Abstract
Vaccine coverage among adults for recommended vaccines is generally low. In Canada and the US, pharmacists are increasingly becoming involved in the administration of vaccines to adults. This study measured the knowledge, attitudes, beliefs, and behaviors of Canadian adults and health care providers regarding pharmacists as immunizers. Geographically representative samples of Canadian adults (n = 4023) and health care providers (n = 1167) were surveyed, and 8 focus groups each were conducted nationwide with adults and health care providers. Provision of vaccines by pharmacists was supported by 64.6% of the public, 82.3% of pharmacists, 57.4% of nurses, and 38.9% of physicians; 45.7% of physicians opposed pharmacist-delivered vaccination. Pharmacists were considered a trusted source of vaccination information by 75.0% of the public, exceeding public health officials (68.3%) and exceeded only by doctors and nurses (89.2%). Public concerns about vaccination in pharmacies centered on safety (management of adverse events), record keeping (ensuring their family physician was informed), and cost (should be no more expensive than vaccination at public health or physicians' offices). Concerns about the logistics of vaccination delivery were expressed more frequently in regions where pharmacists were not yet immunizing than in jurisdictions with existing pharmacist vaccination programs. These results suggest that the expansion of pharmacists' scope of practice to include delivery of adult vaccinations is generally accepted by Canadian health care providers and the public. Acceptance of this expanded scope of pharmacist practice may contribute to improvements in vaccine coverage rates by improving vaccine accessibility.
Introduction
Despite the availability of internationally recommended, safe, and effective vaccines for adults, such as those preventing influenza, pneumococcus, and herpes zoster, adult immunization coverage remains poor.Citation1-4 There are various reasons for the poor coverage, including public apathy, lack of education about vaccines and vaccine-preventable diseases, cost, and convenience.Citation5,6 One strategy used to improve immunization coverage in adults is the use of nontraditional immunization providers, such as pharmacists, to improve the accessibility and convenience of obtaining immunizations.Citation7-9 It is estimated that 55% of adults visit a pharmacy in any given week, which provides pharmacists ample opportunity to interact and assess for vaccine needs.Citation10,11 A pharmacist's recommendation to be immunized has been shown to have a similar effect on a person's decision to be immunized as that of a physician or nurse.Citation12
Pharmacists in the United States began immunizing in 1996 when a national vaccination training program for pharmacists was implemented. Since then, increased public awareness, improved accessibility, and higher rates of adult immunization have been seen in states that allowed pharmacists to vaccinate compared to those that did not.Citation13-17 In the US, more than 260,000 pharmacists have been trained to administer vaccines across the lifespan; most have been trained in a nationally recognized certificate training program.Citation18 In 2007, Alberta became the first province in Canada to expand pharmacists' scope of practice to include administration of vaccines. As of March 2015, 8 provinces have allowed this expanded scope of practice.Citation19
In Canada, pharmacists' primary immunization administration role has been in providing seasonal influenza vaccines. Little is known about the attitudes of pharmacists, other health care providers, or the public regarding the expansion of the role of pharmacists to include a full range of adult immunizations.Citation19
The purpose of this study was to assess the attitudes of a representative national sample of health care providers (HCPs) and the public about pharmacists as immunizers.
Results
Survey
A total of 4023 adults completed the survey; 2252 (56%) were men and 1771 (44%) were women (). More respondents were 45–54 years of age and fewer were younger than 25 years of age or were 75 years and older. Respondents were representative of the Canadian population by province and by urban/suburban residence. For the HCP survey, there were 1167 respondents, comprising 42.8% family physicians, 5.6% internists, 34.3% pharmacists, and 17.3% nurses. Most (83.9%) practised in an urban/suburban setting. Ninety-three percent of physicians, 41% of pharmacists, and 54% of nurses provided direct patient care at least 75% of the time.
Table 1. Characteristics of respondents to the national survey of Canadian public and health care providers (HCPs).
