ABSTRACT
Before the era of immune checkpoint blockade, a meta-analysis encompassing fifteen trials reported that adjuvant IFN-α significantly reduces the risk of relapse and improves survival of ulcerated melanoma (UM) with no benefit for higher doses compared to lower doses. IFNa2b affects many cell intrinsic features of tumor cells and modulates the host innate and cognate immune responses. To better understand the biological traits associated with ulceration that could explain the efficacy of prophylactic type 1 IFN, we performed immunohistochemical analysis of various molecules (major histocompatibility complex class I and class II, MX Dynamin Like GTPase 1 (MX1), inducible Nitric-Oxide Synthase (iNOS) or CD47) in two retrospective cohorts of melanoma patients, one diagnosed with a primary cutaneous melanoma (1995–2013, N = 172, among whom 49% were ulcerated melanoma (UM)) and a second one diagnosed with metastatic melanoma amenable to lymph node resection (EORTC 18952 and 18991 trials, N = 98, among whom 44% were UM). We found that primary and metastatic UM exhibit higher basal expression of MHC class I molecules, independently of Breslow thickness, histology and lymphocytic infiltration compared with NUM and that primary UM harbored higher constitutive levels of the antiviral protein Mx1 at the border of tumor beds than NUM. These findings suggest that UM expand in a tumor microenvironment where chronic exposure to type 1 IFN could favor a response to exogenous IFNs.
Introduction
Ulceration of primary melanoma has first been recognized as a poor prognostic factor in 1953Citation1 and has been steadily incorporated in the American Joint Committee on Cancer (AJCC) staging systems since 2001.Citation2–Citation4 It was originally defined as the absence of intact epidermis overlying a major portion of the primary melanoma, but this definition was refined in 2003 based on the observation that ulceration seems to be more than just the loss of epidermal lining.Citation5 Besides a full-thickness epidermal defect, including absence of stratum corneum and basement membrane, there must also be evidence of a host response (i.e. fibrin deposition, neutrophils) and thinning, effacement or reactive hyperplasia of the surrounding epidermis.Citation5 This definition allows to better distinguish artefactual causes such as biopsy trauma, scratching or technical issues during the preparation of the slides, from actual tumor ulceration. In addition, it firstly implied that an ulcerated melanoma might represent more than just a phenotype, or in other words a biologic entity.
Figure 1. Examples of different immunohistochemical expressions in non-ulcerated primary melanomas (NUM) and ulcerated primary melanomas (UM).
Legend: a. HLA class I expression in NUM, b. HLA class I expression in UM, c. MX-1 expression in NUM, d. MX-1 expression in UM, e. HLA class II expression in NUM, f. HLA class II expression in UM, g. iNOS expression in UM, h. CD47 expression in UM.
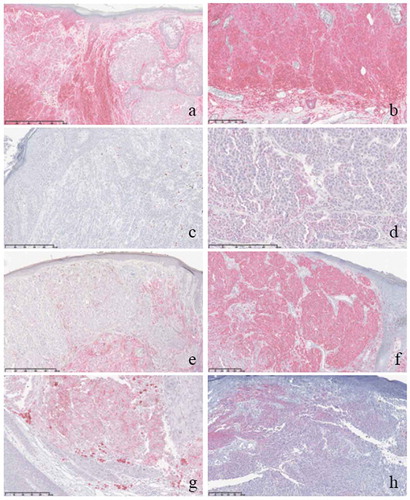
Over the years, researchers have made several attempts to unravel the biological significance of ulcerated melanomas. Several differences have been observed in terms of histopathological, genetic and immunological findings. A common histopathological observation is that ulcerated melanomas have an increased tumor vascularityCitation6-Citation9 and are associated with loss of cell-to-cell adhesion.Citation10 Also, ulcerated melanomas have been shown to have a different gene profile than non-ulcerated melanomas.Citation8,Citation11 Furthermore, the immune microenvironment seems distinct between non-ulcerated and ulcerated melanomas, since ulcerated melanomas have been correlated with the presence of lymphatic invasion,Citation8 increased density of neutrophils, mainly in the superficial part of the tumor,Citation10,Citation12 stronger infiltration of CD11c+ dendritic cells,Citation13 greater intratumoral macrophage countCitation8,Citation13 and an increased number of PD-L1 positive tumors,Citation13 which all may contribute to promoting invasion and tumor cell dispersal. They have also been associated with a lower mature dendritic cell density in sentinel lymph nodes.Citation14
Ulceration as a specific biological entity, represents a very strong prognostic factor associated with dissemination or relapse in stage I, II and III melanoma, and therefore is integrated in multivariable analyses during all systemic therapies currently in randomized trials. Hence, ulcerated primary melanomas seem to exclusively benefit from adjuvant interferon (IFN). Indeed, adjuvant IFN-α significantly reduces the risk of relapse and improves survival with no benefit for higher doses compared to lower doses.This finding was first addressed in the final report of the European Organization for Research and Treatment of Cancer (EORTC) 18991 trial, where 1256 patients with stage III melanoma were randomized between adjuvant pegylated-IFN and observation only.Citation15 Similar findings were published regarding high dose IFN therapy in the Sunbelt Trial.Citation16 Later, it was confirmed in long term outcomes in the EORTC 18991 and EORTC 18952 trials, where 1388 patients with stage IIB-III melanoma were randomized between adjuvant IFN-α and observation only.Citation17–Citation20 The most definite evidence that ulceration may be predictive of response to IFN comes from a recent meta-analysis of all available data from 15 randomized trials of adjuvant IFN versus observation.Citation21
Type 1 IFNs exhibit cell autonomous and host related-fundamental functions for tumor immunosurveillance, including cell proliferation, angiogenesis, antigen presentation, and B and T cell differentiation.Citation22 To further unravel some mechanistic features associated with ulceration that could pave the way for a better and elective therapeutic efficacy of IFN in melanomas, we aimed to explore whether there are significant differences between non-ulcerated melanoma (NUM) and ulcerated melanoma (UM) in the immunohistochemical expression profile for markers related to tumor antigen presentation [Major Histocompatibility Complex Class I and II (HLA class I and class II)], indicative of intrinsic type I IFN signaling [MX Dynamin Like GTPase 1 (MX1)], associated with cytotoxic activity of macrophages [inducible Nitric-Oxide Synthase (iNOS)], or involved in the anti-phagocytotic pathway (CD47).
