ABSTRACT
Invasive non-typhoidal Salmonella (iNTS) infections are a leading cause of bacteremia in Sub-Saharan Africa (sSA), thereby representing a major public health threat. Salmonella Typhimurium clade ST313 and Salmonella Enteriditis lineages associated with Western and Central/Eastern Africa are among the iNTS serovars which are of the greatest concern due to their case-fatality rate, especially in children and in the immunocompromised population. Identification of pathogen-associated features and host susceptibility factors that increase the risk for invasive non-typhoidal salmonellosis would be instrumental for the design of targeted prevention strategies, which are urgently needed given the increasing spread of multidrug-resistant iNTS in Africa. This review summarizes current knowledge of bacterial traits and host immune responses associated with iNTS infections in sSA, then discusses how this knowledge can guide vaccine development while providing a summary of vaccine candidates in preclinical and early clinical development.
1. Introduction
There is growing awareness that foodborne diarrheal agents, such as non-typhoidal Salmonella (NTS), pose a serious threat to public health in resource-limited settings.Citation1 NTS are facultative, anaerobic, intracellular Enterobacteriaceae belonging to the Salmonella enterica (S. enterica) species.Citation2 They are commonly transmitted by the consumption of contaminated food or water and have a broad host-range.
Following ingestion, a proportion of the infectious Salmonella load survives the acidic pH of the stomach and competition with the gut microbiota. Salmonella can penetrate the intestinal epithelial barrier by invading non-phagocytic cells, such as enterocytes, or, preferentially, microfold cells (M cells) of Peyer’s Patches, through the expression of a multiprotein needlelike apparatus (the Type III Secretion System (T3SS) encoded by Salmonella Pathogenicity Island 1 (SPI-1-T3SS)), which is used as a conduit to translocate effector proteins into the host cell cytoplasm. Gut luminal Salmonella can also be taken up by dendritic cells (DCs) or be phagocytized by CD18+ intestinal macrophages.Citation3 Virulent Salmonellae use several strategies to elude the immune system and promote bacterial growth, including interference with the DC-mediated antigen presentation process or modification of the phagosome environment.Citation3
Salmonella-containing macrophages and DCs constitute a vehicle for systemic dissemination, as these cells can rapidly migrateCitation3 and spread through the bloodstream to extra-intestinal sites, such as the spleen and bone marrow.Citation4 However, in otherwise healthy individuals, NTS infections remain localized in the small intestine and colonCitation3 and mostly cause a self-limiting enterocolitis, which is commonly associated with secretory diarrhea, vomiting and abdominal pain. Hepatomegaly, splenomegaly or gastrointestinal complications (which may include cholecystitis, pancreatitis and appendicitis) are not frequently observed in NTS patients.Citation5
Recent estimates report that Salmonella enterocolitis resulted in 95.1 million cases and 50,771 deaths globally in 2017.Citation6 In countries with medium-to-high socio-demographic development, only a small proportion (1–3 cases per 100,000 person-years) of the population develops an invasive Salmonella infection, and this is more likely to occur in children, the elderly and immunocompromised individuals.Citation6 By contrast, NTS species are often responsible for life-threatening systemic infections in low-income settings, where poor sanitation, lack of proper diagnostic tools, malnutrition and other comorbidities can further exacerbate the outcome of the disease.Citation7,Citation8
According to current incidence data, invasive non-typhoidal Salmonella (iNTS) infections particularly haunt Africa. Of the estimated 535,000 cases of iNTS disease and 77,500 deaths that occurred globally in 2017, the highest incidence (34.5 cases per 100,000 person-years) was observed in Sub-Saharan Africa (sSA).Citation6 An average case-fatality rate of 20.6% was recently reported among iNTS patients in sSA, with peaks up to 72% in people presenting infections caused by the human immunodeficiency virus (HIV).Citation9
The typical clinical presentation of iNTS disease in Africa is a nonspecific febrile systemic illness, which is often accompanied by respiratory symptoms and hepatosplenomegaly. For this reason, iNTS infections can be confused with comorbidities commonly reported in sSA, such as malaria and pneumonia. African patients with iNTS infections also display the features of other concurrent conditions which are frequently observed in sSA, such as anemia, malnutrition, and advanced HIV illness. Diarrhea is not a predominant symptom of iNTS disease in Africa.Citation5
The most common invasive S. enterica strains identified in African regions since 1966 belong to the Salmonella serovar Typhimurium (S. Typhimurium) and the Salmonella serovar Enteritidis (S. Enteriditis),Citation9 although other serovariants, such as Salmonella Dublin (S. Dublin), Salmonella Concord (S. Concord), Salmonella Stanleyville (S. Stanleyville) and Salmonella Isangi (S. Isangi) have been reported in sSA.Citation10
During the last 5 decades, several countries of sSA have experienced multiple outbreaks associated with a multidrug-resistant (MDR) iNTS classified as S. Typhimurium sequence type (ST) 313 (ST313).Citation11 Whole genome sequencing (WGS) of iNTS isolates from sSA has identified two closely related, but genetically distinct, ST313 lineages, which emerged in the 1960 s (lineage 1) and late 1970 s (lineage 2).Citation12 It is supposed that around 20 years ago, as a result of the acquisition of chloramphenicol resistance, ST313 lineage 2 gained an evolutionary advantage over lineage 1 and, consequently, clonally replaced it. Additionally, the rapid expansion of lineage 2 MDR ST313 strains across sSA seems to have occurred in parallel with the spread of HIV epidemics,Citation12 suggesting that these iNTS clones may have adapted to a specific human niche characterized by an immunocompromised population and the extensive use of antibiotics.
