ABSTRACT
Blockade of the programmed cell death 1(PD-1)/PD-1 ligand-1(PD-L1) pathway has been exploited therapeutically in many cancer types. Upregulation of PD-L1 in tumor cells contributes to malignancy through suppression of the T cell-mediated antitumor response. Pyruvate dehydrogenase kinase 1 (PDK1), a glycolytic gate-keeping enzyme, is also known to promote tumor development. Here, we have uncovered a mechanism of regulation of PD-L1 by PDK1 through activation of c-Jun-NH2-kinase (JNK)-c-Jun in ovarian cancer cells. Elevated PDK1 expression was correlated with that of PD-L1 in the TCGA ovarian cancer dataset and ovarian cancer tissue array. Overexpression of PDK1 in ovarian cancer cells impaired CD8+ T cell function by suppressing IFN-γ secretion through the PD-1/PD-L1 pathway. Conversely, knockdown of PDK1 in ovarian cancer cells relieved suppression of CD8+ T cell function. CD8+ T cell apoptosis induced by binding of PD-1 with PD-L1 was increased after co-culture with ovarian cancer cells overexpressing PDK1, while depletion of PDK1 exerted the opposite effect. In vivo experiments revealed synergistic improved overall survival and enhanced inhibition of tumor growth upon co-treatment with dichloroacetate (DCA), a PDK inhibitor, and PD-L1 antibody, accompanied by increased IFN-γ secretion by monocytes infiltrating tumor islets. Moreover, PDK1 expression and CD8+ T cell infiltration were inversely correlated in the ovarian cancer tissue array. Our collective findings provide a novel explanation of how PDK1 contributes to upregulation of PD-L1 in ovarian cancer and highlight its potential as a target therapeutic molecule that cooperates with the immune checkpoint blockade.
KEYWORDS:
Introduction
Despite the decrease in ovarian cancer incidence over the last 30 years, fewer than half of the women affected survive longer than five years after diagnosis. Owing to insidious symptoms in the early stages, ovarian cancer remains one of the biggest threats to female health worldwide.Citation1 The currently used first-line treatment for advanced ovarian cancer is debulking surgery followed by adjuvant or neoadjuvant chemotherapy. Despite early responses to first-line treatment, many patients eventually die of disease relapse. Novel and effective therapies are thus urgently required to achieve better clinical outcomes for patients with ovarian cancer.
Immune suppression widely observed in tumors promotes malignancy and therapies aimed at reactivating the immune system are consequently under exploration as promising strategies for management of various types of cancer.Citation2 The immune checkpoint programmed death-1(PD-1)- PD-1 ligand 1 (PD-L1) pathway is a dominant mechanism underlying impairment of T cell function, ultimately leading to immune evasion within the tumor microenvironment.Citation3 PD-1 is expressed in T cells while PD-L1 expression is typically detected in antigen-presenting cells and several cancer cell types.Citation4 Previous studies have reported upregulation of PD-L1 in various malignancies,Citation5 including ovarian cancer,Citation6 and correlation of its expression with unfavorable prognosis and lower survival rates in non-small cell lung cancer (NSCLC),Citation7 melanomaCitation8 and renal cell carcinoma.Citation9 Therapeutic strategies with antibodies targeting PD-1 or PD-L1 aiming to reverse this immune suppression have led to remarkable responses in various cancer types.Citation10 For instance, in a phase II clinical trial involving 20 platinum-resistant ovarian cancer patients, the PD-1 antibody, nivolumab, achieved a 45% disease control rate with controllable treatment-related adverse events,Citation11 clearly demonstrating relatively high clinical efficacy of the antibody. Moreover, PD-L1 expression in tumor cells is correlated with response to the immune checkpoint blockade in NSCLC,Citation12 colorectal cancerCitation13 and ovarian cancer.Citation14 Therefore, elucidation of the mechanisms underlying PD-L1 regulation should facilitate prediction of the clinical response and validation of potential targets that act in cooperation with immunotherapy. However, to our knowledge, limited studies to date have focused on the dynamic regulation of PD-L1 in ovarian cancer.
Distinct from normal cells, tumor cells prefer aerobic glycolysis instead of oxidative phosphorylation for energy production and survival despite low oxygen availability.Citation15 Pyruvate dehydrogenase kinase 1 (PDK1) inhibits the activity of the E1α subunit of pyruvate dehydrogenase complex (PDHA) via phosphorylation, preventing import of pyruvate into the mitochondrion for further metabolism through the Krebs cycle.Citation16 Inhibition of tyrosine phosphorylation of PDK1 is reported to switch the glucose metabolism pathway of non-small lung cancer cells from glycolysis to oxidative phosphorylation.Citation16 Notably, PDK1 promotes the growth of tumors and is predictive of poorer clinical outcomes in head-and-neck squamous cell carcinoma patients.Citation17,Citation18
In this study, we provide evidence of PD-L1 induction by the glycolytic gene, PDK1, in ovarian cancer. PDK1 was positively correlated with PD-L1 expression and negatively correlated with CD8+ T cell infiltration within the tumor microenvironment. In addition, PDK1 impaired CD8+ T cell function and survival through effects on PD-L1. In vivo experiments revealed synergistic prolonged overall survival and enhanced inhibition of tumor growth upon co-treatment with dichloroacetate (DCA), a PDK inhibitor, and PD-L1 antibody, concomitant with enhanced IFN-γ secretion by monocytes infiltrating tumor islets. Low progression-free survival (PFS) rates were associated with high expression of PD-L1 in ovarian cancer based on Kaplan-Meier plotter (ovarian cancer) database analysis. Our collective findings highlight an unexpected function of PDK1 in tumor evasion of the CD8+ T cell-mediated immune response and its utility as a potential target molecule that acts in cooperation with the immune checkpoint blockade.
Materials and methods
Cell lines and culture
Ovarian cancer cells (A2780CP, SKOV-3 and OVCAR3) were used in this studyA2780CP was kindly provided by Professor Benjamin B.K. Tsang from the Department of Obstetrics & Gynecology and Cellular & Molecular Medicine of University of Ottawa (Ottawa Health Research Institute, Ottawa, Ontario, Canada) and SKOV-3 purchased from American Type Culture Collection (ATCC). Both cell lines were maintained in DMEM (Invitrogen, #11965118) supplemented with 10% FBS (Invitrogen, #10270106) and 100 U/ml penicillin-streptomycin (Invitrogen-Gibco, #15140122). OVCAR3 obtained from ATCC was cultured in Medium 199 (Sigma-Aldrich, #M5017) and Medium 105 (Sigma-Aldrich, # M6395) at a ratio of 1:1 supplemented with 15% FBS and 100 U/ml penicillin-streptomycin. All cells were cultured in a humidified incubator at 37°C and 5% CO2.