Providers in all 3 professions surveyed strongly agreed that it was important to inform adults about the benefits and risks of adult immunization and to use other health encounters to check on immunization status (). A majority (54.5–64.3%) of HCPs from the 3 professions agreed that it was difficult to keep up with adult vaccine recommendations and 68.1–83.0% agreed that keeping track of immunization status of their adult patients was difficult. One-third of pharmacists agreed that they did not have time to administer vaccines to adults while only 16% of nurses and 25% of physicians agreed with this statement. Concern about insufficient time to administer vaccines was least in British Columbia and the Prairies where pharmacists were already immunizing (). Substantial differences were detected in support of expansion of the scope of practice of pharmacists to immunize; pharmacists were most supportive, physicians least supportive, and nurses were intermediate. There was a trend toward more hesitancy to expand pharmacist scope of practice to include children over 5 years of age, particularly among physicians. HCPs' willingness to be immunized themselves by a pharmacist and willingness to refer their patients to a pharmacist for immunization closely mirrored the level of support for the expansion of scope of practice. Logistical issues such as sufficient storage facilities to provide adult vaccines did not appear to be a differentiating issue between the health professions. Pharmacists were less likely than doctors and nurses to have a system in place for identifying adults who needed vaccination. Reimbursement for vaccination was more of an issue for pharmacists than physicians and was not an issue for nurses.
Figure 1. (A). Responses by pharmacists by region to the statement “I don't have enough time to administer vaccines to adult patients.” (B). Responses of Canadian adults to the statement “If my pharmacist was trained to vaccinate, I would be willing to have him/her give me my vaccines.” The Prairies includes Alberta, Saskatchewan, and Manitoba; Atlantic includes Nova Scotia, Prince Edward Island, New Brunswick, and Newfoundland and Labrador.
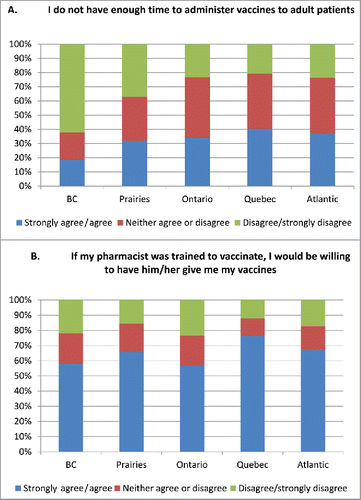
Table 2. Health care provider attitudes about delivery of vaccines by pharmacists.
A majority of the Canadian adult public was willing to receive their vaccines and have their children vaccinated by a pharmacist; support was greater for adult compared to childhood vaccination ().Willingness to be immunized in a pharmacy was similar in all regions of the country, regardless of whether pharmacists were already providing vaccination services (). While 80.4% of the public reported getting their health-related information from their family doctor or nurse, pharmacists were identified as a source of this information by 44.5% of respondents, a level similar to public health officials (48.5%), the media (50.6%), the internet (52.2%) and family members (45.7%). Importantly, however, pharmacists were identified as a trustworthy source of health information by 75% of respondents, similar to public health officials (68.3%) and exceeded only by family doctors and nurses (89.2%). Media (28.5%), the internet (34.0%), family (43.0%), and friends (29.6%) were identified much less frequently as a source of trusted health information. A physician/nurse recommendation to receive a vaccine was reported to be more influential than a pharmacist recommendation for a number of vaccines routinely recommended for adults.
Table 3. Public attitudes about delivery of vaccines by pharmacist delivery.
Only 46.3% of survey respondents reported being up to date on all their adult immunizations and an additional 30% did not know (). The frequency that the public visits a pharmacy compared to a physician's office may provide an opportunity to improve vaccine coverage rates for recommended adult vaccines. Almost 95% of respondents reported visiting a pharmacist compared to 83.9% visiting a physician at least once a year (). More strikingly, 55.2% of respondents reported visiting a pharmacist compared to only 8.1% visiting a physician at least once per month. Respondents were reluctant to pay for the convenience of pharmacy delivered vaccines; 46.4% would be willing to pay up to $5 for the pharmacist vaccine administration and only 16.4% would be willing to pay up to $20 ().
Focus groups
A total of 62 Canadian adults and 45 HCPs participated in the focus groups. The public sessions were held in Charlottetown (Prince Edward Island, n = 8), Montreal (Quebec, n = 8), Toronto and Sudbury (Ontario, n = 8 and n = 10, respectively), Regina (Saskatchewan, n = 8), and Vancouver (British Columbia, n = 7). The two virtual focus groups included participants from Quebec, Nova Scotia, Prince Edward Island, Alberta, British Columbia (n = 10; 2 each), Ontario, Quebec, and Manitoba (n = 3; 1 each). There were 32 women and 30 men in the focus groups; 44 had high daily exposure to children (parents, grandparents, or child care), and 18 had infrequent contact with children. For the HCPs, traditional focus groups were done in Prince Edward Island (Charlottetown), British Columbia (Vancouver), Ontario (Toronto and Sudbury), Quebec (Montreal) and Saskatchewan (Regina). Virtual focus groups included HCPs from Ontario, Saskatchewan, Quebec, British Columbia, and Alberta, while one-on-one interviews included physicians from Nova Scotia, Ontario, and British Columbia. Participants included 16 (36%) family physicians, 12 (27%) pharmacists, 11 (24%) nurses, 2 (4%) general internists, and 2 (4%) pediatricians. One public health physician and 1 emergency room physician were also interviewed.