WGS studies have also identified two related, but geographically and genetically distinct, S. Enteriditis lineages that emerged over the last 90 years in sSA, and which are referred to as the “Western African” (WEA) and “Central/Eastern African” (CEA) clades.Citation13 Like the ST313, these clades almost exclusively cause invasive disease in humans and display resistance to commonly used antibiotics.Citation13
The WEA, CEA and ST313 clades share some virulence features with invasive host-restricted Salmonella serovars such as Salmonella Typhi (S. Typhi) and Salmonella Gallinarum (S. Gallinarum), including genome degradation, the presence of multidrug resistance elements, and a unique prophage repertoire.Citation11,Citation13 Moreover, like the human-restricted S. Typhi, iNTS isolated in sSA rarely cause infections associated with gastrointestinal symptoms, such as inflammatory diarrhea.Citation10,Citation14,Citation15 To date, an animal reservoir for iNTS has not been identified,Citation16 whereas asymptomatic carriage has recently been described in humans.Citation17,Citation18 These observations corroborate the hypothesis that African NTS serovars are increasing their potential for systemic dissemination in humans and can be spread from person to person, in addition to the classic zoonotic transmission.
Along with host susceptibility,Citation15,Citation19 the intrinsic pathogenic signature of African iNTS lineages might explain the high rates of invasive non-typhoidal salmonellosis observed in sSA. Investigating the host- and pathogen-related factors that predispose to iNTS disease may aid the design of efficacious prevention strategies, which are not yet available for implementation in humans.
This review summarizes current knowledge of the bacterial features and host susceptibility factors that contribute to the development of NTS bacteremia in sSA. We also provide a summary of iNTS vaccine candidates currently in the early developmental stage and discuss how information gathered through the study of host-pathogen interactions can be used in iNTS vaccine research.
2. iNTS features associated with invasive infections in Africa
2.1. Multidrug resistance
One of the main drivers of NTS bloodstream infections in Africa is the emergence of MDR iNTS,Citation11,Citation20–Citation22 which can acquire determinants of antimicrobial resistance via plasmid conjugation. The spread of plasmids that harbor both virulence and drug-resistance elements (“Virulence-Resistance Plasmids” -VRP-) has increased alarmingly in recent years within NTS serovars capable of systemic dissemination in humans, such as Salmonella Choleraesuis (S. Choleraesuis), S. Dublin, S. Enteriditis and S. Typhimurium.Citation23
A clinical gastroenteritis-causing MDR S. Enteriditis isolate recovered in Spain in 2003 was found to carry one of these VRP, and was dubbed pUO-SeVR1.Citation24 This is a derivative of the common S. Enteriditis-associated plasmid pSEV and carries the spv virulence locus, together with genetic elements that confer resistance to a range of widely used drugs, such as ampicillin, chloramphenicol, streptomycin, sulfonamides, tetracycline and trimethoprim.Citation24 pUO-SeVR1 also shows accumulation of insertion sequences,Citation25 a feature which is regarded as an evolutionary step toward host restriction.Citation26 The presence of pUO-SeVR1–like plasmids in the WEA and CEA clades,Citation13 as well as in S. Enteriditis isolates from patients of African origin or travelers to Africa,Citation24,Citation27 has been associated with MDR invasive infections in humans.
In the S. Typhimurium ST313 lineage 2, the MDR-associated genotype is mostly encoded by integrons inserted in the pSLT-BT plasmid. pSLT-BT is closely related to pSLT, a virulence plasmid containing the spv genes involved in systemic infection by S. Typhimurium in mice.Citation11 pSLT-BT includes a Tn21-like mobile element harboring multiple resistance loci which resemble those identified in pUO-SEVR1-like plasmids.Citation11,Citation21,Citation24,Citation28 The MDR spectrum of ST313 is further enriched by the presence IncHI2 plasmids.Citation29 IncHI2 plasmids harbored by iNTS strains isolated in Kenya,Citation29,Citation30 Malawi,Citation21 and the Democratic Republic of Congo (DRC)Citation31 have been seen to carry resistance to extended-spectrum β-lactams (ESBLs), including ceftriaxone, a third-generation cephalosporin regarded as one of the last options in the case management of iNTS infections. The presence of ESBL resistance elements has also been reported in MDR S. Typhimurium isolates from the DRC,Citation32 Burkina Faso,Citation33 SenegalCitation34 and Nigeria.Citation35
Along with ceftriaxone, fluoroquinolones (FQ) are one of the few classes of antibiotics currently recommended for use against invasive Salmonella infections.Citation36,Citation37 Although FQ resistance is still rarely reported in iNTS serovars, careful surveillance is necessary, since both FQ-resistant invasive S. Typhimurium and S. Enteriditis have been observed in African countries.Citation13,Citation28–Citation30,Citation32,Citation34,Citation37,Citation38 This highlights the need not only for prudent use of antimicrobials, but, most importantly, for the implementation of preventive strategies against iNTS.
2.2. Genetic differences that influence the pathogenesis of iNTS and NTS strains
The S. Typhimurium ST313 clade is phylogenetically distinct from common gastroenteritis-associated S. Typhimurium lineages such as ST19. Some features, including genome degradation and the presence of novel prophages carrying virulence genes, distinguish ST313 isolates from ST19 strains, and may favor the spread of ST313 toward systemic sites.
