ABSTRACT
Ferroptosis is an iron-dependent form of non-apoptotic cell death that has recently been attributed with antitumor immune effects. Thus, early ferroptotic cells underwent immunogenic cell death that was accompanied by the emission of damage-associated molecular patterns (DAMPs) and triggered dendritic cell maturation in vitro. Furthermore, ferroptotic cells were able to vaccinate against a rechallenge with fibrosarcoma in preclinical models.
Cell death and immunity are two evolutionary conserved processes that maintain homeostasis through complex molecular and cellular interactions.Citation1 On one hand, the effective elimination of cellular debris through phagocyte-mediated efferocytosis is essential to prevent inflammatory and autoimmune diseases. On the other hand, the release or exposure of intracellular molecules from dead or dying cells can elicit adaptive immunity, which favors an immune response against intracellular pathogens as well as against tumor-associated antigens. Regarding these immunological consequences, cell death can therefore be divided into two different types: tolerogenic and immunogenic cell death (ICD).Citation2 While the concept of ICD was originally described for chemotherapy-induced apoptotic cell death, it is now believed that ICD can occur in various types of non-apoptotic cell death caused by chemotherapy, radiotherapy, or other anticancer treatments.Citation3 Notably, the type and activity of damage-associated molecular patterns (DAMPs) emitted during the course of cell death play a key role in determining the characteristics of ICD ().Citation4
Figure 1. Immunogenic ferroptosis as a tool for anticancer treatments. Ferroptosis is a lipid peroxidation-dependent cell death that can be induced by the GPX4 inhibitor RSL3. The release of danger-associated molecular patterns (DAMPs, such as HMGB1 and ATP) from ferroptotic cancer cells contributes to the maturation of DCs, thereby triggering cytotoxic T cell-mediated adaptive immunity
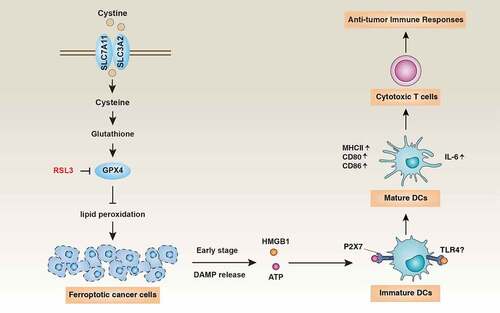
Ferroptosis is a type of non-apoptotic cell death driven by excessive iron accumulation, unrestricted lipid peroxidation, and final plasma membrane damage.Citation5 The solute carrier family 7 member 11 (SLC7A11)-glutathione-glutathione peroxidase 4 (GPX4) axis plays a central role in blocking lipid peroxidation, whereas the endosomal sorting complex required for transport-III (ESCRT-III) machinery limits plasma membrane damage during ferroptosis.Citation6 More recently, accumulating evidence suggested that the biochemical machinery that induces or suppresses ferroptosis is altered in cancer cells due to the activation of oncogenes and the inactivation of tumor suppressor genes.Citation7 The ferroptotic pathway harbors therapeutic targets for sterile inflammation and infectionCitation5and pharmacological induction of ferroptosis by specific agents (such as erastin [a SLC7A11 inhibitor] and RSL3 [a GPX4 inhibitor]) may selectively eliminate cancer cells carrying mutant RAS oncogene.Citation8 Nevertheless, in spite of the wealth of information on ferroptosis, it has remained obscure whether ferroptosis might be immunogenic.
In a recent issue of the Journal for Immunotherapy of Cancer, Efimova and colleagues described a novel approach for the induction of antitumor immunity by triggering ferroptosis-dependent ICD in preclinical models.Citation9 In a first step, the authors confirmed that the GPX4 inhibitor RSL3 induced cell death in mouse fibrosarcoma MCA205 cells via ferroptosis, rather than apoptosis and necroptosis.Citation9 The authors compared the impact of MCA205 cells treated with RSL3 for 1 h (“early ferroptotic cancer cells”) or 24 h (“late ferroptotic cancer cells”) on the maturation of mouse bone-marrow derived dendritic cells (BMDCs) using phagocytosis assays combined with the flow cytometric detection of DC maturation markers (e.g., MHC Class II, CD80, and CD86). These co-culture assays led to the conclusion that early (but not late) ferroptotic cancer cells induced BMDC maturation, whereas late (but not early) ferroptotic cells were eliminated by BMDC-mediated phagocytosis. Subsequently, the authors injected early or late ferroptotic MCA205 cells under the skin of immunocompetent C57BL/6 J mice (or, as a control, immunocompromised Rag2−/− mice). Rechallenge of these “vaccinated” mice with live MCA205 cells revealed that only early (but not late) ferroptotic cancer cells could induce a protective immune response against this fibrosarcoma.Citation9 Finally, the authors proved that extracellular HMGB1 and adenosine triphosphate (ATP) were required for ICD caused by early ferroptotic cells. In particular, the pharmacological blockade of the ATP receptor (P2X7) using oxiATP reversed the tumor-protective effects of vaccination with early ferroptotic cancer cells.