Discussion guides constructed for the HCP and public groups included questions that probed for information regarding the acceptability of the pharmacist's role in administering adult vaccines. Overall, HCPs were open to giving more responsibility to pharmacists for adult vaccination, but many members of the public were skeptical about getting vaccinated in a pharmacy and stated that they would prefer to receive vaccinations from a doctor or public health professional. The public felt that their family physician or nurse was better able to manage adverse events that might require immediate attention and wanted to know whether their doctor recommended the vaccine.
“Sure, absolutely, physicians shouldn't do the immunization anyways” (NS, HCP).
“If it became an option for pharmacists, fabulous, no issue.” (ON, HCP).
“No. With any vaccine there is always a risk of having a reaction I will always want to be either with a doctor or a nurse or with some professional who knows what to do in the case of a reaction and also close to medical staff and facilities. I don't say that they can't do it, I am just saying I would rather not.” (NS, Public).
“If my doctor says it is ok” (ON, Public).
While HCPs were supportive of pharmacy–delivered vaccination, many stated that they would only be willing to refer their patients to a pharmacist for vaccine administration and support the expansion of pharmacists' scope of practice provided certain conditions are met.
“If we are going to allow pharmacies to do it, then I think there needs to be stringent guidelines.” (PEI, HCP).
In keeping with the public's comments concerning the management of adverse events, most HCP participants agreed that all pharmacists who administer vaccines must be properly trained to deliver vaccines and must work in facilities that can support the appropriate to management of vaccine adverse events.
“I would as long as they got the appropriate training by a credited source” (BC, HCP).
“If they have adequate facilities and they can deal with adverse effects, yes, but I don't think this is what they have now.” (ON, HCP).
“Yes, if the person giving the vaccine was properly trained to administer the vaccine and deal with allergic reactions” (AB, Public).
Most HCPs stated that it would be much easier for the public to access vaccines at a pharmacy especially for those adults who do not have a family physician or an HCP whom they visit on a regular basis. The public also agreed with this statement and stated that pharmacy-delivered vaccination would lessen the burden on family physicians.
“But I think the more people you can get on board to administer the Tdap vaccine the better…. If the pharmacy starts giving it, when they go to pick up their monthly prescriptions they could have it administered there.” (PEI, HCP).
“I would prefer a pharmacist over a public health clinic due to lack of line ups and the ability to make an appointment.” (AB, Public).
“Yes if pharmacists can speed up the process, if it's hard to get the vaccine elsewhere or if the hours don't allow you to get it easily.”(QB, Public).
Tracking of vaccination status and costs were raised as concerns by the HCPs. Many HCPs felt that duplication of records would occur and that the family physician would not have complete documentation of their patients' vaccination status if their patients received their vaccines at a pharmacy.
“You don't want duplication, go get this vaccine from the GP and this one at the pharmacy, it would be difficult.” (ON, HCP).
HCPs also suggested that the cost of the vaccine should be the same or lower when administered in a pharmacy as opposed to a physician's office.
“If it is less cost for the patient, yes I would refer the patient” (BC, HCP).
“If they got it for free I would say go there.” (PEI, HCP).
Many pharmacists were quite receptive to the idea of administering adult vaccines to the public, but felt that this practice would be most relevant during times of a crisis such as during a pandemic or flu season.
“I think during a pandemic we can give help because we have to vaccinate a lot of people very quickly.” (ON, HCP).
In British Columbia, participants stated that pharmacists who deliver vaccines are able to vaccinate individuals who are unable to visit their family physicians due to time constraints.
“We are catching those who fall through the cracks or those who do not have the time to go to their family physician.” (BC, HCP).
The public as well as HCPs stated that pharmacists as well as doctors and other HCPs should act as authority figures or spokespeople for the purpose of promoting the vaccine.