Sequence analysis of the representative African ST313 lineage 2 strain D23580 isolated in Blantyre (Malawi)Citation11 revealed the presence of two strain-specific prophages, named Blantyre Prophage 1 (BTP1) and Blantyre Prophage 5 (BTP5),Citation11,Citation39 which are absent in the ST19 cladeCitation39 or in ST313 variants found in the United Kingdom (UK).Citation40 One BTP1 gene, st313-td, has been associated with increased ST313 replication within macrophages, a key feature of the development of systemic S. Typhimurium infections.Citation41 Another BTP1 element, the gtrAC operon, has been found to encode a glycosyltransferase which modifies the composition and length of the O-antigen (OAg),Citation42 the main polysaccharide component of the Gram-negative lipopolysaccharide (LPS). The OAg is well known to be involved in resistance to antibody-mediated killing,Citation39,Citation42–Citation45 and longer OAg chains have been associated with increased iNTS serum resistance.Citation43 Since the gtrC operon has proved to play a role in the retention of full-length OAg,Citation42 and deletion of st313-td has been associated with virulence attenuation in a mouse model of systemic NTS infection,Citation41 it is likely that these genetic elements contribute to the enhanced ability of African ST313 to persist systemically within the host.
Loss of coding sequences and the consequent formation of pseudogenes (PSD) are associated with the adaptation of Salmonella to new host niches, and may facilitate systemic infection. The accumulation of PSD is more frequent in host-specialized or -adapted Salmonella, such as S. Gallinarum (more than 300 PSD),Citation46 S. Typhi and Salmonella Paratyphi A (S. Paratyphi A) (204 and Citation177 PSD, respectively),Citation47,Citation48 Salmonella Paratyphi C (S. Paratyphi C) and S. Choleraesuis (about 160 PSD),Citation49 than in generalist serovars like S. TyphimuriumCitation50 and S. Enteriditis,Citation46 suggesting that the frequency of gene-inactivating mutations may be linked to host adaptation.
The genome of D23580 carries at least 77 PSD, 23 of which are strain-specific.Citation11,Citation51,Citation52 One of the most recently discovered is the sseI pseudogene. When functional, sseI encodes the SseI effector of the Salmonella Pathogenicity Island 2-induced T3SS (SPI-2-T3SS), a kind of T3SS which is expressed by Salmonella following internalization within cells such as macrophages and DCs. The SseI effector has been found to inhibit DC migration by altering chemotaxis.Citation53 In a murine infection model, pseudogenization of sseI resulted in rapid CD11b+ DC-mediated migratory hyper-dissemination toward draining lymph nodes.Citation54 Additionally, sseI is either absent or inactivated in invasive host-restricted or highly adapted Salmonella serovars.Citation46,Citation54–Citation57 This further emphasizes the importance of gene inactivation as a strategy for promoting bacterial spread toward extra-intestinal sites.
In NTS, the fliC gene encodes phase 1 flagellin FliC, a surface antigen which plays a role in motility and cell invasion, but which is also a target of innate and adaptive immune responses. Cummings et al.Citation58 demonstrated that, while extracellular S. Typhimurium abundantly expresses FliC, intracellular Salmonella at systemic sites down-regulates fliC expression below the threshold required for the activation of T cell responses, in order to evade host defenses. Repression of flagellin synthesis has been associated with invasive Salmonella infections, such as those caused by the host-restricted S. DublinCitation59 and S. Typhi.Citation60 Ramachandran and colleaguesCitation61 demonstrated that clinical ST313 with attenuated flagella isolated in Mali were phagocytosed more efficiently by murine J774 macrophages and were less susceptible to macrophage killing than ST19. Carden et al.Citation62 found that, in comparison with ST19, inflammasome activation within macrophages was markedly reduced in the case of ST313 infection, as evidenced by decreased interleukin (IL)-1β production and caspase-1-induced macrophage death. As flagellin can trigger inflammasome activation in macrophages, and FliC production is reduced in ST313 strains,Citation62 it is likely that the decreased sensing of flagellar proteins by the inflammasome complex can promote the enhanced intracellular survival of African ST313 strains.
Genes involved in iron uptake and siderophore secretion within macrophages have proved to be down-regulated to a greater degree in the ST313 D23580 strain than in ST19 strains.51 S. Typhimurium with defects in iron acquisition have shown enhanced ability to grow systemically in a mouse model co-infected with malaria parasites, which are known to increase intracellular iron availability.Citation63 This suggests that repression of genes associated with iron metabolism could be the result of niche adaptation.Citation51
A single nucleotide polymorphism (SNP) which up-regulates the expression of the pgtE gene has recently been identified as a feature of the African ST313 D23580 strain, thereby differentiating it from ST19 strains.Citation64 pgtE encodes the outer membrane protease PgtE, which is highly expressed by Salmonella upon exit from macrophages, and interferes with the complement cascade. The increased production of PgtE results in reduced complement deposition on the surface of D23580, making this ST313 strain more resistant to complement-mediated serum killing.Citation64 The same pgtE mutation has been found in the S. Gallinarum serovar.Citation64 PgtE is also up-regulated in human macrophages during S. Typhi infection,Citation65 strengthening the hypothesis that increased expression of this protease is linked to an invasive phenotype and to host adaptation.
Lower sopE2 mRNA levels have also been observed in ST313 strains than in ST19 strains.Citation62 sopE2 down-regulation results in decreased expression of the SPI-1-T3SS effector protein SopE2 and has been associated with decreased invasion of epithelial cells.Citation62 Interestingly, sopE2 is a pseudogene in the human-restricted S. TyphiCitation66 and shows point mutations in the invasive, avian-adapted S. Gallinarum.Citation46 In addition, deletions in the pipD gene (which is implicated in fluid accumulation in ileal bovine loops) have been associated with a diminished ability of ST313 lineage 2 isolates to elicit inflammation of the mammalian intestinal tract.Citation67
Numerous inactivating mutations in metabolism-related genes (such as allB, allP,Citation11,Citation40,Citation51,Citation67 ttdACitation67 and melRCitation51) and in genes involved in intestinal persistence (such as ratBCitation67,Citation68 and macABCitation40,Citation69) have been described in the ST313 clade but not in the ST19 clade; however, their influence on the pathogenesis of iNTS disease remains to be clarified.