Quantitative real-time PCR
Total RNA was extracted from cells using a NucleoSpin RNA kit (Macherery-Nagel, # 740955) following the recommendations of the supplier. After synthesis of cDNA with the aid of SuperScript VILOTM Master Mix (Invitrogen, #11755250), quantitative real-time PCR was conducted to quantify target gene expression. The specific primers used were as follows: PD-L1 (Forward 5ʹ-TGCCGACTACAAGCGAATTACTG-3ʹ, Reverse 5ʹ-CTGCTTGTCCAGATGACTTCGG-3ʹ), PDK1 (Forward 5ʹ-CCAAGACCTCGTGTTGAGACC-3ʹ, Reverse 5ʹ-AATACAGCTTCAGGTCTCCTTGG-3ʹ) PFKP (Forward 5ʹ-CGGAAGTTCCTGGAGCACCTCTC-3ʹ, Reverse 5ʹ-AAGTACACCTTGGCCCCCACGTA-3ʹ), PFKFB3 (Forward 5ʹ- CAGTTGTGGCCTCCAATATC-3ʹ, Reverse 5ʹ-GCTTCATAGCAACTGATCC-3ʹ) and GAPDH (Forward 5ʹ-TCCATGACAACTTTGGTATCGTG-3ʹ Reverse 5ʹ-ACAGTCTTCTGGGTGGCAGTG-3ʹ). JNK1-specific primers were purchased from Santa Cruz (Santa Cruz, #sc-29380). GAPDH was employed as the internal reference gene.
Immunoblot analysis
Cells were lysed in CelLytic solution (Sigma-Aldrich, #C2978) supplemented with Phosphatase Inhibitor Cocktail (Sigma-Aldrich, #P8340, #P5726, #P0044) at a ratio of 100:1. After separation via SDS-polyacrylamide electrophoresis, the protein was transferred to PVDF membrane. The following antibodies were used to probe the membrane: PD-L1 (Cell Signaling, #29122), PDK1 (Εnzo, #ADI-KAP-PK112-F), β-actin (Abcam, #ab6276), GAPDH (Cell Signaling, #5174S), JNK (Cell Signaling, #9252), c-Jun (Cell Signaling, #9165), and activated JNK (Thr183/Tyr185) (Cell Signaling, #9251S) and c-Jun (Ser73) (Cell Signaling, #3270S). The ECL detection kit was utilized to visualize protein bands.
Knockdown or overexpression of target genes
Cells were transfected with siPDK1 (Santa Cruz, #sc-36203)/siPFKP (Thermo Fisher Scientific,#s10372) and #s10374)/siPFKFB3 (Thermo Fisher Scientific, #4390824 and # 4427038) or their corresponding control siRNAs (Santa Cruz, #sc-37007; Thermo Fisher Scientific, #4390846) using siLenFect (Bio-Rad, #170–3361) and PDK1-expressing (Origene, #RC505127) or control vector(#PS100001) using Lipofectamine 3000 (Invitrogen, #L3000015), according to the manufacturer’s instructions. RNA was extracted at 24 h and protein at 48 h after transfection, and PDK1/PFKP/PFKFB3 expression evaluated using western blot and/or qPCR. Additionally, after 48 h of transfection, cells were collected to perform functional assays and assess PD-L1 expression via flow cytometry.
Purified CD8+ T cell population
Fresh peripheral blood mononuclear cells (PBMCs) were isolated from buffy coat leukocyte concentrates obtained from female healthy donors kindly provided by Hong Kong Red Cross. CD8+ T cells were enriched by negative selection with a RosetteSep human CD8+ T cell Enrichment Cocktail (StemCell Technologies, #15063) according to the instructions of the supplier. Purified CD8+ T cells were maintained in RPMI (Invitrogen, #11875119) containing 10% FBS and 100 U/ml penicillin-streptomycin.
T cell/ovarian cancer cell co-culture systems
Based on the coculture system reported by Xu et al.,Citation19 we stimulated PBMCs with anti-CD3e (2.5 μg/mL) (BioLegend, #300432) and anti-CD28 (1.25 μg/mL) (BioLegend, #302923) and ovarian cancer cell lysis for 3–5 days. Isolated CD8+ T cells were subsequently co-cultured with ovarian cancer cells with knockdown or overexpression of PDK1 or their corresponding control cells at a ratio of 10:1. After 36 h, T cells were collected by washing twice with phosphate-buffered saline (PBS) (Sigma-Aldrich) and flow cytometry used to detect secretion of IFN-γ. To block binding of PD-1 with PD-L1, 20 μg/ml PD-L1 blocking antibody (BioLegend, #329711) or control IgG (BioLegend, #400339) was used.
Flow cytometry
IFN-γ levels were examined via flow cytometry to provide an indication of CD8+ T cell function. Briefly, the protein transport inhibitor, brefeldin A (eBioscience, #00-4506-51), was added to the co-culture system 6 h before termination. CD8+ T cells were harvested and placed into assay tubes at a density of 2 × 105 cells per tube. After staining with the surface markers, FITC-labeled anti-CD8 (BD Pharmingen, #555634) or isotype control (BD Pharmingen, # 555748), cells were permeabilized using Cytofix/Cytoperm (BD Pharmingen, #554723). Cells were further stained with the APC-anti-IFN-γ antibody (BD Pharmingen, #554702) or its corresponding isotype control (BD Pharmingen, #554681). For detection of PD-L1 expression on the ovarian cancer cell surface, cells were harvested after transfection and counted. Equal numbers of cancer cells (2 × 105) were aliquoted into assay tubes and washed with PBS. Following staining of samples with a PE-anti-PD-L1 antibody or isotype control (eBioscience, #12-5983-42), flow cytometry was performed on a BD LSR Fortessa instrument using Flow Jo software for data analysis.
T cell apoptosis assay
For determination of apoptosis, activated CD8+ T-cells were co-cultured with ovarian cancer cells for 48 h at a ratio of 10:1, harvested via centrifugation at 300 g for 10 min and washed with PBS. Next, cells (2 × 105/well) were stained with PE-anti-CD8 antibody (BD Pharmingen, #555635) at 4°C for 30 min according to a previous protocol. After rinsing and centrifugation at 300 g for 10 min, T-cells were resuspended in 100 µL Annexin V binding buffer containing 5 µL Annexin V (BD Pharmingen, #556547) for 15 min at room temperature and protected from light. Annexin V-positive cells under gating of CD8+ were regarded as apoptotic CD8+ T-cells.
Ascites-derived ovarian cancer cells
Ascites fluid was collected at the time of initial cytoreductive surgery from patients with histological diagnosis of ovarian cancer. Written informed consent was obtained from participants before sample collection. This study was conducted under the approval of the Ethics Committee of Hong Kong University. Malignant cells from ascites were isolated based on the protocol of Shepherd et al.Citation20 Cells were maintained in Medium 199 and Medium 105 at a ratio of 1:1 supplemented with 15% FBS and 100 U/mL penicillin-streptomycin and cultured at 37°C in a humidified atmosphere with 5% CO2. After incubation for 2–3 days, expression of CK7, AE1/AE3, and CD45 was assessed via immunofluorescence. Only CK7+ AE1/AE3+ CD45−cells were used in subsequent functional assays, which were performed within 3–4 passages.
Immunohistochemistry
Two ovarian cancer tissue arrays, one containing 102 samples purchased from Pantomics (OVC1021; Richmond, CA) and the other with 100 samples purchased from US Biomax (BC11115c; Rockville, MD), were used for immunohistochemical experiments. Briefly, ovarian cancer tissue slides were deparaffinized and rehydrated in xylene and a series of ethanol solutions. Antigen retrieval was accomplished by exposing the slide to citrate buffer (for anti-PDK1 and anti-CD8 staining) or EDTA (for anti-PD-L1 staining) for 15 min at boiling temperature. Next, sections were incubated with primary antibodies targeting PD-L1 (1:25 dilution, Cell Signaling, #13684), PDK1 (1:200 dilution, Εnzo, #ADI-KAP-PK112-F) or CD8 (DAKO, #M710301) overnight at 4°C. After washing and incubation with the appropriate secondary antibody, the tissue slide was reacted with 3,3ʹ-diaminobenzidine solution and counterstained with hematoxylin. Finally, well-mounted slides were scanned and analyzed with Aperio ImageScope software12.3 (Leia Biosystems, Wetzlar).