“We also have to get doctors, pharmacists and nurses on board because they are providing information to so many people; the more information authority people have the better.” (BC, Public)
“Doctors and pharmacists should promote it basically.” (ON, Public)
“Chief health officer can endorse it, the message can then be carried by family physicians, nurses, pharmacists, if we all carried the same message, and the population doesn't become confused.” (PEI, HCP)
Discussion
Adult vaccination rates for recommended vaccines such as influenza, Tdap, pneumococcal, and herpes zoster remain sub-optimal.Citation20-22 Barriers to achieving high vaccination rates in adults are multifactorial, including lack of education about vaccines and vaccine-preventable diseases, infrastructural issues including access to vaccines and HCPs, financial concerns, and the attitudes of patients and providers toward vaccinations.Citation25 Interventions that enhance access to vaccinations can improve vaccine coverage.Citation23 The addition of pharmacists as immunization providers is one way to expand access and subsequently improve vaccination coverage.Citation13-17
Early studies in Canada have shown the addition of pharmacists as immunizers of influenza vaccine to be successful.Citation24,25 Many provinces are allowing the administration of additional vaccines by pharmacists; however, the uptake is not well known at this time, as many vaccines are purchased privately and are not provided through public health.
Improvements to overall vaccine uptake in Canada arising from the addition of pharmacists as vaccine providers can be optimized by understanding and responding to the factors impacting acceptance of pharmacist immunizers by both the Canadian public and conventional HCPs. The results of our nationwide representative sampling, by survey and focus groups, of HCPs and the public demonstrate moderate support for immunization by pharmacists but also identify some barriers to widespread support among both HCPs and the public which will need to be addressed. While 82% of pharmacists would support the expansion of the pharmacists' scope of practice to include provision of vaccines to adults, a substantial minority of nurses and physicians (32% and 46%, respectively) would not support this change in practice and less than 1/2 of nurses and physicians surveyed would refer their patients to a pharmacist for immunization or feel comfortable themselves receiving vaccines from a pharmacist. While most HCPs acknowledged that it would be more convenient for patients to access adult vaccines at a pharmacy and that administration of vaccines by pharmacists would reduce the burden on family physicians for this service, HCP respondents indicated that they would feel comfortable referring patients to pharmacists for vaccination only if stringent guidelines for training were in place. Notably, HCPs wanted assurance that pharmacists had received training not only to vaccinate but also to safely manage adverse events following immunization, particularly anaphylaxis. Record keeping was also raised as a concern in our study. HCPs wanted assurance that pharmacist records of vaccine administration would be available to the patients' other care providers, and they were concerned that there might be inadequate records or duplication of vaccine delivery if patients received some vaccines in a pharmacy. Pharmacist respondents in provinces that did not allow pharmacists to immunize had concerns about the logistics of administering vaccines in their pharmacy, specifically a lack of time; however, these concerns were noted to a lesser extent in pharmacist respondents in provinces that allowed pharmacists to immunize adults.
In our study, support for pharmacists as immunizers was higher among the public than among HCPs. Almost two-thirds of the public surveyed would be willing to receive their vaccinations from a pharmacist, and more than 1/2 would be willing to have a pharmacist vaccinate their children. More than 1/2 of public respondents agreed or strongly agreed that it would be more convenient to receive vaccines from their pharmacist. Almost 1/2 of public respondents reported receiving their health information from a pharmacist, and 75% considered pharmacists a trusted source of health information. Nonetheless, respondents were slightly more likely to receive vaccines if the vaccines were recommended by their physician or nurse rather than by their pharmacist. Like HCPs, public respondents wanted assurance that pharmacists had received adequate training to administer vaccines and to treat allergic reactions. Our study also revealed cost to be a potential barrier to public acceptance of vaccination by a pharmacist. Less than 1/2 of respondents were willing to pay up to $5 per vaccine administered by a pharmacist and only 16% would pay $20.
Previous studies have shown that the addition of pharmacists as vaccine providers resulted in improved access and higher rates of adult immunization.Citation13-17 There is evidence that pharmacists can increase access and immunization rates specifically in rural areas, areas that are usually underserviced by physicians and/or public health clinics.Citation16,24,26 Other studies have found that patients appreciate pharmacist immunization services, as pharmacists offer convenient locations and times including “off-clinic hours,” such as evenings, weekends, and holidays, when traditional HCPs are often not available.Citation27,28 One study found that almost one-third of pharmacy immunization recipients received vaccines during “off-clinic hours.”Citation27 A Canadian pilot study found that pharmacy-based clinics were the preferred site for receiving immunizations because of the convenience, less waiting time, not having to make an appointment, and easier parking.Citation28
In order to fully realize the potential benefits of pharmacists as immunizers, work remains to be done to educate both conventional immunization providers and the public and to ensure adequate systems for training, record keeping, and reimbursement are in place. While rigorous immunization training programs for pharmacists addressing immunization schedules, risks and benefits of vaccination, immunization technique, record keeping, and adverse event management have now been developed and are required for pharmacists to immunize, our data suggest that conventional HCPs and the public are not fully aware of the comprehensive training that pharmacists receive and that this is contributing to hesitance to refer patients to pharmacists or to accept immunization from a pharmacist. This could be readily addressed through educational and marketing initiatives from pharmacists' professional associations and colleges.