A summary of the main genetic differences and associated phenotypic changes of the S. Typhimurium ST313 D23580 strain, in comparison with ST19 strains, is reported in .
Table 1. Main genetic differences and associated phenotypic changes of S. Typhimurium D23580 (ST313) in comparison with ST19 strains.
3. Host risk factors that increase susceptibility to iNTS disease
Extremes of age are common risk factors for invasive non-typhoidal salmonellosis globally.Citation6,Citation71,Citation72 However, a different bimodal age distribution of iNTS disease cases has been found in sSA, with adults and children aged < 5 years bearing the highest disease burden.Citation6,Citation70–Citation73 The fact that African adults are one of the most severely affected groups may be explained by the higher prevalence of HIV in the middle-aged population.Citation70 Indeed, together with malariaCitation8 and malnutrition,Citation74 HIV has been identified as a major predisposing factor for disseminated NTS infections. This is further emphasized by the significant association between the administration of antiretroviral therapy (ART) and the reduced occurrence of S. Typhimurium bacteremia in South Africa.Citation75
HIV infection causes loss of CD4 + T helper 17 cells in the gastrointestinal mucosa, and therefore reduces local IL-17 levels. This leads to decreased neutrophil infiltration and promotes the spread of Salmonella from the gut toward systemic sites.Citation76 Additionally, the depletion of CD4 + T cells results in an unbalanced T helper 1 (Th1)/T helper 2 (Th2) response, with decreased secretion of Th1 cytokines such as gamma interferon (IFN-γ)Citation77 in favor of skewing toward a Th2 response.Citation78 Susceptibility to iNTS in HIV-infected African adults is also promoted by an excessive production of anti-LPS immunoglobulins G (IgG).Citation79 This dysregulated humoral response interferes with antibody-dependent complement-mediated killing in a concentration-dependent wayCitation80 and probably allows Salmonella to establish an intracellular niche before serum bactericidal antibodies can exert their activity.Citation81
Like HIV-iNTS co-infection, the concurrent presence of iNTS and malaria parasites leads to the suppression of intestinal inflammation. This attenuated immune response within the gut mucosa has been linked to an increased production of the anti-inflammatory cytokine IL-10,Citation82 resulting in an increased availability of intracellular ironCitation83 that can be exploited by S. Typhimurium to promote intracellular growth,Citation63 particularly within malaria-induced hemophagocytic macrophages (which show reduced microbicidal activityCitation84–Citation86) and neutrophils.Citation87,Citation88 The systemic dissemination of iNTS is also facilitated by other malaria-induced perturbations of the immune response: (i) consumption of the C3 complement component, which reduces the efficiency of antibody-mediated bactericidal killing;Citation89 and (ii) inhibition of the production of circulating IL-12, a cytokine which plays an important role in the regulation of IFN-γ release, and hence in the clearance of invading pathogens.Citation85
Malnutrition may increase susceptibility to invasive infections by impairing the integrity and acidity of the gastro-intestinal barrierCitation90 and by negatively affecting the functions of the immune system. Reduced phagocytosis, neutrophil chemotaxis and bactericidal activity have been reported in children affected by protein-energy malnutrition.Citation91,Citation92 Lower proportions of circulating effector CD4+ and CD8 + T cells,Citation93 and CD4 + T cells,Citation94 and the reduced ability of these latter to mount a protective memory response against infectious agents,Citation95 have been observed in malnourished infected children in comparison with well-nourished infected children.
Additionally, a number of genetic risk factors (reviewed in referenceCitation19 andCitation96) have been associated with increased susceptibility to iNTS disease. This particularly applies to primary immunodeficiencies, such as Chronic Granulomatous Disease (CGD), Mendelian Susceptibility to Mycobacterial Disease (MSMD) and Sickle Cell Disease (SCD).
CGD is a hereditary disorder in which impaired ROS production induces a hyper-inflammatory state that is characterized by phagocytes with defective microbicidal activity and reduced control over innate and T cell responses.Citation97 MSMD is a collection of inherited deficiencies that affect the IL-12/23-IFN-γ pathway, with consequent impairment of IFN-γ-mediated immunity;Citation98 The fact that NTS infections in patients with CGD or MSMD can be successfully treated with antibiotic therapy and IFN-γCitation98 highlights the contribution of IFN-γ to immunity against Salmonella and the importance of a functional innate immune system in protecting against invasive Salmonella infections.Citation99
SCD is a group of genetic blood disorders characterized by altered neutrophil activity,Citation100,Citation101 impaired splenic functions,Citation102 reduced CD4/CD8 ratioCitation103 and reduced serum bactericidal activity (resulting from the impairment of the alternative complement pathway).Citation104 A large study involving Kenyan children with SCD revealed that 18% of bacteremia cases reported in this group were attributed to NTS species.Citation105 Similar results were observed in SCD patients from a Tanzanian tertiary-level hospital.Citation106 In Burkina FasoCitation107 and CameroonCitation108 around 30% of bacterial infections occurring in SCD patients were associated with Salmonella species. Between 2010 and 2015, S. Typhimurium was recovered in more than 50% of Gambian SCD children presenting with an invasive bacterial infection.Citation109 These data further emphasize the importance of targeted immunization programs in subjects with SCD; yet no clinical trial to assess the efficacy and safety of Salmonella vaccines is ongoing in SCD patients.Citation110