Results
TIL infiltration does not contrast ulcerated from non-ulcerated primary melanoma
We analyzed two retrospective cohorts of melanoma bearing patients, one diagnosed with a primary cutaneous melanoma (1995–2013, N = 172) and a second one diagnosed with metastatic melanoma amenable to lymph node resection (EORTC 18952Citation18,Citation19 and 18991Citation15,Citation20 trials, N = 98).
The primary melanoma cohort from Gustave Roussy was composed of 172 patients, of which 87 patients had NUM and 85 ulcerated melanoma UM (). Median Breslow thickness was significantly higher in UM compared with NUM, namely 4.00 mm (IQR 2.55–6.00) and 1.70 mm (IQR 1.30–3.20), respectively (P < .001). In UM and NUM, a diffuse TIL infiltrate was observed in similar frequencies, namely 25.0% (21/84) and 15.7% (13/83), respectively (P = .33). When adjusted for Breslow thickness and histology, there was still no association between ulceration status and TIL infiltrate ().
Table 1. Baseline characteristics.
Table 2. Logistic regression analysis for evaluation of the association between TIL pattern in primary melanomas and ulceration.
The metastatic lymph node (LN) cohort was composed of 95 patients who participated in the EORTC 18952 & 18991 trials, of which 53 patients had NUM and 42 UM (). Median Breslow thickness was significantly higher in UM compared with NUM, namely 3.05 mm (IQR 2.05–6.01) and 1.95 mm (IQR 1.02–3.08), respectively (P < .001). For both UM and NUM, most patients had macroscopic nodal disease, namely 73.8% (31/42) and 81.1% (43/53), respectively (P = .39).
In two independent cohorts of early stage melanoma, ulceration characterized 44–49% cases, was associated with higher Breslow thickness but not distinguishable from the non ulcerated lesions according to T cell infiltration.
MHC class I molecules are overexpressed in ulcerated melanoma
MHC class I expression is gradually lost with tumor progression,Citation23 as a result of positive selection following CTL pressure.Citation24–Citation27 Type 1 IFNs are known to restore or upregulate transcription and translation of MHC molecules on epithelial and antigen presenting cells.Citation28,Citation29 Hence, we explored intra- and peri-tumoral expression of MHC class I molecules in NUM and UM in our cohorts (examples in ).
In the primary melanoma cohort, the median H-score for intratumoral HLA class I expression, which takes into account staining intensity as well as percentages of positive cells, tended to be significantly higher in UM than in NUM, namely 208 (IQR 120–260) versus 180 (IQR 83–230), respectively, (P = .05) (, ). In multivariable logistic regression analysis, the H-score for intratumoral HLA class I expression was independently associated with UM (P = .004) (). This trend was confirmed in the LN cohort. The median H-score for intratumoral HLA class I expression was significantly higher in UM than in NUM, namely 160 (IQR 120–237) and 140 (IQR 50–200), respectively (P < .05) (, ). In multivariable analysis, the H-score for intratumoral HLA class I expression tended to be independently associated with UM (P = .08) (). Of note, there was no difference in the peritumoral expression levels of MHC class I molecules in the primary melanoma cohort, with 63.8% (51/80) and 61.0% (50/82) strong/moderate peritumoral HLA class I expression in NUM and UM respectively (P = .72) (, ). Also, it was not independently associated with UM in multivariable logistic regression analysis (P = .79) ().
Table 3. Association between detection of HLA class I, HLA class II, Mx1, iNOS and CD47 in the primary melanoma cohort and ulceration.
Table 4. Association between detection of HLA class I and MX1 in the lymph node cohort and ulceration.
Figure 2. H-scores for intratumoral expression of HLA class I, HLA class II, Mx1, iNOS and CD47 in the primary melanoma cohort and of HLA class I and Mx1 in the lymph node cohort.
Legend: the colored bars represent the median H-score (middle line) with interquartile range (two outer lines of the colored bar). The horizontal line endings represent the range of the H-score.NUM, non-ulcerated melanomas; UM, ulcerated melanomas
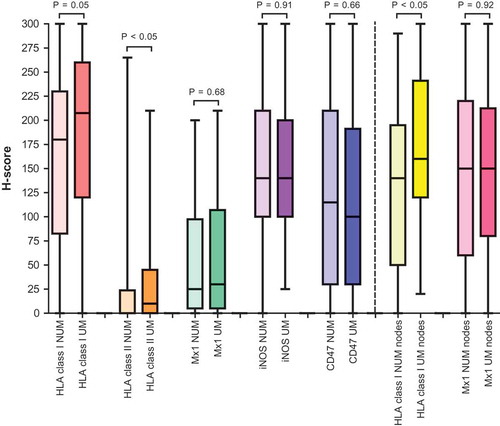
Figure 3. Percentage of strong/moderate peritumoral expression of HLA class I, HLA class 2, Mx1, iNOS and CD47 in primary melanomas.