Concerns about cost and reimbursement will require systematic change to ensure reimbursement schemes are in place to allow pharmacists to provide vaccines to patients under the same funding model as other HCPs, such as physicians and public health. If the vaccine is funded by the public health care system, it should be available to patients from all authorized immunization providers and reimbursement schedules should be negotiated to ensure delivery by pharmacists is not impeded by a need to pass administration costs on to patients.
Both HCPs and the public expressed concerns about record keeping and data sharing to ensure that vaccine receipt was documented and available to all of a patient's care providers. Without a national vaccination registry in Canada, this will remain an issue with all HCPs.Citation29 A national vaccine registry to capture immunization data from all HCPs would ensure vaccination efforts are optimized and not duplicated with the addition of other HCP immunizers. In the absence of a national or regional registry, pharmacists in some provinces are required to provide written notification of vaccination administration to the patient's primary care provider to improve the communication between providers.Citation30 This or similar interim solutions may be a necessity in all jurisdictions in which pharmacists immunize in order to address patient and HCP concerns.
In summary, low vaccine coverage in adults remains a concern. The addition of pharmacists as vaccine providers has been shown to improve access and vaccination coverage. We have shown that there is general support from the Canadian public and health care providers to expand the scope of pharmacists' practice to include immunization but that several key issues must be addressed to ensure optimal impact of pharmacists to improve coverage rates among adults.
While expanding the number of immunizers to include pharmacists is an important step, fundamental changes that must occur in order to address low vaccine coverage in adults have been identified. Among the issues that must be considered in a comprehensive adult vaccination strategy are the changing demographics of an increasingly aging population, addressing the bias that may prioritize vaccination programs for children in favor of adult programs, undertaking research and development into understanding the decreased immune response to immunization with increasing age (immunosenescence) and increasing translational research to develop vaccines that overcome these issues, improving measurement and reporting of vaccine coverage rates in adults, implementing innovative and transformative adult immunization programs, and designing creative education programs.Citation31 Infrastructure for providing vaccines to adults is inadequate,Citation32 and improving access to vaccinations is an important component of improving vaccine coverage. Given the current degree of support for pharmacists as immunizers in Canada and the excellent training and legislative processes already in place in some jurisdictions, optimizing pharmacist delivery of vaccination to adults as a means to increase accessibility and coverage is a logical next step in the development of a comprehensive adult immunization strategy in Canada.
Methods
We used a mixed method, sequential, explanatory design consisting of quantitative data collection and analysis (survey) followed by qualitative data collection and analysis (focus groups);Citation33,34 details of the methodology have been published previously and are summarized here.Citation35,36 The study was approved by the Research Ethics Board, IWK Health Centre, Halifax, Nova Scotia.
Quantitative stage (survey)
The national public survey was administered by Leger Marketing (Montreal, QC) which maintains email addresses for 350,000 Canadian adults (150,000 in Quebec) aged 18 years and older who are representative of the Canadian general population of adults and who have provided contact information to Leger for the purpose of participating in market and other research. A national sampling of Canadian adults was invited to participate in the public survey. Sampling was designed to ensure appropriate representation based on regional population, age, gender, and urban and rural residence. Inclusion criteria were being 18 years of age or older, having internet access, and being willing and able to complete the self-administered questionnaire. A subset of HCPs within this database was invited to participate in the HCP survey; sampling was based on regional representation, age, gender, urban and rural practice, and specialty (general practice physicians, internal medicine specialists, nurses, and pharmacists). Inclusion criteria were being in practice for a minimum of 3 years, responsibility for immunization delivery and/or patient consultation concerning vaccines in their province or territory, internet or telephone access, and willingness/ability to complete the interview. Participants received an email invitation to the survey outlining the purpose of the study, its voluntary nature, and the time commitment involved. Consent to participate was implied by completion of the web-based survey.