Carriage of an SNP on the STAT4 gene was recently associated with an increased risk of iNTS bacteremia during a Genome Wide Association Study (GWAS) involving Malawian and Kenyan children with iNTS disease.Citation111 The presence of the STAT4 locus rs13390936 in this population affected the ability of NK cells to produce IFN-γ following IL-12 stimulation, and resulted in decreased IFN-γ serum levels. The presence of the risk allele did not modify the proportion of IFN-γ-producing CD4 + T cells, suggesting that the STAT4 variant enhances the probability of developing invasive non-typhoidal salmonellosis independently from the HIV status. Additionally, no association of the rs13390936 locus with malaria or malnutrition was found. This emphasizes once again the importance of IFN-γ in the response against iNTS.Citation111
4. Protection against iNTS: the role of antibodies and T cell-mediated immunity
The early response against Salmonella relies on innate immunity within the gut mucosa. NeutrophilsCitation112 and macrophagesCitation113 are key players in this phase, since they produce, among other antimicrobial peptides, ROS that are involved in the respiratory burst, an essential defense mechanism for killing intracellular pathogens.Citation114,Citation115 Recent studies have also shown that neutrophils are an important source of mucosal IFN-γ during S. Typhimurium-induced colitis in mouse models.Citation112 The depletion of neutrophils allows S. Typhimurium to grow extracellularly and increases bacterial burden in murine spleenCitation112 and liver,Citation116 suggesting that the neutrophilic compartment can curb bacterial dissemination to extra-intestinal sites.Citation82,Citation116
However, as the infection proceeds, effective immune responses against Salmonella depend on the generation of T cells and antibody responses.Citation45,Citation99,Citation117–Citation119 At a later stage of infection, T cells are among the main producers of IFN-γCitation120 and can delay the spread of bacteria during the intracellular phase of Salmonella. Additionally, CD4+ T cells stimulate B cells to produce antibodiesCitation118 which are able to control extracellular Salmonellae via classical/alternative complement pathways and opsonophagocytosis.Citation45,Citation121
CD4 + T helper cells seem to play a greater role in immunity against Salmonella infections than CD8 + T cells.Citation119 Loss of CD4 + T cells (e.g. as occurs in advanced HIV) is one of the primary correlates of susceptibility to NTS bacteremiaCitation4 and can also perturb antibody responses to Salmonella.Citation79,Citation80 African iNTS are reported to elicit bactericidal antibodies against OAg,Citation122 and the acquisition of antibodies to iNTS LPS OAg in Malawian children has been seen to correlate with a decline in the incidence of invasive disease,Citation123 suggesting that serum killing may have an important role in protecting against iNTS. Decreased levels of anti-LPS IgG and immunoglobulins A (IgA) have also been observed in CD4 + T cell-depleted mice,Citation124 emphasizing the importance of T cell-mediated immunity in the induction of anti-iNTS humoral responses.
The critical role of NTS-specific antibodies in preventing bacteremia is also deducible from various observations: subjects with deficiencies in the IL-12/IL-23/IFN-γ axis, despite impairment in this pathway, do not suffer fatal Salmonella infections, most probably because of the functional antibody activity against NTS;Citation125 the age-dependent decline in the occurrence of iNTS, corresponding to sequential acquisition of antibodies;Citation123 the relatively low incidence of iNTS disease observed in newborns, a protection probably afforded by maternal antibodies;Citation45,Citation123 and the finding that anti-LPS antibodies from both HIV and non-HIV infected subjects are bactericidal in vitro at very low titers.Citation126
These observations, along with the fact that humoral responses elicited by NTS vaccination are preserved in many iNTS-predisposing conditions,Citation127–Citation129 suggest that vaccine-induced protection against iNTS should focus on the induction of both antibodies and T cell-mediated immunity.
5. iNTS vaccine development
5.1 Live attenuated vaccines
Salmonella live attenuated vaccines (LAVs) contain strains that carry attenuating mutations but maintain their immunogenic capacity. These vaccines can deliver multiple antigens that stimulate the immune system and elicit both humoral and cell-mediated responses.Citation130 Other advantages of Salmonella LAVs are their potential to induce cross-protection as well as mucosal immunity, and their convenient oral administration.Citation131
While an oral live attenuated vaccine against S. Typhi (Ty21a) has been available for more than 2 decades, it shows moderate immunogenicity and efficacy and has not been pre-qualified by the World Health Organization (WHO).Citation132
With regard to NTS, the only live attenuated vaccine that has entered the clinical stage is a vaccine based on S. Typhimurium attenuated by means of deletion of the aroC and ssaV genes (WT05).Citation133 The introduction of these mutations has yielded strains with defective aromatic acid biosynthesis, which are unable to properly form the SPI-2-T3SS apparatus required for the invasion of phagocytic cells. Although WT05 has displayed good tolerability and elicited high anti-LPS antibody responses in healthy human volunteers, the vaccine strains have proved to be excreted in stools for more than 3 weeks.Citation133 Prolonged fecal shedding of viable organisms constitutes a problem for vaccine development, as it increases the risk of food or water contamination, and consequently of transmission. For this reason, the WT05 candidate has not been tested further.