Legend: the colored bars represent the percentage of strong/moderate peritumoral expression per protein.NUM, non-ulcerated melanomas; UM, ulcerated melanomas
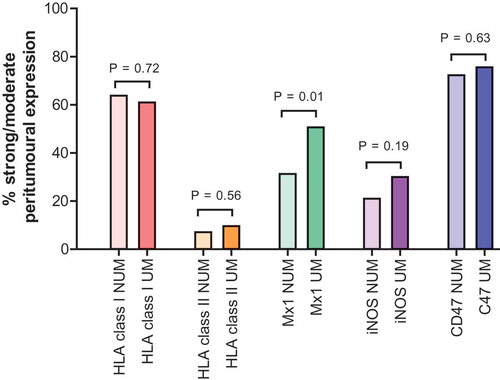
Recent articles revealed that MHC class II associated molecules (such as its invariant chain CD74) and more generally the IFNγ-regulated antigen processing machinery confered a favorable prognosis and was a predictor of response to nivolumab in metastatic melanoma.Citation30,Citation31 Here, the median H-score for intratumoral expression of HLA class II was significantly higher in UM compared with NUM, namely 10 (IQR 0–45) and 0 (IQR 0–24) respectively, (P < .05). However, in multivariable logistic regression analysis, the H-score for intratumoral HLA class II expression was not independently associated with UM (P = .84) (). The number of strong/moderate peritumoral HLA class II expression was not significantly different between NUM and UM (7.1% (6/84) and 9.6% (8/83), respectively (P = .56)) and in addition, it was not independently associated with UM in multivariable logistic regression analysis (P = .35) (, ).
In conclusion, primary and metastatic ulcerated melanoma exhibit higher basal expression of MHC class I molecules, independently from Breslow thickness, histology and TILs.
Constitutive expression of Mx1 in the peritumoral areas of ulcerated melanoma
Given the observed overexpression of MHC class I molecules on UM, we hypothesized that constitutive IFN signaling might take place in this immunopathology. Dynamin-like GTPase myxovirus resistance protein 1 (Mx1) is an intracellular anti-viral protein following the activation of type I and type III interferon signaling. Mx1 inhibits viral replication by blocking the transcription of viral RNA, and a deficiency in this protein enhances susceptibility to viral infection.Citation32 Higher Mx1/MxA expression in breast or glioblastoma than in normal tissue has been reported, suggesting that IFN signaling is constitutively active in these tumors.Citation33,Citation34 Therefore, we explored Mx1 expression in our two cohorts (examples in ).
In the primary melanoma cohort, the median H-score for intratumoral Mx1 expression was relatively low and not significantly different between NUM and UM, namely 25 (IQR 5–98) and 30 (IQR 5–108), respectively (P = .68) (, ). The H-score for intratumoral Mx1 expression was also not independently associated with UM in multivariable logistic regression analysis (P = .55) (). Similar findings were observed in the LN cohort. Median H-scores for intratumoral Mx1 expression were relatively higher than in the primary melanomas, but not significantly different between NUM and UM (130 (IQR 60–220) and 150 (80–205), respectively, P = .92) (, ). In addition, the H-score for intratumoral Mx1 expression was not independently associated with UM in multivariable logistic regression analysis (P = .78) ().
Interestingly, in contrast to intralesional stainings, peritumoral expression in primary melanomas was different between UM and NUM. Moderate or strong peritumoral Mx1 expression was dominant in UM compared with NUM (50.6% (42/83) and 31.3% (25/80), respectively, P = .01) (, ). It was also independently associated with UM in multivariable logistic regression analysis (P = .02) ().
In conclusion, as expected from the upregulation of MHC class I molecules, we observed a constitutive high level of antiviral protein Mx1 at the epidermal border of tumor beds in primary melanoma.
No relevance of other key molecules involved in scavenging and phagocytosis
We next analyzed the expression of nitric oxide synthase 2 (NOS2) in NUM versus UM. The production of nitric oxide (NO) in cells results from the conversion of L-arginine to L-citrulline by the enzyme NOS. NO regulates neurotransmission, immune-responses and antimicrobial responses. In addition, its role during the various stages of oncogenesis has been well exemplified. The anti-tumor effects of NO produced by the immune defense were reported in various human tumors, while the pro-tumorigenic and immunosuppressive effects of NO were demonstrated in progressive tumors and metastases.Citation35,Citation36 NOS2 is primarily regulated at the expression level by inflammatory cytokines (TNF-α, IL-1β, IL-6 and IFNγ), lipopolysaccharide, hypoxia, oxidative stress and HSP70.Citation35 NOS2 can be dependent on type I IFN signaling, especially STAT1, STAT2, Irf3 and NF-κBCitation37 leading to various outcomes during infection.Citation38–Citation40 In melanoma, NOS2 activation in γδT cells promoted dissemination in mouse melanoma modelsCitation41 and was associated with poor survival in melanoma patients.Citation30
Therefore, we examined whether NOS2/iNOS would be associated with ulceration and chronic inflammation in our cohorts. The median H-score for intratumoral iNOS expression was not significantly different between NUM and UM (140 (IQR 100–210) and 140 (IQR 100–200), P = .91) (, ). Also, peritumoral expression was not significantly different between NUM and UM (strong/moderate expression in 21.0% (17/81) and 30.0% (24/83), respectively, P = .19) (, ). In multivariable logisitic regression analysis, both the H-score for intratumoral expression of iNOS as the peritumoral expression were not independently associated with ulceration (P = .80 and P = .29, respectively) ().
CD47 is a “don’t eat me signal” associated molecule with an immunoglobulin-like domain that is expressed on the tumor cell surface and inhibits macrophage phagocytosis via binding the signal regulatory protein α (SIRPα) on phagocytes.Citation42 In gastric and ovarian cancer as well as in uveal melanoma, CD47 expression is an independent negative prognostic factor,Citation43 often upregulated following exposure to inflammatory stimuli.Citation44 Anti-CD47 Ab could blunt melanoma dissemination.Citation45 We next explored the potential discriminative value of this don’t eat me signal in UM vers NUM in our two cohorts. The median H-score for intratumoral CD47 expression was not significantly different between NUM and UM (115 (IQR 30–210) and 100 (IQR 30–191), P = .66) (, ). Also, peritumoral expression was not significantly different between NUM and UM (strong/moderate expression in 72.3% (60/84) and 75.6% (62/82), respectively, P = .63) (, ). In multivariable logisitic regression analysis, both the H-score for intratumoral expression of CD47 as the peritumoral expression were not independently associated with ulceration (P = .15 and P = .33, respectively) ().