Quantification of CD8+ T cells
Aperio ImageScope software was applied to evaluate the entire slide of ovarian cancer tissue array stained with the CD8 antibody. We selected the most abundant CD8+ T cell areas (5 cancer epithelium and 5 stromal areas) and counted cell numbers under 40× magnification (area of 0.0625 mm2). The total number of CD8+ T cells for each patient was used for statistical analysis.
In vivo experiments
After receiving approval from the Committee of the Use of Live Animals in Teaching and Research of Hong Kong, we obtained immunocompetent C57BL/6J mice from the Laboratory Animal Unit of the University of Hong Kong. ID8 mice-derived ovarian cancer cells (5 × 106) in 200 μl PBS were inoculated into the abdomen of 4–6 week-old female mice. After 28 days, mice were randomized into four groups receiving: a) DCA + IgG of anti-mouse PD-L1 antibody (LEAFTM, #400637), b) DCA+ anti-PD-L1 (LEAFTM, #124309, c) vehicle + anti-PD-L1 or d) vehicle + IgG of anti-PD-L1 via intraperitoneal injection. DCA (Sigma-Aldrich, #347795) was dissolved in H2O and administered at a dose of 50 mg/kg/d daily for 14 days, followed by anti-PD-L1 (200 μg per mouse) daily for five days. Mice were monitored daily for body weight and examined for symptoms such as depression, lethargy, inactivity or non-ambulatory, abnormal breathing, hunched back or ruffled fur, pale extremities, abdominal distension, muscle atrophy, emaciation, hypothermia or dehydration. Animals were euthanized following loss of >20% original body weight or appearance of any four symptoms on three consecutive days. Tumors excised from mouse abdomen were counted, weighed and subjected to dissociation using the MinuteTM cell suspension isolation kit (Invent, #SC-012). Red blood cells were lysed with ACK lysis buffer (GibcoTM, #A1049201) after filtration through a 40 μm cell strainer. Subsequently, cancer cells and monocytes were separated via Percoll gradient centrifugation (Sigma-Aldrich, #17-0891-01). PE-Cyanine 7-IFN-γ antibody (XMF1.2) (eBioscienceTM, #25-7311-82) or its isotype control (eBioscienceTM, #25-4301-82) was used for staining of IFN-γ in monocytes as described above.
Ovarian cancer database analyzes
A dataset including 489 ovarian serous cystadenocarcinoma (TCGA, Nature 2011) samples was downloaded from cbioportal (https://www.cbioportal.org/) to evaluate PD-L1, hexokinase 2 (HK2), lactate dehydrogenase A (LDHA), PDK1, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 and 3 (PFKFB2 and 3), phosphofructokinase platelet (PFKP), and pyruvate kinase M (PKM) mRNA expression patterns. Spearman’s rho test was applied to assess the relationships between PD-L1 and these key glycolytic genes. Additionally, cases with BRCA mutation information were downloaded from the cbioportal to evaluate PD-L1 and PDK1 expression patterns. Kaplan-Meier survival analysis of PD-L1/PDK1/c-Jun/JNK1/JNK2 in ovarian cancer was conducted based on the Kaplan-Meier plotter (ovarian cancer) database (https://www.tcpaportal.org/tcpa/survival_analysis.html) and the cutoff value taken as median mRNA expression of the genes examined.Citation21 The predictive value of c-Jun and JNK phosphorylation in ovarian cancer was additionally evaluated on the Cancer Proteome Atlas (https://www.tcpaportal.org/tcpa/survival_analysis.html). Differences were regarded as significant at P < .05.
Statistical analysis
Data were analyzed using GraphPadPrism 6 (San Diego, CA). Representative results from three independent experiments are presented. Comparisons between two groups were performed using Student’s t-test or Mann-Whitney test and Kruskal-Wallis rank test applied to compare data from three groups. For correlation analysis, Spearman’s rho test was used. P values <.05 were considered significant.
Results
PD-L1 expression is positively correlated with key glycolytic genes
We initially analyzed the correlations between PD-L1 and key glycolytic genes (HK2, PFKFB2, PFKFB3, PFKP, PKM, LDHA, and PDK1) in the TCGA dataset comprising 489 ovarian adenocarcinoma samples. Statistically significant associations were observed between expression of PD-L1 and PFKFB3 (r = 0.2229, P < .0001), PFKP (r = 0.1917, P < .0001), PKM (r = 0.1538, P = .0006), LDHA (r = 0.2162, P < .0001) and PDK1 (r = 0.1574, P = .0005) in this cohort (supplementary Figure 1(a,b)). Regulation of PD-L1 by PKM and LDHA has been reported in melanoma and colon cancer cells.Citation22,Citation23 Accordingly, we focused on the regulatory activities of PFKP, PFKFB3 and PDK1 on PD-L1. Further examination of the potential correlations between immune checkpoint genes (PD-L1, PD-1, CTLA4, and CD80) and PDK1 in the TCGA ovarian cancer dataset revealed a positive association between PDK1 and CD80 (r = 0.1136, P = .0119; Supplementary Figure 2).
PDK1 is involved in PD-L1 induction
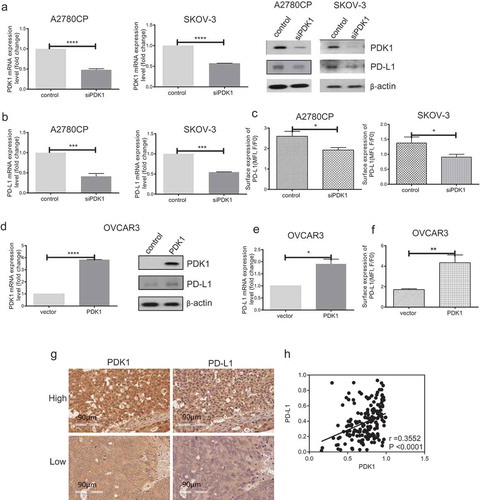
Notably, knockdown of PDK1 in A2780CP and SKOV-3 cells (P < .0001, P < .0001; )) led to significant downregulation of PD-L1 at both mRNA and protein levels (P = .0001, P = .0001; ). Consistently, flow cytometry analysis revealed decreased surface expression of PD-L1 in ovarian cancer cells depleted of PDK1, compared with control cells (P = .0122, P = .0208; )). Conversely, PD-L1 expression was increased after transfection of OVCAR3 cells with PDK1-expressing vector (P < .0001; )), as confirmed based on qPCR, western blot and flow cytometry findings (P = .0227, P = .0041; ). Further examination of PD-L1 mRNA levels after knockdown of PFKFB3/PFKP in A2780CP cells revealed no significant differences in expression (Supplementary Figure 1(c)).