Other oral Salmonella LAVs based on attenuated S. Typhimurium and S. Enteriditis strains are under investigation.Citation134,Citation135 Among the most recently developed are CVD 1931 (S. Typhimurium D65 ΔguaBA ΔclpX) and CVD 1944 (S. Enteritidis R11 ΔguaBA ΔclpX), which carry mutations in the guaBA and clpX genes. These mutations reduce bacterial virulence by impairing the guanine synthesis pathway and increasing the expression of flagella. Both vaccine strains have shown excellent immunogenicity after two and three immunization doses, and provide both protection in BALB/c mice following homologous challenge and cross-protection against heterologous Salmonella serovars.Citation135 The protection against mortality conferred by S. Enteriditis CVD 1944 exceeds 80% in mice challenged with the heterologous strain S. Typhimurium D65 (ST313). Similarly, CVD 1931 vaccine efficacy is high against S. Stanleyville, which causes sporadic antibiotic-resistant clinical cases in sSA.Citation135 More recently, it has been suggested that NTS LAVs carrying heterologous OAg may be effective against Salmonella infections, although the protection they confer is highly O-serotype-specific.Citation136,Citation137
No data are currently available on the fecal shedding of CVD 1931, CVD 1944 or OAg-based NTS LAVs. In monkeys infected with the ST313 strain S. Typhimurium D65, excretion of bacteria in the feces stopped 10 days post-infection, whereas in most of the rhesus macaques infected with the ST19 S. Typhimurium I77 fecal shedding was observed up to 18 days after infection.Citation138 The short-lasting stool excretion of the ST313 clade may justify the use of these organisms, rather than gastroenteritis-associated strains, for the construction of LAVs against S. Typhimurium. Alternatively, multiple attenuations that reduce intestinal persistence can be introduced into vaccine strains derived from commonly spread Salmonella. In this regard, Ghany and colleaguesCitation139 found that introducing mutations into hdA, misL, or ratB genes could reduce the fecal shedding of S. Typhimurium in mice without affecting immunogenicity. Moreover, Wang et al.Citation140 reported that the expression of the Salmonella bactericidal yncE gene induced by oral administration of arabinose 24 hours post-vaccination with an S. Typhimurium-based live attenuated vaccine was able to eliminate bacteria in the murine intestinal tract, with no significant impact on anti-LPS and anti-flagellin IgG titers or on protection upon challenge.
Along with the risk of prolonged fecal shedding, the use of Salmonella LAVs raises additional concerns. One of these is the possibility of an in vivo reversion of LAVs to a wild type phenotype through the re-acquisition of deleted genes; however, the presence of double (or triple) genetically distinct mutations in the vaccine strain should prevent the risk of regaining virulence.Citation141 Moreover, a good balance between vaccine immunogenicity and reactogenicity must be achieved in humans, especially in immunocompromised hosts. Indeed, one of the main problems associated with LAVs is their potentially harmful effect in individuals with immune suppression; this could hamper the use of LAVs in sSA, where malaria and HIV infections are common.
A temporary loss of vaccine-mediated protection has been observed in mice co-infected with an S. Typhimurium live attenuated vaccine and malaria parasites.Citation127 This absence of protective immunity was mostly attributed to suppression of T cell effector responses and to an increased IL-10 expression.Citation127 While LAV responses depend on cellular immunity, the loss of T cell-mediated effector immunity would not be expected to affect responses to vaccines such as the glycoconjugates.Citation127 Indeed, these rely on antibody-mediated immunity as their main mechanism of action, while still requiring T cell help in order to induce immunological memory.Citation131
5.2. Glycoconjugate vaccines
Protein-polysaccharide vaccines (also known as glycoconjugates) show some advantages over pure glycans in terms of vaccine-induced immunity, as they can stimulate a T cell-dependent antibody response and immune memory.Citation131 Additionally, they can overcome the safety issues associated with the use of LAVs.
5.2.1. Glycoconjugate vaccines against S. Typhi
Several glycoconjugate vaccines against typhoid fever have been developed in the last decade. The first typhoid conjugate vaccine (TCV) developed was based on the S. Typhi Vi capsular polysaccharide and the recombinant mutant of Pseudomonas aeruginosa exotoxin A as a carrier protein (Vi-rEPA). This vaccine displayed almost 90% efficacy in Vietnamese children aged 2 to 5 years, and was submitted for in-country licensure in China in 2013.Citation132 Three formulations based on the S. Typhi Vi polysaccharide conjugated to tetanus toxoid (TT) (Vi-TT) are currently licensed in India for use in subjects aged > 6 years. One of these, the Typbar-TCV, which was WHO-prequalified in late 2017, is being used in India and Pakistan, and is under evaluation in many countries.Citation132 Recently, this vaccine was found to be immunogenic and effective in reducing the burden of typhoid fever in a clinical field trial conducted in children aged 9 months to 16 years living in an endemic setting.Citation142 Additionally, a vaccine containing the Vi antigen conjugated with the recombinant nontoxic mutant of diphtheria toxin (CRM197) (Vi-CRM197) is currently under investigation and has proved to be safe and immunogenic in phase 2 clinical trials conducted in both endemic and non-endemic regions.Citation132 Furthermore, a vaccine based on the Vi polysaccharide conjugated to diphtheria toxoid (DT) (Vi-DT) has recently proved to be safe and immunogenic both in a phase 1 clinical trial involving 2- to 45-year-old participantsCitation143 and in a phase 2 trial conducted in children aged 6 to 23 months.Citation144
The Vi-rEPA-, Vi-TT-, Vi-CRM197- and Vi-DT-based formulations can overcome the limitations of the previously licensed unconjugated Vi capsular polysaccharide vaccines, which suffer from poor immunogenicity in young children and require repeated doses.Citation132
5.2.2. Glycoconjugate vaccines against iNTS
Being the main surface-associated polysaccharide in NTS, the OAg has been identified as a possible candidate for the development of iNTS glycoconjugate vaccines. Preclinical evidence has shown that OAg is poorly immunogenic and does not elicit immunological memory if administered alone,Citation145 owing to its polysaccharide nature (usually associated with a T-independent immune response). However, studies in animal models have revealed that conjugation of Salmonella OAg with carrier proteins induces bactericidal antibodies that confer protection against invasive infections.Citation145–Citation147
5.2.2.1. iNTS OAg plus CRM197 (iNTS OAg-CRM197) conjugate vaccines
Rondini and colleaguesCitation148 showed that conjugation of S. Typhimurium D23580 OAg with CRM197 (OAg-CRM197) was able to induce a protective antibody response in mice and to reduce bacterial load in systemic sites. Among the different D23580 OAg-CRM197 candidates tested, the greatest immunogenicity was associated with OAg populations with the highest acetylation and glycosylation levels along with low or mixed molecular weight.Citation148 Since the composition of the D23580 OAg is influenced by the gtr operon,Citation42 the expression of which is not controlled by phase variation in D23580, it is unlikely that this strain can escape the immune surveillance provided by OAg-specific antibodies.Citation42 This further supports the use of D23580 OAg as an iNTS vaccine component, and justifies additional research into other surface-exposed non-phase-variable targets.