Discussion
Over the past decade, researchers have made several attempts to unravel the biological significance of ulcerated melanomas. There are several explanations for the adverse prognostic value of ulceration in melanoma: ulceration is a surrogate of an intrinsic biological attribute of the tumor or the host that favors its dissemination, or ulceration directly favors the dissemination of the tumor, for example, by modifying the local environment. Proliferation of the tumor in the vicinity of the epidermis may erode it by contact and thus favor tumor expansion. Among the intrinsic properties of melanoma that might favor ulceration, proliferation and dissemination, the most convincing evidence is for the loss of E-cadherin expression, Citation10 the dual role of the matrix protein osteopontin,Citation46–Citation50 and/or the lack of the matricellular CCN3 which inhibits melanocyte proliferation and stimulates adhesion to collagen type IV.Citation51–Citation53 In addition, ulceration may have a direct influence on the local microenvironment that subsequently may favor dissemination. The presence of increased density of activated tumor-associated neutrophils in the superficial part of the lesionCitation10 and gene signatures associated with the wound healing pathway and pro-inflammatory cytokines (such as IL-1b, IL6 and IL8)Citation8 in ulcerated melanomas, compared with non ulcerated ones reenforce this hypothesis. However, our study did not support the fact that ulceration may result from defects in clearance of dying cells or phagocytosis, since the main don’t eat me signal CD47 was not differentially expressed between the two melanoma entities. However, our results fuel the assumption that ulceration may result from chronic exposure to IFNs. Indeed, we observed increased MHC class I and Mx1 expression in ulcerated lesions compared with non ulcerated melanoma. But this Mx1-associated inflammation found in ulcerated lesions may unlikely be associated with type II IFN since MHC class II molecules and inducible NOS were not relevant in our analyses.
Our study revealed unexpected findings. First, the observation that higher tumor HLA class I expression represented a hallmark of ulceration could appear surprising. Indeed, IFNγ-mediated cell surface expression of MHC molecules predicts superior antigen presentation, subsequent activation of CD8+ cytotoxic T lymphocytes, and better prognosis, ensuring prediction to respond to immune checkpoint blockade in numerous studies.Citation31,Citation54,Citation55 As a matter of fact, the presence of tumor infiltrating lymphocytes has been reported as an independent favorable prognostic factor for survival in ulcerated melanomas, though the quantity of TILs was similar in both non-ulcerated and ulcerated melanomas.Citation56 It is conceivable that higher MHC class I expression may represent a surrogate marker for IFNγ-related hallmarks of immunoresistance, such as PD-L1 or IDO and other ligands for inhibitory receptors. Supporting this view, higher proportion of tumor cell PD-L1 expression, associated with increased intratumoral CD163+ macrophage infiltrates suggesting a dominance of M2 geered-tumor microenvironment were reported in ulcerated melanomas .Citation13
Secondly, the notion that preexisting type 1 IFN fingerprint could be associated with ulceration and dismal prognosis were not expected either. Indeed, type I IFNs, secreted by malignant cells or tumor-infiltrating dendritic cells, mainly function by stimulating host anticancer immune responses.Citation22 Moreover, intratumoral expression levels of type I IFNs or their downstream effectors such as IFN-stimulated genes (ISGs) correlate with favorable disease outcome in melanoma,Citation54 in contrast to what was reported for breast cancer and glioblastoma.Citation33,Citation34 The antiviral factor Mx dynamin-like GTPase 1 (Mx1) is one of the various type I ISGs. The current study did not show a difference in the intratumoral expression of MX1 between non-ulcerated and ulcerated primary melanomas. This is in accordance with a previous study that also showed no difference in intratumoral expression of MxA (alternative name for Mx1) between ulcerated and non-ulcerated primary melanomas.Citation13 Interestingly, the current study did show upregulation of peritumoral MX1 expression in ulcerated melanomas, as indicated by a significantly higher percentage of strong/moderate expression in the adjacent epidermis. Many articles demonstrate the deleterious effects of chronic exposure to type 1 IFN or TLR3 signaling. Hence, IFN or STAT1 -mediated epigenetic imprinting in tumor cells leads to a marked upregulation of major ligands for inhibitory receptors (such as Galectin 9 for TIM3). Crippling the program interfered with multiple inhibitory pathways and expanded distinct T cell populations expressing exhaustion markers.Citation57 Moreover, an IFN signature associated with DNA damage response paved the way to resistance to therapies inducing immunogenic cell death pathways.Citation58 Finally, TLR3 signaling stimulates stemcellness in many malignant processes or pathophysiological circumstances.Citation59
Notewithstanding, ulceration is the overriding determinant of activity of adjuvant IFN therapy. Kirkwood’s group evaluated STAT1 and STAT3 jointly as mediators of type 1 IFN effects in the setting of a prospective neoadjuvant trial of high dose IFNa2b (HDI), in which tissue samples were obtained before and after 20 doses of HDI therapy. Double immunohistochemistry for pSTAT1 and pSTAT3 was performed on paired fixed or frozen biopsies in about 20 patients. HDI tilted the balance between pSTAT1 and pSTAT3, upregulating the former and down regulating the latter. Higher pSTAT1/pSTAT3 ratios in tumor cells or lymphocytes pretreatment were associated with longer overall survival. Moreover, TAP2 was augmented by HDI (but not TAP1 and MHC class I/II).Citation60 pSTAT1 results from IFNAR1/IFNAR2 receptor signaling cascade, culminating in type 1 IFN or ISG transcription. Our data showing that MHC class I molecules and surrounding Mx1 expression characterized ulcerated lesions imply that the IFNAR signaling cascade may be intact and trained in tumor cells of ulcerated lesions, accounting for the ability of IFNa2b to promptly phosphorylate STAT1 downstream of this cascade.