In summary, Efimova et al. reported that RSL3 stimulates ferroptosis that results in ICD, which occurs in an ATP- and P2X7-dependent manner.Citation9 It will be interesting to investigate whether this type of ICD can be induced by other ferroptosis activators, especially SLC7A11 inhibitors. Beyond this pharmacological question, a few general issues arise. Cancer cell immunogenicity depends on two factors, adjuvanticity (which is dictated by DAMPs) and antigenicity (which depends on tumor-associated antigens, many of which reflect the result of oncogenic stress).
With respect to immunogenicity, the question comes up whether ferroptotic ICD relies on additional DAMPs beyond ATP and HMGB1Citation10 (such as release of annexin A1, secretion of type-1 interferons and surface exposure of calreticulin) as this is the case for apoptotic ICD.Citation4 As a possibility, a specific panel of other, yet-to-be-defined DAMPs might come into action in the context of different types of ICD. Hence, further research is required to confirm or refute the hypothesis that different cell death modalities, including apoptosis, ferroptosis, necroptosis, and pyroptosis, may induce ICD through distinct mechanisms, using an only partially overlapping set of DAMPs.
With respect to antigenicity, the question arises whether the specific biochemical context of ferroptosis with massive oxidative modifications of the plasma membrane may generate an “altered self” that favors later immune recognition of tumor antigens. It is conceivable that the premortem stress responses occurring before tumor cells activate the irreversible phase of apoptosis, ferroptosis or necroptosis, differentially affect their antigenic makeup. Future studies must address this possibility. Indeed, it will be interesting to see whether different ICD modalities would induce immune response against distinct tumor-associated antigens. If this is the case, one might conceive “mixed” vaccines composed by cancer cells that succumb to distinct ICD types that might achieve superior anticancer immune responses.
Disclosure of potential conflicts of interest
O.K. and G.K. are cofounders of Samsara Therapeutics. G.K. is cofounder everImmune and Therafast Bio.
Additional information
Funding
References
- Yatim N, Cullen S, Albert ML. Dying cells actively regulate adaptive immune responses. Nat Rev Immunol. 2017;17:262–2. doi:10.1038/nri.2017.9.
- Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–363. doi:10.1038/nri2545.
- Kepp O, Marabelle A, Zitvogel L, Kroemer G. Oncolysis without viruses - inducing systemic anticancer immune responses with local therapies. Nat Rev Clin Oncol. 2020;17(49–64):49–64. doi:10.1038/s41571-019-0272-7.
- Galluzzi L, Vitale I, Warren S, Adjemian S, Agostinis P, Martinez AB, Chan TA, Coukos G, Demaria S, Deutsch E, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. 2020;8:e000337. doi:10.1136/jitc-2019-000337.
- Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2020. doi:10.1038/s41422-020-00441–1
- Tang D, Kroemer G. Ferroptosis. Curr Biol. 2020;30:R1292–R1297. doi:10.1016/j.cub.2020.09.068.
- Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2020:1–28. doi:10.1080/15548627.2020.1810918.
- Dixon SJ, Lemberg K, Lamprecht M, Skouta R, Zaitsev E, Gleason C, Patel D, Bauer A, Cantley A, Yang W, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(1060–1072):1060–1072. doi:10.1016/j.cell.2012.03.042.
- Efimova I, Catanzaro E, Van der Meeren L, Turubanova VD, Hammad H, Mishchenko TA, Vedunova MV, Fimognari C, Bachert C, Coppieters F, et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer. 2020;8:e001369. doi:10.1136/jitc-2020-001369.
- Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510:278–283. doi:10.1016/j.bbrc.2019.01.090.