Figure 1. PDK1 influences PD-L1 expression in ovarian cancer. (a–c) Knockdown efficiency of PDK1 assessed via qPCR and immunoblotting in A2780CP and SKOV-3 cells (a). PD-L1 expression in A2780CP and SKOV-3 cells after knockdown of PDK1 evaluated via immunoblotting (a), qPCR (b) and flow cytometry (c). (d–f) Overexpression of PDK1 in OVCAR3 cells assessed via qPCR and immunoblotting (d). PD-L1 expression in OVCAR3 cells overexpressing PDK1 detected via immunoblotting (d), qPCR (e) and flow cytometry (f). (g) Representative immunohistochemistry samples showing similar expression patterns of PDK1 and PD-L1 within ovarian cancer. (h) Statistical analysis showing a positive correlation between PDK1 and PD-L1 in an ovarian cancer tissue array. Representative data from three experiments are shown (*P < .05, **P < .01, ***P < .001, ****P < .0001).
PDK1 expression is positively correlated with that of PD-L1 in ovarian cancer tissue arrays
To confirm bioinformatic data, .PDK1 and PD-L1 expression levels were further evaluated via immunohistochemistry within two ovarian cancer tissue arrays comprising 202 samples. Consistently, PDK1 and PD-L1 levels were positively correlated in ovarian cancer tissues (r = 0.36, P < .0001, ). Based on the collective findings, we hypothesize that PDK1 and PD-L1 are positively associated, in turn, influencing the immune response in ovarian cancer.
PDK1 expressed in ovarian cancer cells impairs CD8+ T cell function through the PD-1/PD-L1 axis
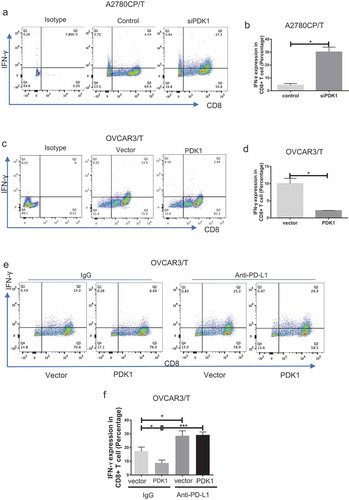
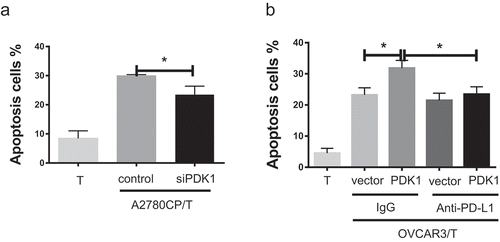
To determine CD8+ T cell function, secretion of IFN-γ was assessed. Flow cytometry results showed increased IFN-γ secretion by CD8+ T cells after co-culture with A2780CP cells depleted of PDK1, compared with the respective control group (P = .0115; ). IFN-γ secretion by CD8+ T cells co-cultured with OVCAR3 cells overexpressing PDK1 was lower than that by T cells co-cultured with control cells (P = .0186; ). To investigate whether PDK1 influences CD8+ T cell function through PD-L1, we repeated the co-culture assay as above with OVCAR3 cells in the presence of anti-PD-L1. Compared with the control group, the percentage of IFN-γ-positive CD8+ T cells decreased after co-culture with OVCAR3 cells overexpressing PDK1. Notably, however, this inhibition was relieved in the presence of the PD-L1 antibody (). Our results suggest that PDK1 expressed on ovarian cancer cells influences CD8+ T cell function through regulation of PD-L1.
Figure 2. PDK1 expressed in ovarian cancer cells impairs CD8+ T cell function through PD-L1 regulation. CD8+ T cells collected from the co-culture system were stained with antibodies against CD8A and IFN-γ. (a, c) Flow cytometry analyses showing IFN-γ secretion by CD8+ T cells co-cultured with A2780CP cells depleted of PDK1 or control cells (a), OVCAR3 cells overexpressing PDK1 or control cells (c). (b, d) Statistical analysis of IFN-γ detected via flow cytometry. (e) IFN-γ secretion by CD8+ T cells co-cultured with OVCAR3 cells with PDK1 overexpression in the presence of anti-PD-L1. (f) Statistical analysis of . Representative data from three experiments are shown (*P < .05, ***P < .001).
PDK1 expressed in ascites-derived ovarian cancer cells impairs CD8+ T cell function
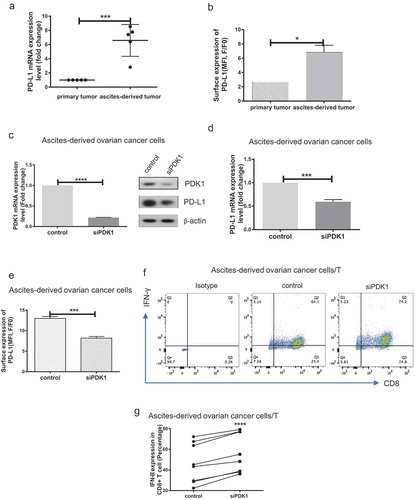
Firstly, we evaluated PD-L1 expression in primary and ascites-derived ovarian cancer cells via qPCR and flow cytometry analyses. PD-L1 was highly expressed in ascites-derived cancer cells, compared with primary ovarian cancer cells (P = .0005, P = .0321; ). Knockdown of PDK1 expression was achieved via transfection of ascites-derived cancer cells with targeted siRNAs (P < .0001; )), and PD-L1 expression assessed using qPCR, immunoblot and flow cytometry. Similar to the data obtained with ovarian cancer cell lines, knockdown of PDK1 led to suppression of PD-L1 expression (P = .0001, P = .0002; ). Moreover, IFN-γ secretion by CD8+ T cells was enhanced after co-culture with cancer cells depleted of PDK1, compared with their control cell counterparts (P < .0001; ). Our findings clearly suggest that PDK1 influences CD8+ T cell function in clinical ovarian cancer.
Figure 3. PDK1 expressed in ascites-derived ovarian cancer cells impairs CD8+ T cell function. (a) PD-L1 mRNA expression in paired primary ovarian cancer and ascites-derived ovarian cancer cells. (b) Representative results of flow cytometry showing PD-L1 surface expression in primary ovarian cancer and ascites-derived cancer cells. c-e Transient knockdown of PDK1 mRNA and protein expression (c) in representative cases of ascites-derived ovarian cancer cells. PD-L1 protein (c), mRNA (d) and surface expression (e) in representative cases of ascites-derived ovarian cancer cells with knockdown of PDK1. (f) Representative results showing IFN-γ secreted by CD8+ T cells co-cultured with ascites-derived ovarian cancer cells depleted of PDK1 or control cells. g Statistical analysis of (n = 8) (*P < .05, ***P < .001, **** P < .0001).
PDK1 promotes CD8+ T cell apoptosis through the PD-1/PD-L1 axis
In addition to cytokine secretion, lymphocyte apoptosis induced by PD-1/PD-L1 binding contributes to immunosuppression.Citation24 Accordingly, we examined whether PDK1 expressed in ovarian cancer affects CD8+ T cell apoptosis. As shown in ), the apoptotic rate of CD8+ T cells decreased after co-culture with A2780CP cells depleted of PDK1 relative to co-cultures with control cells (P = .0186; )), while increased apoptosis of CD8+ T cells was observed in co-cultures with OVCAR3 cells overexpressing PDK1, compared with control cells (P = .0109; )). However, this effect was blocked in the presence of the PD-L1 antibody ()). Based on the data, we suggest that PDK1 upregulates PD-L1 expression and promotes PD-1/PD-L1 mediated apoptosis of CD8+ T cells.