We acknowledge the limitations of our study. Due to the retrospective nature of the analysis, the lack of clinical follow up of patients operated from their primary lesions, and the technology that failed to be multiplexed to encompass the type 1/type 2 IFN pathways and the whole MHC antigen processing machinery, it remains difficult to conclude on the clinical relevance of endogenous IFN signaling in ulcerated melanoma. Importantly, during the time of this study, adjuvant therapies for stage III melanoma have been revolutionized by the introduction of immune checkpoint inhibitors, such as ipilimumab followed by pembrolizumab,Citation61,Citation62 which are both distinctly governed by melanoma expression of MHC class I or class II molecules,Citation31 suggesting that future studies using in depth tissue profiling through multiplexed immunohistochemical consecutive or concomittant stainings should revisit these notions accurately.Citation63
Methods and patients
Sample cohorts
Primary melanomas from institute gustave-roussy
The study group was derived from 1373 consecutive resections of primary cutaneous melanomas between October 1995 and October 2013 from the Institute Gustave-Roussy. Samples were excluded based on chart review (e.g. wrong coding, history of resection with previous biopsy, mucosal melanoma, in situ melanoma, Breslow thickness <1.0 mm) and based on pathology review (e.g., biopsies, non-confirmed ulceration status, traumatic ulceration, and Breslow thickness >10.0 mm). A total of 172 samples were eligible for staining of which 87 samples represented NUM and 85 UM. Data on patient characteristics (e.g., age, gender) and tumor characteristics (e.g. ulceration, Breslow thickness, histology) were retrieved from electronic patient files. Follow-up information was not available. Codes were used to maintain patient confidentiality throughout the study. Human archival tissue specimens were used. Informed consent was obtained prior to storage of the human tissue specimens.
Lymph nodes from EORTC 18952 & 18991 trials
The EORTC 18952 trial evaluated the role of intermediate doses of regular IFN-α2b in 1388 stage IIB-III patients compared with observation only.Citation18,Citation19 The EORTC 18991 trial evaluated pegylated-IFN (PEG-IFN) in 1256 stage III patients compared with observation only.Citation15,Citation20 From both trials, 98 positive lymph nodes were selected, of which 55 samples belonged to patients with NUM and 43 to UM. Data on patient characteristics (e.g., age, gender) and tumor characteristics (e.g. ulceration, Breslow thickness, histology, microscopic or macroscopic involvement) were retrieved. Follow-up information was not retrieved.
Immunohistochemical staining
Formalin-fixed paraffin-embedded tumor samples were stained with hematoxylin-eosin (H&E) and assed immunohistochemically on a Ventana BenchMark Ultra Platform, using antibodies against HLA class I (clone: EMR8-5, supplier: MBL, catalog: D226-3, source: mouse, concentration: 1:2000), antibodies against HLA class II (clone: CR3/43, supplier: Dako, catalog: M0775, source: mouse, concentration: 1:200), antibodies against MX1 (clone: polyclonal, supplier: Sigma, catalog: HPA030917, source: rabbit, concentration: 1:100), antibodies against iNOS (clone: L14-B, supplier: Creative Diagnostics, catalog: DMAB5712RH, source: rabbit, concentration: 1:420) and antibodies against CD47 (clone: polyclonal, supplier: Sigma, catalog: HPA044659, source: rabbit, concentration: 1:50). An UltraView detection kit was used with diaminobenzidine (DAB) as a chromogen for HLA class I and alkaline phosphatase (AP) red as a chromogen for HLA class II, MX1, iNOS and CD47. Human colon carcinoma tissue sections were used as positive control for HLA class I, HLA class II and iNOS. Human pancreas tissue sections were used as positive control for MX1 and CD47. Negative controls were obtained by omitting the primary antibody.
Immuno-histochemicalscoring
Evaluation of immunohistochemistry was performed by two pathologists (KC and GT), who were blinded to the subject’s ulceration status. Disagreement was resolved by discussion. For each slide objective fields at 5x, 10x, 20x or 40x magnification were reviewed using a Leica DM 2000 microscope. Immunohistochemical staining of the primary melanomas was evaluated in two ways: 1) evaluation of intratumoral staining using semi-quantitative scoring for the intensity of positive staining (0, negative; 1, weak; 2, moderate; 3, strong) and for the percentage of positive staining (0–100%). A cumulative “H-score” was obtained by multiplying the intensity score (0–3) by the percentage of positive staining (0–100%; with any intensity of staining). The final score ranges from 0 to 300. This approach gives more weight to higher-intensity staining in a given tumor sample; and 2) evaluation of staining in the peritumoral epidermis adjacent to and/or overlying the primary melanoma, by assigning the following score: absent, weak, moderate or strong. In addition, lymphocyte infiltrate was evaluated on the H&E slides, and the following score was assigned: diffuse, (pluri)focal or absent. Diffuse was defined as presence of lymphocytes throughout the vertical growth phase or across the entire base of the tumor. (Pluri)focal was defined as lymphocytes focally infiltrating the tumor. Absent was defined as absent lymphocytes or when they were present but not infiltrating the tumor. Immunohistochemical staining of the metastatic lymph nodes was evaluated using only the semi-quantitative H-score.
Statistical analyses
Continuous data according to the ulceration status were presented as median with interquartile range (IQR) and statistically compared using the Mann-Whitney U test. Categorical data according to the ulceration status were presented by number and percentage and statistically compared using the chi-square test. Associations between intra- and peritumoral staining in primary melanomas and ulceration status, were tested in multivariable logistic regression models, adjusting for Breslow thickness, histology and TILs. Associations between intratumoral staining in lymph nodes and ulceration status, were also tested in multivariable logistic regression models, adjusting for Breslow thickness and type of lymph node involvement. Associations with ulceration status were reported as odds ratios (OR) and their 95% confidence intervals (CI), and the corresponding P-values. For all statistical tests the threshold for significance was set a P < .05. All statistical analyses were performed in SPSS, version 24.