Antibodies elicited by the D23580 OAg-CRM197 conjugates have been seen to inhibit the growth of both invasive and noninvasive S. Typhimurium strains at very low concentrations; however, they do not offer cross-protection against S. Enteriditis.Citation148 The serovar-specific response has been attributed to the presence of additional O-acetyl groups on the rhamnose of the D23580 OAg, and it has been suggested that partially acetylated O-antigens could cover a wider range of OAg specificities.Citation149 However, conjugate vaccines against a single serovar have never been intended to provide broad coverage. Given the co-endemicity of S. Typhimurium and S. Enteriditis in sSA, a bivalent formulation that includes both serogroups would be a more suitable strategy for the development of iNTS glycoconjugate vaccines.Citation150
OAg levels of invasive S. Typhimurium isolates collected in Kenya have proved to correlate with increased resistance to human serum, whereas no similar association has been observed in invasive S. Enteriditis. This latter displays less susceptibility to antibody-mediated killing than S. Typhimurium,Citation43 suggesting that immune mechanisms other than OAg-antibody interaction (such as the antibody-dependent oxidative burst mediated by phagocytesCitation121) may be more important in protection against S. Enteriditis in sSA.
Importantly, the dissimilar resistance to serum killing observed in the two serovars may have an impact on vaccine efficacy.Citation149,Citation151 Introducing proper adjuvants, such as aluminum hydroxide (AlOH),Citation150 cytosine-phosphorothioate-guanine oligodeoxynucleotideCitation150 or liposomesCitation152 into the vaccine formulation can boost the immune response against Salmonella, potentially offering broader protection. This is well exemplified by a recent immunogenicity study in mice, which showed that Typbar-TCV formulated with AlOH elicited significantly higher anti-Vi IgG titers and greater IL4 and IFN-γ expression than the unadjuvanted version.Citation153
5.2.2.2. iNTS Core O-Polysaccharides plus FliC (iNTS COPS:FliC) conjugate vaccines
The administration of Salmonella O-polysaccharide linked with proteins of the homologous strain, such as the phase 1 flagellin FliC, is an attractive alternative to conjugation with exogenous carriers. There are several reasons for using FliC as a carrier in iNTS vaccines. First, this enables carrier-induced epitopic suppression to be avoided. Second, anti-flagellin antibodies have proved to be protective against invasive African iNTS in animal models.Citation134,Citation154,Citation155 Additionally, as FliC is a phase 1 flagellar protein, its inclusion in the vaccine preparation might allow coverage of uncommon monophasic African variants, such as the invasive S. Typhimurium I:4,[5],12:i:-, which does not express phase 2 flagella.Citation156
S. Typhimurium and S. Enteriditis Core O-PolySaccharides (COPS) coupled with FliC (COPS:FliC) have shown similar immunogenicity to OAg-CRM197 conjugates in mice.Citation149,Citation157 Protection against challenge with the invasive Malian blood isolate S. Enteriditis R11 was achieved in both infant and adult mice following immunization with 2 doses of S. Enteriditis COPS:FliC adjuvanted with monophosphoryl lipid A, thus providing the rationale for a possible evaluation of this formulation in the youngest.Citation157 Additionally, the passive transfer of S. Typhimurium COPS:FliC-induced maternal antibodies to infant mice proved to confer to the offspring nearly complete protection against lethal challenge with the Malian isolate S. Typhimurium D65, thereby providing further preclinical evidence that this vaccine may protect against pediatric iNTS disease in sSA.Citation158
More recently, a trivalent formulation containing iNTS COPS:FliC and the licensed S. Typhi Vi antigen-based conjugate vaccine Typbar-TCV has shown high immunogenicity and efficacy.Citation159 Immunization of rabbits with the trivalent typhoid-iNTS conjugate formulation elicited high serum IgG titers against all three polysaccharide antigens. Anti-COPS IgG were primarily directed against serogroup-specific O-polysaccharide epitopes. Post-vaccination rabbit sera mediated substantial bactericidal activity (SBA) in vitro against the invasive Malian S. Typhimurium D65, whereas lower SBA was reported against an invasive Malian prototype of S. Enteriditis.Citation159 This is in line with the findings of previous studiesCitation151 which hypothesized that invasive S. Enteriditis isolates were more resistant than invasive S. Typhimurium strains to anti-COPS antibodies-induced complement-mediated killing. Nevertheless, the efficacy of the trivalent typhoid-iNTS conjugate vaccine in vivo was high against both iNTS serovars, as the passive transfer of antibodies from the post-vaccination sera of rabbits was able to protect 100% of S. Typhimurium D65-infected mice and 88% of those challenged with the S. Enteriditis R11 isolate.Citation159 The trivalent conjugate vaccine is currently under evaluation in a Phase 1 clinical study involving healthy adults in the United States (clinicaltrials.gov identifier: NCT03981952).