Figure 4. PDK1 expressed in ovarian cancer cells promotes CD8+ T cell apoptosis through PD-L1. (a) Apoptosis rates of CD8+ T cells co-cultured with A2780CP cells with PDK1 knockdown or control cells. (b) Apoptosis rates of CD8+ T cells co-cultured with OVCAR3 cells overexpressing PDK1 or control cells in the presence of anti-PD-L1 or control IgG. Representative data from three experiments are shown (*P < .05 **P < .01).
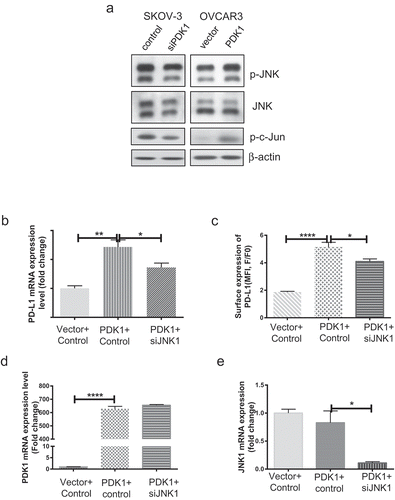
PDK1 regulates PD-L1 expression through activation of JNK-c-Jun
The flexible expression of PD-L1 may be influenced by a number of intrinsic and extrinsic factors. To date, several studies have addressed the essential functions of the JNK signaling pathway in tumorigenesis, cellular metabolism and inflammation.Citation25,Citation26 JNK signaling is reported to contribute to upregulation of PD-L1 in melanoma and bladder cancer cells.Citation27,Citation28 Recently, our group demonstrated that PDK1 is one of the factors activating JNK signaling (paper under review). To this end, we further explored whether PDK1 regulates PD-L1 through JNK signaling. As presented in , JNK and c-Jun phosphorylation were decreased upon knockdown of PDK1 and, conversely, enhanced upon PDK1 overexpression. Next, JNK1 was depleted using a specific siRNA in OVCAR3 cells overexpressing PDK1 (Supplementary Figure 3) and the effects on PD-L1 expression determined. Overexpression of PDK1 led to increased PD-L1 expression in ovarian cancer cells but this effect was partially blocked after knockdown of JNK1 (), supporting the involvement of JNK-c-Jun in modulation of PD-L1 by PDK1 in ovarian cancer.
Figure 5. JNK-c-Jun activation is involved in PDK1-induced PD-L1 expression in ovarian cancer cells. (a) Immunoblotting of total and phosphorylated forms of JNK and c-Jun in OVCAR3 cells overexpressing PDK1 and SKOV-3 cells depleted of PDK1. (b, c) PD-L1 mRNA and surface expression in PDK1-overexpressing OVCAR3 cells with knockdown of JNK1. (d) PDK1 mRNA expression in OVCAR3 cells transfected with PDK1-expressing plasmid followed by siRNAs targeting JNK1. (e) qPCR assessment of JNK1 mRNA expression. Representative data from three experiments are shown (*P < .05, **P < .01, ****P < .0001).
Combination treatment with DCA and anti-PD-L1 antibody suppresses tumor growth through increasing IFN-γsecretion in vivo
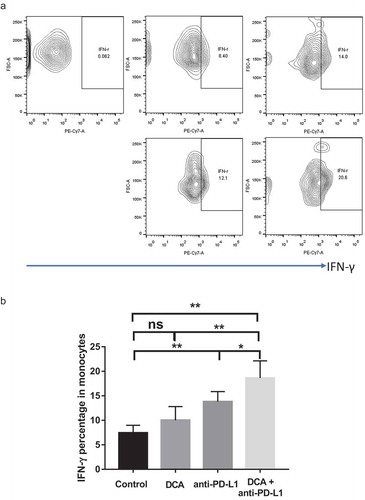
Next, we examined the effects of co-treatment with DCA (a PDK inhibitor) and anti-PD-L1 in the xenograft model of ovarian cancer. DCA and anti-PD-L1 were administered intraperitoneally to C57BJ/6J mice 28 days after injection of ID8 ovarian cancer cells into mouse abdomen. Mice were divided into four groups to receive DCA and anti-PD-L1 alone or in combination. Overall survival of mice receiving DCA or anti-PD-L1 was significantly longer than that of their control counterparts. Moreover, the combination treatment induced prolonged survival time, compared with each treatment alone (median survival times of 45, 57, 63, and 70 days for control, DCA, anti-PD-L1 and combination treatment groups, respectively; ). Both the number and weight of tumors excised from the peritoneal cavity of mice in the co-treatment group were significantly decreased, compared with those in single treatment or control groups ()).
Figure 6. Co-treatment with the PDK inhibitor, DCA, and anti-PD-L1 antibody suppresses tumor growth in an ID8 ovarian cancer model. (a). Typical presentation of ID8 ovarian cancer tumors in C57BL/6 mice treated with DCA and/or anti-PD-L1 antibody. (b). Scheme of the therapeutic strategy. (c). Overall survival of mice from the four groups. (d). Peritoneal tumors were counted and weighed following euthanization. Each dot represents a single mouse (* P < .05, ** P < .01).
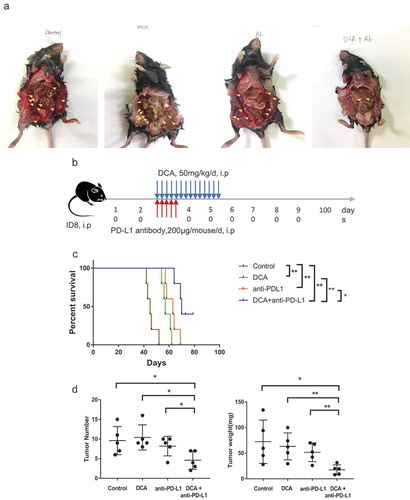
Tumors excised from mice were subjected to flow cytometry for detecting IFN-γ secreted from isolated monocytes. Anti-PD-L1 treatment led to increased IFN-γ secretion (P = .0038; ) and DCA alone also induced a slight increase in IFN-γ secretion of monocytes, but not to a significant extent. Interestingly, co-treatment with both agents synergistically promoted IFN-γ secretion of monocytes (P = .0039, P = .0045, P = .0359; ). Our collective results indicate that combined treatment with DCA and anti-PD-L1 presents an ideal approach to improve treatment outcomes for ovarian cancer.
PD-L1 overexpression is an indicator of poor prognosis in ovarian cancer
Next, we analyzed the correlation of PD-L1 with clinical parameters in ovarian cancer tissue. PD-L1 was expressed at higher levels in both the cytoplasm and membrane of ovarian cancer cells relative to normal tissue (Supplementary Figure 4 and Supplementary Table 1). Although differences were not statistically significant, higher expression of PD-L1 was observed in tissues with advanced grade tumors (Supplementary Table 1). No significant correlation was observed between PD-L1 and disease stage (Supplementary Table 1). Interestingly, PD-L1 expression in the endometrioid subtype was higher than that in other subtypes (P < .0001; Supplementary Table 1).
The Kaplan-Meier plotter ovarian cancer dataset was used to conduct survival analysis in relation to PD-L1, PDK1, c-Jun, JNK1, and JNK2. The log-rank test revealed poorer progression-free survival rates in patients with higher than median expression of PD-L1/PDK1/c-Jun and poorer overall survival rates in patients with higher than median expression of c-Jun (Supplementary Figure 5(a)). Moreover, pS73_c-Jun was positively correlated with ovarian cancer patient survival according to data from the TCGA ovarian cancer protein dataset on the Cancer Proteome Atlas (TCPA) (Supplementary Figures 5, 6). However, we observed no significant differences in survival rates among patients showing differential expression of JNK1 or JNK2. Moreover, pT183Y185_JNK was not significantly correlated with ovarian cancer patient survival (Supplementary Figures 5, 6).