Disclosure of Potential Conflicts of Interest
Danielle Verver: consultancy/advisory board for Amgen, unrelated to this work. Dirk Grunhagen: honoraria for Amgen, BMS, and Abbvie, all unrelated to this work. Caroline Robert: has received personal fees from GSK, Roche, MSD, BMS and Amgen for serving as a consultant. Laurence Zitvogel: founder of a Biotech, cie involved in the cancer/microbiome space: everImmune, Editor in chief: OncoImmunology, Member of the board of director of Transgene, member of the scientific advisory board of Transgene, EpiVax, Lytix Biopharma, past research contracts with: Merus, Roche, Tusk, current research contracts with: Innovate Pharma, Kaleido, Pileje, Incyte, BMS, GSK, Transgene. Alexander Eggermont: has received personal fees from Actelion, Agenus, Amgen, Bayer, BMS, Catalym, CellDex, Gilead, GSK, HalioDX, Incyte, IO Biotech, ISA Pharmaceuticals, MedImmune, MSD, Nektar, Novartis, Pfizer, Polynoma, Regeneron, Sanofi, SkylineDx. He has equity in RiverDx, SkylineDx and Theranovir. All other authors declare that they have no other potential or real competing interests.
Additional information
Funding
References
- Allen AC, Spitz S. Malignant melanoma; a clinicopathological analysis of the criteria for diagnosis and prognosis. Cancer. 1953;6:1–11. doi:10.1002/1097-0142(195301)6:1<1::aid-cncr2820060102>3.0.co;2-c.
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A, Kirkwood JM, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi:10.1200/JCO.2001.19.16.3635.
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi:10.1200/JCO.2009.23.4799.
- American Joint Committe on Cancer (AJCC). AJCC Cancer Staging Manual Eight Edition. 2017.
- Spatz A, Cook MG, Elder DE, Piepkorn M, Ruiter DJ, Barnhill RL. Interobserver reproducibility of ulceration assessment in primary cutaneous melanomas. Eur J Cancer. 2003;39:1861–1865. doi:10.1016/s0959-8049(03)00325-3.
- Kashani-Sabet M, Sagebiel RW, Ferreira CM, Nosrati M, Miller JR 3rd. Tumor vascularity in the prognostic assessment of primary cutaneous melanoma. J Clin Oncol. 2002;20:1826–1831. doi:10.1200/JCO.2002.07.082.
- Straume O, Salvesen HB, Akslen LA. Angiogenesis is prognostically important in vertical growth phase melanomas. Int J Oncol. 1999;15:595–599. doi:10.3892/ijo.15.3.595.
- Jewell R, Elliott F, Laye J, Nsengimana J, Davies J, Walker C, Conway C, Mitra A, Harland M, Cook MG, et al. The clinicopathological and gene expression patterns associated with ulceration of primary melanoma. Pigment Cell Melanoma Res. 2015;28:94–104. doi:10.1111/pcmr.12315.
- Depasquale I, Thompson WD. Microvessel density for melanoma prognosis. Histopathology. 2005;47:186–194. doi:10.1111/j.1365-2559.2005.02193.x.
- Bonnelykke-Behrndtz ML, Steiniche T, Norgaard P, Danielsen AV, Damsgaard TE, Christensen IJ, Bastholt L, Møller HJ, Schmidt H. Loss of E-cadherin as Part of a Migratory Phenotype in Melanoma Is Associated With Ulceration. Am J Dermatopathol. 2017;39:672–678. doi:10.1097/DAD.0000000000000750.
- Winnepenninckx V, Lazar V, Michiels S, Dessen P, Stas M, Alonso SR, Avril M-F, Ortiz Romero PL, Robert T, Balacescu O, et al. Gene expression profiling of primary cutaneous melanoma and clinical outcome. J Natl Cancer Inst. 2006;98:472–482. doi:10.1093/jnci/djj103.
- Antonio N, Bonnelykke-Behrndtz ML, Ward LC, Collin J, Christensen IJ, Steiniche T, Schmidt H, Feng Y, Martin P. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. Embo J. 2015;34:2219–2236. doi:10.15252/embj.201490147.
- Koelblinger P, Emberger M, Drach M, Cheng PF, Lang R, Levesque MP, Bauer JW, Dummer R. et al. Increased tumour cell PD-L1 expression, macrophage and dendritic cell infiltration characterise the tumour microenvironment of ulcerated primary melanomas. J. Eur Acad Dermatol Venereol. 2019; 33(4):667-675.
- Elliott B, Scolyer RA, Suciu S, Lebecque S, Rimoldi D, Gugerli O, Musat E, Sharma RN, Lienard D, Keilholz U, et al. Long-term protective effect of mature DC-LAMP+ dendritic cell accumulation in sentinel lymph nodes containing micrometastatic melanoma. Clin Cancer Res. 2007;13:3825–3830. doi:10.1158/1078-0432.CCR-07-0358.
- Eggermont AM, Suciu S, Santinami M, Testori A, Kruit WH, Marsden J, Punt CJA, Salès F, Gore M, Mackie R, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet. 2008;372:117–126. doi:10.1016/S0140-6736(08)61033-8.
- McMasters KM, Edwards MJ, Ross MI, Reintgen DS, Martin RC 2nd, Urist MM, Noyes RD, Sussman JJ, Stromberg AJ, Scoggins CR. Ulceration as a predictive marker for response to adjuvant interferon therapy in melanoma. Ann Surg. 2010;252:460–465. discussion 5-6. doi:10.1097/SLA.0b013e3181f20bb1.
- Eggermont AM, Suciu S, Testori A, Kruit WH, Marsden J, Punt CJ, Santinami M, Salès F, Schadendorf D, Patel P, et al. Ulceration and stage are predictive of interferon efficacy in melanoma: results of the phase III adjuvant trials EORTC 18952 and EORTC 18991. Eur J Cancer. 2012;48:218–225. doi:10.1016/j.ejca.2011.09.028.
- Eggermont AM, Suciu S, Rutkowski P, Kruit WH, Punt CJ, Dummer R, Salès F, Keilholz U, de Schaetzen G, Testori A. Long term follow up of the EORTC 18952 trial of adjuvant therapy in resected stage IIB-III cutaneous melanoma patients comparing intermediate doses of interferon-alpha-2b (IFN) with observation: Ulceration of primary is key determinant for IFN-sensitivity. Eur J Cancer. 2016;55:111–121. doi:10.1016/j.ejca.2015.11.014.