For the public survey, a sample size of 4000 adults was calculated to provide an acceptable precision by region (95% confidence interval (CI) around the point estimate) of ±5%. For the HCP survey, a sample size of 500 family physicians and 400 pharmacists was calculated to provide an acceptable precision (95% CI around the point estimate) of ±5%; a sample size of 100 internal medicine specialists and 200 nurses was calculated to provide an acceptable precision of ±5–10% for each practitioner type. Point estimates with 95% CIs were calculated. The first level of analysis comprised a review of the descriptive, summative statistics for trends in the data. The second level of analysis involved tests of association. Data were divided by public and by HCP profession (physician, nurse, pharmacist) and locale (province/territory). In general, continuous variables were presented by summary statistics (i.e., mean and standard error) and the categorical variables by frequency distributions (i.e., frequency counts, percentages, and their 2-sided 95% exact binomial CIs). Differences in survey responses between groups were assessed using Fisher's exact tests. For continuous variables, logistic regression was used. Associations between attitude questions, behavioral responses, and demographics were estimated using ordinal logistic regression or Fisher's exact tests. P-values <0.05 were considered statistically significant.
Qualitative stage (Focus groups)
Focus groups were administered by Leger Marketing in multiple locations across Canada using a semistructured facilitation guide. For the public, 6 traditional face-to-face focus groups and 2 “virtual” focus groups (web-based teleconferences) were undertaken. For HCPs, 6 traditional face-to-face focus groups, 2 “virtual” web-based focus groups, and 4 one-on-one interviews were undertaken. Regional representation was sought with a balance of rural and urban residence for the public and large and small urban areas, suburban, and rural practices for HCP. Inclusion criteria for the public survey were being an adult aged 21–65 years, with 2/3 of participants per focus group having frequent contact with children. HCPs invited to participate in the focus groups included physicians, nurses, and pharmacists. Inclusion criteria for participation were being a HCP who routinely provides immunizations or advice about immunization to their patients and being in practice for a minimum of 3 years. HCPs included nurses, pharmacists, and physicians (including general practitioners, internists, and emergency room physicians). A maximum quota of 2 pharmacists and 1 physician per group was imposed.
All focus groups were recorded and transcribed verbatim. A debriefing by investigators with the moderator team took place immediately following the focus group. Thematic analysis was initiated concurrent with the first focus group as previously described.Citation35,36 Transcripts were labeled and categorized by 2 investigators according to similarities and related patterns as well as for differences, followed by combining and cataloguing similar patterns into subthemes (NUD*IST software version N9, Sage Publications Ltd, London, UK).
Disclosure of potential conflicts of interest
JI, SAM, JML, and SAH have received grants and contracts for clinical trials and epidemiological studies from GlaxoSmithKline and/or Sanofi Pasteur and have served on ad hoc scientific advisory panels for these vaccine manufacturers. DM, BAH, DM-C, and LL have no conflicts of interest to disclose.
Acknowledgments
The authors thank Dr. Bruce Smith for his assistance with the statistical analysis and Kristine Webber for her assistance with the thematic analysis of the focus groups.
Funding
This study was funded by research grants from GlaxoSmithKline and Sanofi Pasteur. The funders played no role in the collection or analysis of the data.