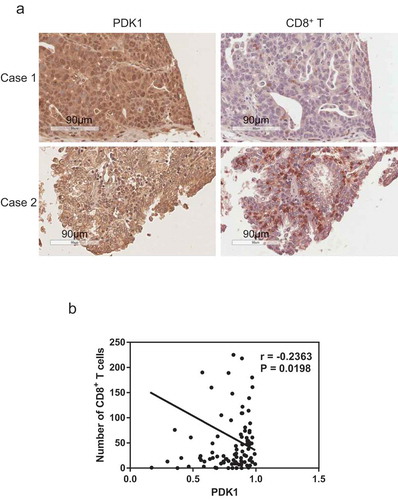
PDK1 is negatively correlated with infiltration of CD8+ T cells within ovarian cancer
CD8+ T cells play a central role in adaptive immunity and their infiltration reflects response to anti-PD-1/PD-L1 therapy.Citation29 Thus, we further examined the potential correlation between CD8+ T cell infiltration and PDK1 expression in ovarian cancer via immunohistochemical analysis. Our data showed a negative correlation (r = 0.2363, P = .0198, ), suggesting that PDK1 expression facilitates the development of an immunosuppressive microenvironment and promotes survival of ovarian cancer cells.
Figure 8. Expression of PDK1 in ovarian cancer is negatively correlated with CD8+ T cell infiltration. (a) Representative immunohistochemical staining image of PDK1 (left panel) and CD8+ T cells (right panel) in ovarian cancer tissue. (b) Spearman’s rho analysis of PDK1 staining density in relation to the number of CD8+ T cells in ovarian cancer. PDK1 staining density was calculated using Image Scope software and CD8+ T cell numbers counted manually.
BRCA mutation does not influence PD-L1 or PDK1 expression within the TCGA ovarian cancer dataset
In view of the finding that BRCA1/2 genes play crucial roles in progression of ovarian cancer,Citation30 we additionally evaluated PD-L1 and PDK1 expression in patients with mutant or wild-type BRCA according to the TCGA ovarian cancer dataset. Cases with mutated BRCA1 and/or 2 genes were classified as the ‘mutation’ group. PD-L1 and PDK1 expression in tissues with mutant or wild-type BRCA was not significantly different (Supplementary Figure 7).
Discussion
The finding that tumor-infiltrating lymphocytes in ovarian cancer are associated with better clinical outcomes endows immunogenic characteristics to this disease type and sheds light on the effective application of immunotherapy as a therapeutic option.Citation31 Binding of PD-1 expressed on T cells to its ligand PD-L1, which is mainly expressed on tumor cells, suppresses the proliferation, survival and effector functions of T cells.Citation32 Blockade of this pathway is regarded as a promising strategy in the treatment of various malignancies,Citation13 including ovarian cancer.Citation33 Moreover, improved clinical response has been reported in clinical trials evaluating combination of anti-PD-1/anti-PD-L1 therapy with other strategies, such as chemotherapy.Citation34 Thus, elucidation of the mechanisms regulating PD-L1 expression within tumors is essential.
Altered metabolism within cancer cells not only sustains their proliferation but also promotes resistance to currently available treatments.Citation35 Accumulating evidence suggests that immune metabolism plays a fundamental role in the process of adaptive immunity, especially the anti-tumor T cell response.Citation36 Moreover, metabolic interplay between cancer and immune cells contributes to the dysfunction of T cells, supporting immune escape.Citation37 IFN-γ secreted by activated T cells plays a pivotal role in innate and adaptive immunity.Citation38 Interactions of PD-L1 with PD-1 are proposed to transmit a powerful negative signal that blocks CD8+ T cell activation and decreases IFN-γ secretion.Citation39 Data from this study showed that the highly expressed glycolytic gene, PDK1, in ovarian cancer affects PD-L1 expression which, in turn, impairs CD8+ T cell function by inhibiting IFN-γ secretion and promoting apoptosis. Palsson-McDermott and colleagues demonstrated that knockdown of pyruvate kinase isoform M2 (PKM2), another critical glycolysis gene abrogates PD-L1 expression in colon carcinoma cells. This mechanism may be attributed to the shared binding sites of PKM2 with HIF-1α on the PD-L1 promoter.Citation23 The collective data suggest that glycolysis genes upregulated in cancer cells suppress T cell immunity via upregulation of PD-L1.
PDK1 is a crucial glycolytic gene involved in several cancer cell processes, such as proliferation, apoptosis and invasion.Citation17,Citation40 In this study, we showed that PDK1 expressed in ovarian cancer regulates PD-L1 through the JNK-c-Jun pathway, consequently influencing CD8+ T cell function and survival. While its oncogenic function has been broadly documented, JNK also participates in the regulation of immune responses.Citation41 For instance, in melanoma cells, c-Jun is reported to directly regulate PD-L1 as an inducible transcription factor, which may be enhanced by cooperation with STAT3. Inhibition of JNK upstream kinase or knockdown of JNK led to reduced PD-L1 expression in melanoma cells,Citation27,Citation42 while after treatment with the JNK inhibitor, SP600125, PD-L1 expression was attenuated in bladder cancer,Citation28 clearly indicating that JNK influences PD-L1 expression. In our experiments, phosphorylation of JNK and c-Jun was inhibited in ovarian cancer cells with knockdown of PDK1 and, conversely, enhanced with PDK1 overexpression. Furthermore, upon siRNA-induced JNK1 knockdown in OVCAR3 cells overexpressing PDK1, PD-L1 overexpression was partially inhibited, supporting the theory that PDK1 influences PD-L1 expression through the JNK-c-Jun pathway.
Consistent with the in vitro functional assays, in vivo experiments showed that co-treatment with the PDK inhibitor, DCA, and anti-PD-L1 antibody synergistically prolongs overall survival in mice with ovarian tumor, reduces tumor growth and increases IFN-γ secretion by monocytes infiltrating tumors, compared to treatment with the individual agents. These results support the potential utility of this combined approach in the clinic. An earlier study similarly showed that DCA treatment induced a significant increase in IFN-γ-producing CD8+ T cells and Natural killer cells isolated from spleen of lymphoma-bearing mice.Citation43 Additionally, DCA enhanced the antitumor effect of Poly(I:C) (a ligand for Toll-like receptor 3) in a lymphoma or melanoma-bearing mouse model.Citation43 The collective results suggest that DCA acts synergistically with immunotherapy, leading to improved response rates.
We additionally analyzed the correlation of PD-L1 with clinical parameters in ovarian cancer using immunohistochemistry. Our results demonstrated a significant correlation between PD-L1 immunoreactivity and ovarian cancer differentiation. Although not statistically significant, PD-L1 expression was higher in Grade 3 tumor tissues, compared with that in Grade 1–2 tumor tissues. However, we observed no significant association of PD-L1 expression with tumor stage. Similar to our findings, recent studies have reported higher expression of PD-L1 in high-grade ovarian tumors relative to that in low-grade tumors.Citation6 Furthermore, we observed lower PD-L1 expression in serous, mucinous and clear cell histological subtypes relative to endometrial adenocarcinoma. These collective results should facilitate selection of patients who will benefit from anti-PD-1/PD-L1 therapy.Citation44 In the present study, analysis of the Kaplan-Meier plotter (ovarian cancer) dataset disclosed a significant correlation between upregulation of PD-L1 and lower progression-free survival rate of ovarian cancer, in agreement with Hamanishi et al.Citation45 who reported that PD-L1 can be effectively used to predict unfavorable outcome in ovarian cancer patients. Additionally, we analyzed the predictive value of PDK1,c-Jun, JNK1/2, and phosphorylated c-Jun and JNK. Expression levels of PDK1 and c-Jun were negatively correlated with clinical outcomes (progression-free and/or overall survival) and phosphorylation of c-Jun was predictive of poor survival of ovarian cancer patients.