- Eggermont AM, Suciu S, MacKie R, Ruka W, Testori A, Kruit W, Punt CJA, Delauney M, Sales F, Groenewegen G, et al. Post-surgery adjuvant therapy with intermediate doses of interferon alfa 2b versus observation in patients with stage IIb/III melanoma (EORTC 18952): randomised controlled trial. Lancet. 2005;366:1189–1196. doi:10.1016/S0140-6736(05)67482-X.
- Eggermont AM, Suciu S, Testori A, Santinami M, Kruit WH, Marsden J, Punt CJA, Salès F, Dummer R, Robert C, et al. Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J Clin Oncol. 2012;30:3810–3818. doi:10.1200/JCO.2011.41.3799.
- Ives NJ, Suciu S, Eggermont AMM, Kirkwood J, Lorigan P, Markovic SN, Garbe C, Wheatley K. Adjuvant interferon-alpha for the treatment of high-risk melanoma: An individual patient data meta-analysis. Eur J Cancer. 2017;82:171–183. doi:10.1016/j.ejca.2017.06.006.
- Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15:405–414. doi:10.1038/nri3845.
- Milling SW, Silvers WK, Sai T, Mintz B. Decline in MHC class I expression with increasing thickness of cutaneous melanomas in standard-strain transgenic mouse models. Melanoma Res. 2002;12:221–230.
- Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–208. doi:10.1016/j.coi.2007.02.001.
- Engels B, Engelhard VH, Sidney J, Sette A, Binder DC, Liu RB, Kranz DM, Meredith SC, Rowley DA, Schreiber H. Relapse or eradication of cancer is predicted by peptide-major histocompatibility complex affinity. Cancer Cell. 2013;23:516–526. doi:10.1016/j.ccr.2013.03.018.
- Agrawal S, Reemtsma K, Bagiella E, Oluwole SF, Braunstein NS. Role of TAP-1 and/or TAP-2 antigen presentation defects in tumorigenicity of mouse melanoma. Cell Immunol. 2004;228:130–137. doi:10.1016/j.cellimm.2004.04.006.
- Seliger B, Ritz U, Abele R, Bock M, Tampe R, Sutter G, Drexler I, Huber C, Ferrone S. Immune escape of melanoma: first evidence of structural alterations in two distinct components of the MHC class I antigen processing pathway. Cancer Res. 2001;61:8647–8650.
- Halloran PF, Urmson J, Van der Meide PH, Autenried P. Regulation of MHC expression in vivo. II. IFN-alpha/beta inducers and recombinant IFN-alpha modulate MHC antigen expression in mouse tissues. J Immunol. 1989;142:4241–4247.
- Garofalo R, Mei F, Espejo R, Ye G, Haeberle H, Baron S, Ogra PL, Reyes VE. Respiratory syncytial virus infection of human respiratory epithelial cells up-regulates class I MHC expression through the induction of IFN-beta and IL-1 alpha. J Immunol. 1996;157:2506–2513.
- Ekmekcioglu S, Davies MA, Tanese K, Roszik J, Shin-Sim M, Bassett RL Jr., Milton DR, Woodman SE, Prieto VG, Gershenwald JE, et al. Inflammatory Marker Testing Identifies CD74 Expression in Melanoma Tumor Cells, and Its Expression Associates with Favorable Survival for Stage III Melanoma. Clin Cancer Res. 2016;22:3016–3024. doi:10.1158/1078-0432.CCR-15-2226.
- Rodig SJ, Gusenleitner D, Jackson DG, Gjini E, Giobbie-Hurder A, Jin C, Chang H, Lovitch SB, Horak C, Weber JS, et al. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci Transl Med. 2018;10(450).
- Verhelst J, Parthoens E, Schepens B, Fiers W, Saelens X. Interferon-inducible protein Mx1 inhibits influenza virus by interfering with functional viral ribonucleoprotein complex assembly. J Virol. 2012;86:13445–13455. doi:10.1128/JVI.01682-12.
- Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remédios C, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–1309. doi:10.1038/nm.3708.
- Silginer M, Nagy S, Happold C, Schneider H, Weller M, Roth P. Autocrine activation of the IFN signaling pathway may promote immune escape in glioblastoma. Neuro Oncol. 2017;19:1338–1349. doi:10.1093/neuonc/nox051.
- Vannini F, Kashfi K, Nath N. The dual role of iNOS in cancer. Redox Biol. 2015;6:334–343. doi:10.1016/j.redox.2015.08.009.
- Lu G, Zhang R, Geng S, Peng L, Jayaraman P, Chen C, Xu F, Yang J, Li Q, Zheng H, et al. Myeloid cell-derived inducible nitric oxide synthase suppresses M1 macrophage polarization. Nat Commun. 2015;6:6676. doi:10.1038/ncomms7676.
- Farlik M, Reutterer B, Schindler C, Greten F, Vogl C, Muller M, Decker T. Nonconventional initiation complex assembly by STAT and NF-kappaB transcription factors regulates nitric oxide synthase expression. Immunity. 2010;33:25–34. doi:10.1016/j.immuni.2010.07.001.
- Diefenbach A, Schindler H, Donhauser N, Lorenz E, Laskay T, MacMicking J, Röllinghoff M, Gresser I, Bogdan C. Type 1 interferon (IFNalpha/beta) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity. 1998;8:77–87.
- Mattner J, Schindler H, Diefenbach A, Rollinghoff M, Gresser I, Bogdan C. Regulation of type 2 nitric oxide synthase by type 1 interferons in macrophages infected with Leishmania major. Eur J Immunol. 2000;30:2257–2267. doi:10.1002/1521-4141(2000)30:8<2257::AID-IMMU2257>3.0.CO;2-U.
- Moreira-Teixeira L, Sousa J, McNab FW, Torrado E, Cardoso F, Machado H, Castro F, Cardoso V, Gaifem J, Wu X, et al. Type I IFN Inhibits Alternative Macrophage Activation during Mycobacterium tuberculosis Infection and Leads to Enhanced Protection in the Absence of IFN-gamma Signaling. J Immunol. 2016;197:4714–4726. doi:10.4049/jimmunol.1600584.