References
- Public Health Agency of Canada. Canadian Immunization Guide [Internet]. Ottawa, ON: Public Health Agency of Canada; 2012 Dec 3 [ updated 2014 Apr 24; cited 2015 Mar 9]. Available from: http://www.phac-aspc.gc.ca/publicat/cig-gci/p01-12-eng.php
- US. Department of Health & Human Services. US. National Vaccine Plan. [Internet]. Washington, DC: US. Department of Health & Human Services; 2010 [ updated 2013; cited 2015 Mar 9]. Available from: http://www.hhs.gov/nvpo/vacc_plan/
- World Health Organization. What are some of the myths – and facts – about vaccination? [Internet]. Geneva, Switzerland: World Health Organization; 2013 April [cited 2015 Mar 9]. Available from: http://www.who.int/features/qa/84/en/index.html
- Skelton JB, American Pharmacists Association, Academy of Managed Care Pharmacy. Pharmacist-provided immunization compensation and recognition: White paper summarizing APhA/AMCP stakeholder meeting. J Am Pharm Assoc (2003) 2011; 51:704-12; PMID:22068191; http://dx.doi.org/10.1331/JAPhA.2011.11544
- Colgrove, J. Immunity for the People: The challenge of achieving high vaccine coverage in American history. Public Health Rep 2007; 122:248-57; PMID:17357368
- Kamal KM, Madhavan SS, Amonkar MM. Determinants of adult influenza and pneumonia immunization rates. J Am Pharm Assoc (2003) 2003; 43:403-11; PMID:12836791
- Kau L, Sadowski CA, Hughes C. Vaccinations in older adults: focus on pneumococcal, influenza and herpes zoster infections. Can Pharm J (Ott) 2011; 144:132-41; http://dx.doi.org/10.3821/1913-701X-144.3.132
- Shen AK, Bridges CB, Tan L. The first national adult immunization summit 2012: implementing change through action. Vaccine 2013; 31:279-84; PMID:23174197; http://dx.doi.org/10.1016/j.vaccine.2012.11.033
- Yarnall K.S., Pollak K.I., Østbye T., Krause K.M., Michener J.L. Primary care: is there enough time for prevention? Am J Public Health 2003; 93:635-41; PMID:12660210; http://dx.doi.org/10.2105/AJPH.93.4.635
- Grabenstein JD, Guess HA, Hartzema AG, Koch GG, Konrad TR. Attitudinal factors among adult prescription recipients associated with choice of where to be vaccinated. J Clin Epidemiol 2002; 55:279-84; PMID:11864799; http://dx.doi.org/10.1016/S0895-4356(01)00452-8
- Steyer TE, Ragucci KR, Pearson WS, Mainous AG. The role of pharmacists in the delivery of influenza vaccinations. Vaccine 2004; 22:1001-6; PMID:15161077; http://dx.doi.org/10.1016/j.vaccine.2003.08.045
- Grabenstein JD, Hartzema AG, Guess HA, Johnston WP, Rittenhouse BE. Community pharmacists as immunization advocates. Cost-effectiveness of a cue to influenza vaccination. Medical Care 1992; 30:503-13; PMID:1593916; http://dx.doi.org/10.1097/00005650-199206000-00004
- Ashby-Hughes B, Nickerson N. Provider endorsement: the strongest cue in prompting high-risk adults to receive influenza and pneumococcal immunizations. Clin Excell Nurse Pract 1999; 3:97-104; PMID:10646398
- Higginbotham S, Stewart A, Pfalzgraf A. Impact of a pharmacist immunizer on adult immunization rates. J Am Pharm Assoc (2003); 2012; 52:367-71; PMID:22618978; http:dx.doi.org/10.1331/JAPhA.2012.10083
- Loughlin SM, Mortazavi A, Garey KW, Rice GK, Birtcher KK. Pharmacist-managed vaccination program increased influenza vaccination rates in cardiovascular patients enrolled in a secondary prevention lipid clinic. Pharmacotherapy 2007; 27:729-33; PMID:17461708; http://dx.doi.org/10.1592/phco.27.5.729
- Ernst ME, Chalstrom CV, Currie JD, Sorofman B. Implementation of a community pharmacy-based influenza vaccination program. J Am Pharm Assoc (Wash) 1997; NS37:570-80; PMID:9479410
- Van Amburgh JA, Waite NM, Hobson EH, Migden H. Improved influenza vaccination rates in a rural population as a result of a pharmacist-managed immunization campaign. Pharmacotherapy 2001; 21:1115-22; PMID:11560201; http://dx.doi.org/10.1592/phco.21.13.1115.34624
- American Pharmacists Association. Guidelines for pharmacy-based immunization advocacy. [Internet]. Washington, DC: American Pharmacists Association; 1997 August [cited 2015 August 26]. Available from: http://www.pharmacist.com/guidelines-pharmacy-based-immunization-advocacy.
