzi M, Spada M, Giammarioli AM, Malorni W, Fais S, Belardelli F. Expression of CCR-7, MIP-3beta, and Th-1 chemokines in type I IFN-induced monocyte-derived dendritic cells: importance for the rapid acquisition of potent migratory and functional activities. Blood. 2001;98(10):3022–3029. doi:10.1182/blood.v98.10.3022.
- Kirkwood JM, Richards T, Zarour HM, Sosman J, Ernstoff M, Whiteside TL, Ibrahim J, Blum R, Wieand S, Mascari R. Immunomodulatory effects of high-dose and low-dose interferon alpha2b in patients with high-risk resected melanoma: the E2690 laboratory corollary of intergroup adjuvant trial E1690. Cancer. 2002;95(5):1101–1112. doi:10.1002/cncr.10775.
- Wenjun Wang HDE, Uma NM, Rao DM, Jukic SR, Land SF, John M. Kirkwood, modulation of signal transducers and activators of transcription 1 and 3 signaling in melanoma by high-dose IFNα2b. Clin Cancer Res. 2007;13:1523–1531.
- Carballido JA, Moltó ML, Manzano L, Olivier, C, Salmerón, OJ, de Mon, MA. Interferon-alpha-2b enhances the natural killer activity of patients with transitional cell carcinoma of the bladder. Cancer. 1993;72(5):1743–1748.
- Brunet JF, Denizot, F, Luciani, MF, Roux-Dosseto, M, Suzan, M, Mattei, MG, Golstein, P. A new member of the immunoglobulin superfamily–CTLA-4. Nature. 1987;328(6127):267–270.
- Khattri R, Auger, JA, Griffin, MD, Sharpe, AH, Bluestone, JA Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J Immunol. 1999;162(10):5784–5791.
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–547.
- Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–988. doi:10.1126/science.270.5238.985.
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182(2):459–465. doi:10.1084/jem.182.2.459.
- Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448.
- van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–366. doi:10.1084/jem.190.3.355.
- Tarhini AA, Cherian J, Moschos SJ, Tawbi HA, Shuai Y, Gooding WE, Sander C, Kirkwood JM. Safety and efficacy of combination immunotherapy with interferon alfa-2b and tremelimumab in patients with stage IV melanoma. J Clin Oncol. 2012;30(3):322. doi:10.1200/JCO.2011.37.5394.
- Ribas A, Comin-Anduix B, Chmielowski B, Jalil J, de la Rocha P, TA M, Ochoa MT, Seja E, Villanueva A, Oseguera DK, et al. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin Cancer Res. 2009;15:6267–6276. doi:10.1158/1078-0432.CCR-09-1254.
- Huang RR, Jalil J, JS E, Chmielowski B, RC K, Mok S, Sazegar H, Seja E, Villanueva A, Gomez-Navarro J, et al. CTLA4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans. Clin Cancer Res. 2011;17:4101–4109. doi:10.1158/1078-0432.CCR-11-0407.
- Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, Saw RP, Thompson JF. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30(21):2678–2683. doi:10.1200/JCO.2011.37.8539.
- Simeone E, Gentilcore G, Giannarelli D, Grimaldi AM, Caracò C, Curvietto M, Esposito A, Paone M, Palla M, Cavalcanti E, et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother. 2014;63(7):675–683. doi:10.1007/s00262-014-1545-8.
- Ascierto PA, Kalos M, Schaer DA, Callahan MK, Wolchok JD. Biomarkers for Immunostimulatory Monoclonal Antibodies in Combination Strategies for Melanoma and Other Tumor Types. Clinical Cancer Research. 2013;19(5):1009-1020.
- Bjoern J, Juul Nitschke N, Zeeberg Iversen T, Schmidt H, Fode K, Svane IM. Immunological correlates of treatment and response in stage IV malignant melanoma patients treated with Ipilimumab. Oncoimmunology. 2016;5(4):e1100788. doi:10.1080/2162402X.2015.1100788.
- Hopkins AM, Rowland A, Kichenadasse G, Wiese MD, Gurney H, McKinnon RA, Karapetis CS, Sorich MJ. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br J Cancer. 2017;117(7):913. doi:10.1038/bjc.2017.274.
- Robert L, Tsoi J, Wang X, Emerson R, Homet B, Chodon T, Mok S, Huang RR, Cochran AJ, Comin-Anduix B, Koya RC. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clinical Cancer Research. 2014;20(9):2424-32.
- Postow MA, Manuel M, Wong P, Yuan J, Dong Z, Liu C, Perez S, Tanneau I, Noel M, Courtier A, et al. Peripheral T cell receptor diversity is associated with clinical outcomes following ipilimumab treatment in metastatic melanoma. J Immunother Cancer. 2015;3(1):23. doi:10.1186/s40425-015-0070-4.
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568. doi:10.1038/nature13954.
- Roh W, Chen P-L, Reuben A, Spencer CN, Prieto PA, Miller JP, Gopalakrishnan V, Wang F, Cooper ZA, Reddy SM, et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med. 2017;9(379):eaah3560. doi:10.1126/scitranslmed.aah3560.
- Page DB, Yuan J, Redmond D, Wen YH, Durack JC, Emerson R, Solomon S, Dong Z, Wong P, Comstock C, et al. Deep sequencing of T-cell receptor DNA as a biomarker of clonally expanded TILs in breast cancer after immunotherapy. Cancer Immunol Res. 2016;4(10):835–844. doi:10.1158/2326-6066.CIR-16-0013.
- Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, Hodi FS, Martín-Algarra S, Mandal R, Sharfman WH, et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell. 2017;171(4):934–949.e16. doi:10.1016/j.cell.2017.09.028.