- Douguet L, Bod L, Lengagne R, Labarthe L, Kato M, Avril MF, Prévost-Blondel A. Nitric oxide synthase 2 is involved in the pro-tumorigenic potential of gammadelta17 T cells in melanoma. Oncoimmunology. 2016;5:e1208878. doi:10.1080/2162402X.2016.1208878.
- Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90. doi:10.1038/nature18935.
- Tong B, Wang M. CD47 is a novel potent immunotherapy target in human malignancies: current studies and future promises. Future Oncol. 2018;14:2179–2188. doi:10.2217/fon-2018-0035.
- Basile MS, Mazzon E, Russo A, Mammana S, Longo A, Bonfiglio V, Fallico M, Caltabiano R, Fagone P, Nicoletti F, et al. Differential modulation and prognostic values of immune-escape genes in uveal melanoma. PLoS One. 2019;14:e0210276. doi:10.1371/journal.pone.0210276.
- Ngo M, Han A, Lakatos A, Sahoo D, Hachey SJ, Weiskopf K, Beck AH, Weissman IL, Boiko AD. Antibody Therapy Targeting CD47 and CD271 Effectively Suppresses Melanoma Metastasis in Patient-Derived Xenografts. Cell Rep. 2016;16:1701–1716. doi:10.1016/j.celrep.2016.07.004.
- Buback F, Renkl AC, Schulz G, Weiss JM. Osteopontin and the skin: multiple emerging roles in cutaneous biology and pathology. Exp Dermatol. 2009;18:750–759. doi:10.1111/j.1600-0625.2009.00926.x.
- Conway C, Mitra A, Jewell R, Randerson-Moor J, Lobo S, Nsengimana J, Edward S, Sanders DS, Cook M, Powell B, et al. Gene expression profiling of paraffin-embedded primary melanoma using the DASL assay identifies increased osteopontin expression as predictive of reduced relapse-free survival. Clin Cancer Res. 2009;15:6939–6946. doi:10.1158/1078-0432.CCR-09-1631.
- Haritoglou I, Wolf A, Maier T, Haritoglou C, Hein R, Schaller UC. Osteopontin and ‘melanoma inhibitory activity’: comparison of two serological tumor markers in metastatic uveal melanoma patients. Ophthalmologica. 2009;223:239–243. doi:10.1159/000206139.
- Mandelin J, Lin EC, Hu DD, Knowles SK, Do KA, Wang X, Sage EH, Smith JW, Arap W, Pasqualini R. Extracellular and intracellular mechanisms that mediate the metastatic activity of exogenous osteopontin. Cancer. 2009;115:1753–1764. doi:10.1002/cncr.24170.
- Wu Y, Jiang P, Lin Y, Chen S, Lin N, Li J. Expression of phosphorylated-STAT3 and osteopontin and their correlation in melanoma. J Huazhong Univ Sci Technolog Med Sci. 2009;29:246–250. doi:10.1007/s11596-009-0223-0.
- Fukunaga-Kalabis M, Martinez G, Telson SM, Liu ZJ, Balint K, Juhasz I, Elder DE, Perbal B, Herlyn M. Downregulation of CCN3 expression as a potential mechanism for melanoma progression. Oncogene. 2008;27:2552–2560. doi:10.1038/sj.onc.1210896.
- McCallum L, Irvine AE. CCN3–a key regulator of the hematopoietic compartment. Blood Rev. 2009;23:79–85. doi:10.1016/j.blre.2008.07.002.
- Vallacchi V, Rodolfo M. Regulatory role of CCN3 in melanoma cell interaction with the extracellular matrix. Cell Adh Migr. 2009;3:7–10. doi:10.4161/cam.3.1.6836.
- Gajewski TF, Corrales L, Williams J, Horton B, Sivan A, Spranger S. Cancer Immunotherapy Targets Based on Understanding the T Cell-Inflamed Versus Non-T Cell-Inflamed Tumor Microenvironment. Adv Exp Med Biol. 2017;1036:19–31. doi:10.1007/978-3-319-67577-0_2.
- Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran V, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127:2930–2940. doi:10.1172/JCI91190.
- de Moll EH, Fu Y, Qian Y, Perkins SH, Wieder S, Gnjatic S, Remark R, Bernardo SG, Moskalenko M, Yao J, et al. Immune biomarkers are more accurate in prediction of survival in ulcerated than in non-ulcerated primary melanomas. Cancer Immunol Immunother. 2015;64:1193–1203. doi:10.1007/s00262-015-1726-0.
- Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DSM, Pauken KE, Huang AC, et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell. 2016;167:1540–54 e12. doi:10.1016/j.cell.2016.11.022.
- Weichselbaum RR, Ishwaran H, Yoon T, Nuyten DS, Baker SW, Khodarev N, Su AW, Shaikh AY, Roach P, Kreike B, et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci U S A. 2008;105:18490–18495. doi:10.1073/pnas.0809242105.
- Lee J, Sayed N, Hunter A, Au KF, Wong WH, Mocarski ES, Pera RR, Yakubov E, Cooke JP. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012;151:547–558. doi:10.1016/j.cell.2012.09.034.
- Wang W, Edington HD, Rao UNM, Jukic DM, Land SR, Ferrone S, Kirkwood JM. Modulation of signal transducers and activators of transcription 1 and 3 signaling in melanoma by high-dose IFNalpha2b. Clin Cancer Res. 2007;13:1523–1531. doi:10.1158/1078-0432.CCR-06-1387.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med. 2016;375:1845–1855. doi:10.1056/NEJMoa1611299.
- Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A, Carlino MS, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med. 2018;378:1789–1801. doi:10.1056/NEJMoa1802357.
- Remark R, Merghoub T, Grabe N, Litjens G, Damotte D, Wolchok JD, Kreutzfeldt M, Page N, Zimmer G, Geier F, et al. In-depth tissue profiling using multiplexed immunohistochemical consecutive staining on single slide. Sci Immunol. 2016;1:aaf6925. doi:10.1126/sciimmunol.aah6817.