Interestingly, immunohistochemical results consistently showed that CD8+ T cell infiltration is negatively correlated with PDK1 expression in ovarian cancer. In line with this finding, GLUT-1 expression in renal cell carcinoma was shown to be inversely correlated with the number of CD8+ T cells in tumor lesions.Citation46 Examination of T cell infiltration and GLUT-1 expression in 308 head-and-neck squamous cell carcinoma patients similarly revealed a negative relationship between the number of CD3+ T or CD8+ T cells and GLUT-1 expression.Citation47 Elevated lactate dehydrogenase A (LDHA) expression was additionally associated with lower CD3D expression in melanoma tissue samples.Citation48 The collective results support the contribution of glycolytic genes to immune balance in the tumor microenvironment and aid in elucidating the complex pathways underlying immune evasion by tumor cells.
BRCA mutations occur in 5–15% of all ovarian cancer casesCitation49 with significant effects on overall survivalCitation50and diverse metabolic consequences, such as enhanced glycolysis in ovarian cancer.Citation51 Cases with BRCA mutations showed overall increased mutational burden harboring higher response rates to immunotherapies, including immune checkpoint blockade.Citation52 However, comparison of the expression patterns of PD-L1 and PDK1 in cases with mutant and wild-type BRCA in this study revealed no influence of BRCA mutation on PD-L1 or PDK1 levels in ovarian cancer.
Conclusions
We have shown for the first time that PDK1 expressed in ovarian cancer cells influences CD8+ T cell function by regulating PD-L1 expression via the JNK-c-Jun pathway. Considering the enhanced expression of PDK1 in ovarian cancer and its positive correlation with the immune checkpoint protein, PD-L1, and inverse relationship with CD8+ T cell infiltration, we speculate that the glycolytic gene influences the immune response in the tumor microenvironment. The synergistic effects of DCA with the anti-PD-L1 antibody on prolonging overall survival and suppression of tumor growth in vivo supports the clinical utility of this combinational approach in treating ovarian cancer. Our findings shed light on a novel mechanistic pathway underlying regulation of PD-L1 in ovarian cancer and provide a rationale for combining PDK inhibitors with immunotherapy to improve ovarian cancer treatment options.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Supplemental Material
Download PDF (127.7 KB)Supplemental Material
Download PDF (1 MB)Acknowledgments
We are grateful to our colleagues, Dr Lee Kai Fai Calvin and Dr Lee Cheuk Lun Keith, for providing buffy coat samples that greatly assisted this research. We thank Dr. Katherine Roby (Department of Anatomy and Cell Biology, University of Kansas Medical Center) for providing ID8 mice-derived ovarian cancer cells.
Disclosure statement
No potential conflicts of interest exist.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:1–14. doi:10.3322/caac.21456.
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi:10.1016/j.ccell.2015.03.001.
- Feng J, Yang H, Zhang Y, Wei H, Zhu Z, Zhu B, Yang M, Cao W, Wang L, Wu Z. Tumor cell-derived lactate induces TAZ-dependent upregulation of PD-L1 through GPR81 in human lung cancer cells. Oncogene. 2017;36:5829–5839. doi:10.1038/onc.2017.188.
- Quezada SA, Peggs KS, Simpson TR, Allison JP. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev. 2011;241:104–118. doi:10.1111/j.1600-065X.2011.01007.x.
- Zerdes I, Matikas A, Bergh J, Rassidakis GZ, Foukakis T. Genetic, transcriptional and post-translational regulation of the programmed death protein ligand 1 in cancer: biology and clinical correlations. Oncogene. 2018. doi:10.1038/s41388-018-0303-3.
- Drakes ML, Mehrotra S, Aldulescu M, Potkul RK, Liu Y, Grisoli A, Joyce C, O’Brien TE, Stack MS, Stiff PJ. Stratification of ovarian tumor pathology by expression of programmed cell death-1 (PD-1) and PD-ligand- 1 (PD-L1) in ovarian cancer. J Ovarian Res. 2018;11:43. doi:10.1186/s13048-018-0414-z.
- Ma G, Deng Y, Jiang H, Li W, Wu Q, Zhou Q. The prognostic role of programmed cell death-ligand 1 expression in non-small cell lung cancer patients: an updated meta-analysis. Clin Chim Acta. 2018;482:101–107. doi:10.1016/j.cca.2018.03.038.
- Madore J, Vilain RE, Menzies AM, Kakavand H, Wilmott JS, Hyman J, Yearley JH, Kefford RF, Thompson JF, Long GV, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res. 2015;28:245–253. doi:10.1111/pcmr.12340.
- Wang Z, Peng S, Xie H, Guo L, Cai Q, Shang Z, Jiang N, Niu Y. Prognostic and clinicopathological significance of PD-L1 in patients with renal cell carcinoma: a meta-analysis based on 1863 individuals. Clin Exp Med. 2018;18:165–175. doi:10.1007/s10238-018-0488-3.
- Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375:1767–1778. doi:10.1056/NEJMra1514296.
- Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33:4015–4022. doi:10.1200/JCO.2015.62.3397.
- Kim J, Cho J, Lee MH, Lim JH. Relative efficacy of checkpoint inhibitors for advanced NSCLC according to programmed death-ligand-1 expression: a systematic review and network meta-analysis. Sci Rep. 2018;8:11738. doi:10.1038/s41598-018-30277-0.
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi:10.1056/NEJMoa1200690.
- Disis ML, Patel MR, Pant S, Hamilton EP, Lockhart AC, Kelly K, Beck JT, Gordon MS, Weiss GJ, Taylor MH, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN solid tumor phase Ib trial: safety and clinical activity. Journal of Clinical Oncology. 2016;34:5533. doi:10.1200/JCO.2016.34.15_suppl.5533.
- Li X, Jiang Y, Meisenhelder J, Yang W, Hawke DH, Zheng Y, Xia Y, Aldape K, He J, Hunter T, et al. Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis. Mol Cell. 2016;61:705–719. doi:10.1016/j.molcel.2016.02.009.
- Hitosugi T, Fan J, Chung TW, Lythgoe K, Wang X, Xie J, Ge Q, Gu TL, Polakiewicz RD, Roesel JL, et al. Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism. Mol Cell. 2011;44:864–877. doi:10.1016/j.molcel.2011.10.015.
- McFate T, Mohyeldin A, Lu H, Thakar J, Henriques J, Halim ND, Wu H, Schell MJ, Tsang TM, Teahan O, et al. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J Biol Chem. 2008;283:22700–22708. doi:10.1074/jbc.M801765200.
- Golias T, Papandreou I, Sun R, Kumar B, Brown NV, Swanson BJ, Pai R, Jaitin D, Le QT, Teknos TN, et al. Hypoxic repression of pyruvate dehydrogenase activity is necessary for metabolic reprogramming and growth of model tumours. Sci Rep. 2016;6:31146. doi:10.1038/srep31146.