- Canadian Pharmacists Association. Pharmacists' expanded scope of practice. [Internet]. Ottawa, Ontario: Canadian Pharmacists Association; 2015 [ updated 2014 Dec; cited 2015 Mar 9]. Available from: http://www.pharmacists.ca/index.cfm/pharmacy-in-canada/scope-of-practice-canada
- Parkins MD, McNeil SA, Laupland K. Routine immunization of adults in Canada: Review of the epidemiology of vaccine-preventable diseases and current recommendations for primary prevention. Can J Infect Dis Med Microbiol 2009; 20(3):e81-90
- Public Health Agency of Canada. Vaccine coverage amongst adult Canadians: results from the 2012 adult National Immunization Coverage (aNIC) survey [Internet]. Ottawa, ON: Public Health Agency of Canada; 2012 [ updated 2014 Apr 10; cited 2015 Mar 29]. Available from: http://www.phac-aspc.gc.ca/im/nics-enva/vcac-cvac-eng.php
- Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med 2013; 10(4):e1001420; PMID:23585738; http://dx.doi.org/10.1371/journal.pmed.1001420
- Briss PA, Rodewald LE, Hinman AR, Shefer AM, Strikas RA, Bernier RR, Carande-Kulis VG, Yusuf HR, Ndiaye SM, Williams SM, The Task Force on Community Preventive Services. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med 2000; 18(1 Suppl):97-140; PMID:10806982; http://dx.doi.org/10.1016/S0749-3797(99)00118-X
- Marra F, Kaczorowski J, Gastonguay L, Marra CA, Lynd LD, Kendall P. Pharmacy-based Immunization in Rural Communities Strategy (PhICS): A community cluster-randomized trial. Can Pharm J (Ott) 2014; 147:33-44; PMID:24494014; http://dx.doi.org/10.1177/1715163513514020
- Isenor JE, Alia T, Billard B, McNeil S, Slayter K, MacDougall D, Halperin B, Holmes E, Oliver G, Bowles S. The impact of pharmacists as immunizers on influenza vaccination coverage in Nova Scotia [abstract]. In: Poster Presentation Abstracts of the 11th Canadian Immunization Conference; 2014 Dec 2–4; Ottawa, ON. Abstract Poster 96
- Ndiaye SM, Madhavean S, Washington ML, Shui I, Tucker J, Rosenbluth S, Richards T. The use of pharmacy immunization services in rural communities. Public Health 2003; 117(2):88-97; PMID:12802974; http://dx.doi.org/10.1016/S0033-3506(02)00022-7
- Goad JA, Taitel MS, Fensterheim LE, Cannon AE. Vaccinations administered during off-clinic hours at a national community pharmacy: implications for increasing patient access and convenience. Ann Fam Med 2013; 11(5):429-36; PMID:24019274; http://dx.doi.org/10.1370/afm.1542
- Bowles SK, Strang RA, Wissmann E. A pilot program of community pharmacy-based influenza immunization clinics. Can Pharm J (Ott) 2005; 138(6):38; http://dx.doi.org/10.1177/171516350513800606
- Eggertson L. Experts call for national immunization registry, coordinated schedules. CMAJ 2011; 183:e143-e144; PMID:21242267; http://dx.doi.org/10.1503/cmaj.109-3778
- Nova Scotia College of Pharmacists. Standards of Practice: Drug Administration [Internet]. Halifax, NS: Nova Scotia College of Pharmacists; 2014 Jun [cited 2015 Mar 29]. Available from: www.nspharmacists.ca/standards/documents/DrugAdministrationStandardsofPracticeJun302014.pdf
- Poland GA, Belmin J, Langley J, Michel JP, Van Damme P, Wicker S. A global prescription for adult immunization: time is catching up with us. Vaccine 2010; 28:7137-9; PMID:20937435; http://dx.doi.org/10.1016/j.vaccine.2010.09.045
- Huot C, Sauvageau C, Tremblay G, Dubé E, Ouakki M. Adult immunization services: steps have to be done. Vaccine 2010; 28:1177-80; PMID:19945413; http://dx.doi.org/10.1016/j.vaccine.2009.11.038
- Ivankova NV, Stick SL. Students' persistence in a distributed doctoral program in educational leadership in higher education: a mixed methods study. Res Higher Education 2007; 48:93-135; http://dx.doi.org/10.1007/s11162-006-9025-4
- Creswell JW. Education research: Planning, conducting, and evaluating quantitative and qualitative approaches to research. 2nd edition. Upper Saddle River, NJ: Merrill/Pearson Education, 2005
- MacDougall D, Halperin BA, MacKinnon-Cameron D, Li L, McNeil SA, Langley JM, Halperin SA. Universal tetanus, diphtheria, acellular pertussis (Tdap) vaccination of adults: what Canadian health care providers know and need to know. Hum Vaccin Immunother 2015; 11:2167-79; PMID:2609861; http://dx.doi.org/10.1080/21645515.2015.1046662
- Halperin BA, MacDougall D, MacKinnon-Cameron D, Li L, McNeil SA, Langley JM, Halperin SA. Universal tetanus, diphtheria, acellular pertussis (Tdap) vaccination of adults: What the Canadian public knows and wants to know. Vaccine 2015; 33:6840-8; PMID:26392011; http://dx.doi.org/10.1016/j.vaccine.2015.09.012