- Vilain RE, Menzies AM, Wilmott JS, Kakavand H, Madore J, Guminski A, Liniker E, Kong BY, Cooper AJ, Howle JR, et al. Dynamic changes in PD-L1 expression and immune infiltrates early during treatment predict response to PD-1 blockade in Melanoma. Clin Cancer Res. 2017;23(17):5024–5033. doi:10.1158/1078-0432.CCR-16-0698.
- Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, Miller JP, Bassett RL, Gopalakrishnan V, Wani K, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 2016;6(8):827–837. doi:10.1158/2159-8290.CD-15-1545.
- Wei-Shen Chen MCA, Spencer C, Tawbi HA-H, Lazar A, Tetzlaff MT, Tetzlaff MT, Patel SP, Hwu P, Hwu W-J, Diab A, et al. Molecular and immune predictors of response and toxicity to combined CTLA-4 and PD-1 blockade in metastatic melanoma (MM) patients (pts). J Clin Oncol. 2017;35(15):9579. doi:10.1200/JCO.2017.35.15_suppl.9579.
- Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334(6181):395–402. doi:10.1038/334395a0.
- Li B, Li T, Pignon J-C, Wang B, Wang J, Shukla SA, Dou R, Chen Q, Hodi FS, Choueiri TK, et al. Landscape of tumor-infiltrating T cell repertoire of human cancers. Nat Genet. 2016;48(7):725–732. doi:10.1038/ng.3581.
- Pollack SM, He Q, Yearley JH, Emerson R, Vignali M, Zhang Y, Redman MW, Baker KK, Cooper S, Donahue B, et al. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer. 2017;123(17):3291–3304. doi:10.1002/cncr.30726.
- Riaz N, Havel JJ, Kendall SM, Makarov V, Walsh LA, Desrichard A, Weinhold N, Chan TA. Recurrent SERPINB3 and SERPINB4 mutations in patients who respond to anti-CTLA4 immunotherapy. Nat Genet. 2016;48(11):1327–1329. doi:10.1038/ng.3677.
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi:10.1126/science.aaa4971.
- Hopkins AC, Jacobsen KM, Henry CJ, Huey MG, Parker RE, Page LS, Hill AA, Wang X, Frye SV, Earp HS, et al. T cell receptor repertoire features associated with survival in immunotherapy-treated pancreatic ductal adenocarcinoma. JCI Insight. 2018;3(13). doi:10.1172/jci.insight.97941.
- Wang W, Edington HD, Rao UNM, Jukic DM, Land SR, Ferrone S, Kirkwood JM. Modulation of signal transducers and activators of transcription 1 and 3 signaling in melanoma by high-dose IFNα2b. CCR. 2007;06:1387.
- Yurkovetsky ZR, Kirkwood JM, Edington HD, Marrangoni AM, Velikokhatnaya L, Winans MT, Gorelik E, Lokshin AE. Multiplex analysis of serum cytokines in melanoma patients treated with interferon-alpha2b. Clin Cancer Res. 2007;13(8):2422–2428. doi:10.1158/1078-0432.CCR-06-1805.
- Snyder A, Wordsworth S, Fermont JM, Page S, Kaur K, Camps C, Kaisaki P, Gupta A, Talbot D, Middleton M, et al. Contribution of systemic and somatic factors to clinical response and resistance to PD-L1 blockade in urothelial cancer: an exploratory multi-omic analysis. PLoS Med. 2017;14(5):e1002309. doi:10.1371/journal.pmed.1002230.
- Oh DY, Cham J, Zhang L, Fong G, Kwek SS, Klinger M, Faham M, Fong L. Immune toxicities elicted by CTLA-4 blockade in cancer patients are associated with early diversification of the T-cell repertoire. Cancer Res. 2017;77(6):1322–1330. doi:10.1158/0008-5472.CAN-16-2324.
- Subudhi SK, Aparicio A, Gao J, Zurita AJ, Araujo JC, Logothetis CJ, Tahir SA, Korivi BR, Slack RS, Vence L, et al. Clonal expansion of CD8 T cells in the systemic circ
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–216. doi:10.1093/jnci/92.3.205.
- Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, Riddell SR, Warren EH, Carlson CS. Comprehensive assessment of T-cell receptor β-chain diversity in αβ T cells. Blood. 2009;114(19):4099–4107. doi:10.1182/blood-2009-04-217604.
- Robins H, Desmarais C, Matthis J, Livingston R, Andriesen J, Reijonen H, Carlson C, Nepom G, Yee C, Cerosaletti K. Ultra-sensitive detection of rare T cell clones. J Immunol Methods. 2012;375(1–2):14–19. doi:10.1016/j.jim.2011.09.001.
- Carlson CS, Emerson RO, Sherwood AM, Desmarais C, Chung M-W, Parsons JM, Steen MS, LaMadrid-Herrmannsfeldt MA, Williamson DW, Livingston RJ, et al. Using synthetic templates to design an unbiased multiplex PCR assay. Nat Commun. 2013;4:2680. doi:10.1038/ncomms3680.
- Kirsch I, Vignali M, Robins H. T-cell receptor profiling in cancer. Mol Oncol. 2015;9(10):2063–2070. doi:10.1016/j.molonc.2015.09.003.
- Rempala GA, Seweryn M. Methods for diversity and overlap analysis in T-cell receptor populations. J Math Biol. 2013;67:1339–1368.
- DeWitt WS, Emerson RO, Lindau P, Vignali M, Snyder TM, Desmarais C, Sanders C, Utsugi H, Warren EH, McElrath J, Makar KW. Dynamics of the cytotoxic T cell response to a model of acute viral infection. J Virol. 2015;89(8):4517–4526.