- Xu S, Tao Z, Hai B, Liang H, Shi Y, Wang T, Song W, Chen Y, OuYang J, Chen J, et al. miR-424(322) reverses chemoresistance via T-cell immune response activation by blocking the PD-L1 immune checkpoint. Nat Commun. 2016;7:11406. doi:10.1038/ncomms11406.
- Shepherd TG, Theriault BL, Campbell EJ, Nachtigal MW. Corrigendum: primary culture of ovarian surface epithelial cells and ascites-derived ovarian cancer cells from patients. Nat Protoc. 2015;10:1457. doi:10.1038/nprot0915-1457b.
- Gyorffy B, Lanczky A, Szallasi Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr Relat Cancer. 2012;19:197–208. doi:10.1530/ERC-11-0329.
- Seth P, Csizmadia E, Hedblom A, Vuerich M, Xie H, Li M, Longhi MS, Wegiel B. Deletion of lactate dehydrogenase-A in myeloid cells triggers antitumor immunity. Cancer Res. 2017;77:3632–3643. doi:10.1158/0008-5472.CAN-16-2938.
- Palsson-McDermott EM, Dyck L, Zaslona Z, Menon D, McGettrick AF, Mills KHG, O’Neill LA. Pyruvate kinase M2 is required for the expression of the immune checkpoint PD-L1 in immune cells and tumors. Front Immunol. 2017;8:1300. doi:10.3389/fimmu.2017.01300.
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi:10.1038/nm730.
- Zhang JY, Selim MA. The role of the c-Jun N-terminal kinase signaling pathway in skin cancer. Am J Cancer Res. 2012;2:691–698. https://www.ncbi.nlm.nih.gov/pubmed/23226615.
- Lopez-Bergami P, Kim H, Dewing A, Goydos J, Aaronson S, Ronai Z. c-Jun regulates phosphoinositide-dependent kinase 1 transcription: implication for Akt and protein kinase C activities and melanoma tumorigenesis. J Biol Chem. 2010;285:903–913. doi:10.1074/jbc.M109.075630.
- Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, Saudemont A, Quesnel B. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi:10.1182/blood-2006-10-051482.
- Qian Y, Deng J, Geng L, Xie H, Jiang G, Zhou L, Wang Y, Yin S, Feng X, Liu J, et al. TLR4 signaling induces B7-H1 expression through MAPK pathways in bladder cancer cells. Cancer Invest. 2008;26:816–821. doi:10.1080/07357900801941852.
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi:10.1038/nature13954.
- Neff RT, Senter L, Salani R. BRCA mutation in ovarian cancer: testing, implications and treatment considerations. Ther Adv Med Oncol. 2017;9:519–531. doi:10.1177/1758834017714993.
- Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124:192–198. doi:10.1016/j.ygyno.2011.09.039.
- Schutz F, Stefanovic S, Mayer L, von Au A, Domschke C, Sohn C. PD-1/PD-L1 pathway in breast cancer. Oncol Res Treat. 2017;40:294–297. doi:10.1159/000464353.
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi:10.1056/NEJMoa1200694.
- Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497–1508. doi:10.1016/S1470-2045(16)30498-3.
- Icard P, Shulman S, Farhat D, Steyaert JM, Alifano M, Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist Updat. 2018;38:1–11. doi:10.1016/j.drup.2018.03.001.
- Kouidhi S, Elgaaied AB, Chouaib S. Impact of metabolism on T-cell differentiation and function and cross talk with tumor microenvironment. Front Immunol. 2017;8:270. doi:10.3389/fimmu.2017.00270.
- Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. doi:10.1016/j.cell.2015.08.016.
- Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Anti-PD-L1/PD-1 immune therapies in ovarian cancer: basic mechanism and future clinical application. Int J Clin Oncol. 2016;21:456–461. doi:10.1007/s10147-016-0968-y.
- Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, Haining WN, Freeman GJ, Sharpe AH. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. 2017;214:895–904. doi:10.1084/jem.20160801.
- Zhang SL, Yang Z, Hu X, Chakravarty H, Tam KY. Anticancer effects of some novel dichloroacetophenones through the inhibition of pyruvate dehydrogenase kinase 1. Eur J Pharm Sci. 2018;123:43–55. doi:10.1016/j.ejps.2018.07.026.
- Zhang Y, Blattman JN, Kennedy NJ, Duong J, Nguyen T, Wang Y, Davis RJ, Greenberg PD, Flavell RA, Dong C. Regulation of innate and adaptive immune responses by MAP kinase phosphatase 5. Nature. 2004;430:793–797. doi:10.1038/nature02764.
- Jiang X, Zhou J, Giobbie-Hurder A, Wargo J, FS H. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res. 2013;19:598–609. doi:10.1158/1078-0432.CCR-12-2731.
- Ohashi T, Akazawa T, Aoki M, Kuze B, Mizuta K, Ito Y, Inoue N. Dichloroacetate improves immune dysfunction caused by tumor-secreted lactic acid and increases antitumor immunoreactivity. International Journal of Cancer. 2013;133:1107–1118. doi:10.1002/ijc.28114.
- Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, Curiel TJ, Colon-Otero G, Hamid O, Sanborn RE, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34:3119–3125. doi:10.1200/JCO.2016.67.9761.
- Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi:10.1073/pnas.0611533104.
- Singer K, Kastenberger M, Gottfried E, Hammerschmied CG, Buttner M, Aigner M, Seliger B, Walter B, Schlosser H, Hartmann A, et al. Warburg phenotype in renal cell carcinoma: high expression of glucose-transporter 1 (GLUT-1) correlates with low CD8(+) T-cell infiltration in the tumor. Int J Cancer. 2011;128:2085–2095. doi:10.1002/ijc.25543.
- Ottensmeier CH, Perry KL, Harden EL, Stasakova J, Jenei V, Fleming J, Wood O, Woo J, Woelk CH, Thomas GJ, et al. Upregulated glucose metabolism correlates inversely with CD8+ T-cell infiltration and survival in squamous cell carcinoma. Cancer Res. 2016;76:4136–4148. doi:10.1158/0008-5472.CAN-15-3121.
- Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24:657–671. doi:10.1016/j.cmet.2016.08.011.
- Ramus SJ, Gayther SA. The contribution of BRCA1 and BRCA2 to ovarian cancer. Mol Oncol. 2009;3:138–150. doi:10.1016/j.molonc.2009.02.001.
- Huang YW. Association of BRCA1/2 mutations with ovarian cancer prognosis: an updated meta-analysis. Medicine (Baltimore). 2018;97:e9380. doi:10.1097/MD.0000000000009380.
- Chiyoda T, Hart PC, Eckert MA, McGregor SM, Lastra RR, Hamamoto R, Nakamura Y, Yamada SD, Olopade OI, Lengyel E, et al. Loss of BRCA1 in the cells of origin of ovarian cancer induces glycolysis: a window of opportunity for ovarian cancer chemoprevention. Cancer Prev Res (Phila). 2017;10:255–266. doi:10.1158/1940-6207.CAPR-16-0281.
- Matsuo K, Spragg SE, Ciccone MA, Blake EA, Ricker C, Pham HQ, Roman LD. Nivolumab use for BRCA gene mutation carriers with recurrent epithelial ovarian cancer: a case series. Gynecol Oncol Rep. 2018;25:98–101. doi:10.1016/j.gore.2018.06.011.