5.3. OMV-based vaccines
Outer Membrane Vesicles (OMVs) are blebs spontaneously released by Gram-negative bacteria; they contain Outer Membrane Proteins (OMPs) and other components, including LPS and OAg. These vesicles can present multiple protective antigens and innate signaling molecules (such as Toll-Like Receptors ligands) to the immune system; thus, they are capable of stimulating different branches of the immune response and potentially possess an intrinsic self-adjuvanting activity.Citation160 OMVs have been used in vaccine development for the prevention of bacterial infections, such as those caused by Neisseria meningitidis (N. meningitidis) serogroup BCitation161 or Shigella flexneri,Citation162 and NTS-derived OMVs have recently been tested in animal models, yielding promising immunogenicity and protection data.Citation163–Citation165
One of the main problems of both Salmonella OMVs and LAVs is the toxicity of LPS. Several strategies, including dephosphorylation of lipid ACitation166 or mutation of the wzy gene encoding the OAg polymerase involved in LPS synthesisCitation167 can be applied in order to detoxify LPS without compromising immunogenicity. OMVs shed by S. Typhimurium strains carrying targeted LPS mutations have proved to be an effective vaccine candidate, with the potential to cover several serovars.Citation165
Another problem associated with the purification of OMVs is the residual presence of flagellin, which can induce deleterious over-activation of the innate immune system via an excessive Toll-Like-Receptor-5-mediated pro-inflammatory responseCitation168 and may cause interference with the immune response elicited against other antigens.Citation163 Immunization with OMVs derived from flagellin-deficient S. Typhimurium has proved able to provide protection against homologous and heterologous serovars (S. Enteriditis and S. Choleraesuis), suggesting that it might also be a suitable means of achieving cross-reactive immunity.Citation163,Citation164
5.3.1. Generalized Modules for Membrane Antigens (GMMA) vaccines
A promising approach to the development of a safe and affordable vaccine against iNTS is the use of OMVs in their native conformation (nOMVs) as a vehicle to deliver iNTS OAg. The shedding level of nOMVs is generally too low for them to be used in vaccine production, but deletion of the tolR gene in ShigellaCitation169 and NTSCitation170 species or deletion of the gna33 gene in N. meningitidisCitation171 can substantially increase the nOMV yield during the blebbing process. Additional mutations, such as detoxification of lipid A,Citation170 are generally introduced into nOMV-producing organisms in order to reduce the toxicity of LPS. nOMVs shed by these genetically modified bacteria are called “GMMA” and have been used in the development of vaccines against shigellosisCitation169 and meningococcal diseaseCitation171 in African countries.
In very recent years, a bivalent S. Typhimurium and S. Enteritidis GMMA-based formulation has been proposed as a vaccine candidate against iNTS disease in sSA. Preclinical studies showed that S. Enteriditis and S. Typhimurium GMMA elicited high OAg-specific IgG and bactericidal responses.Citation172 The immunogenicity of iNTS GMMA proved to be at least comparable to that observed following immunization with OAg-CRM197 glycoconjugates. Significantly higher bactericidal titers were elicited by monovalent S. Typhimurium and S. Enteriditis GMMA than by the OAg-CRM197 conjugate vaccines. Importantly, the immunization of mice with a bivalent GMMA formulation substantially reduced the Salmonella load at systemic sites following iNTS challenge. In contrast to the OAg-CRM197 conjugates, which predominantly elicited IgG1 antibodies, the iNTS GMMA induced a broad IgG antibody response.
Unlike OAg-CRM197 glycoconjugates, in which variable amounts of high-, mixed- and low-molecular weight O-antigens can be present, the GMMA OAg population is mainly composed of highly glycosylated mixed-molecular weight molecules,Citation170,Citation172 which have been associated with high immunogenicity.Citation148 Together with the possibility of inducing an immune response against surface components other than the OAg (such as porins),Citation173 these features may account for the enhanced immunogenicity and efficacy of GMMA vaccines in comparison with the OAg-CRM197 formulations. Schager and colleaguesCitation173 showed that the protection conferred by iNTS GMMA could be achieved even in the absence of OAg, was predominantly B cell-dependent, and was long-standing, thereby further highlighting the potential of GMMA as an iNTS vaccine candidate. The power of this formulation is exemplified by the success of the GMMA-based Shigella sonnei vaccine, which is currently in clinical phase 2 and has yielded promising immunogenicity and safety data.Citation169,Citation174,Citation175
In contrast to LAVs and glycoconjugates vaccines, which have been authorized for human use against several pathogens, including S. Typhi,Citation132,Citation142 no formulation based on the GMMA technology has yet been licensed.
5.4. OMP- and T3SS-based vaccines
5.4.1. OMP-based vaccines
OMPs are under investigation for the development of vaccines against Salmonella species. The high expression of the outer membrane protein (OMP) PgtECitation64,Citation65 and its ability to elicit CD4 + T cell responses in miceCitation176 have recently prompted research into PgtE B- and T-cell epitopes that can be recognized by vaccine-induced immunity.Citation176–Citation178 However, the suitability of PgtE as a vaccine antigen against NTS infections has yet to be assessed.
Barat and colleaguesCitation176 showed that, despite its undetectable expression, the siderophore receptor IroN, when used as a vaccine component, provided the longest post-challenge survival times within a set of 37 surface-associated antigens, making it a promising candidate against invasive Salmonella infections.
Another OMP, the porin OmpD, has been shown to induce a T-independent B cell-mediated antibody response capable of limiting the disease caused by invasive S. Typhimurium.Citation179 However, Ashton and colleaguesCitation40 recently found that acquisition of the bla CTX-M-15 gene (responsible for resistance against ESBLs) caused disruption of the ompD locus in the UK-isolated U60 strain belonging to lineage 2 ST313, which probably originated in Kenya. The absence of OmpD in strains such as U60 may hamper the use of these strains as broad-spectrum vaccines against non-typhoidal salmonellosis in sSA.
5.4.2. T3SS-based vaccines
Components of the T3SS are currently under evaluation as an option for the development of vaccines against iNTS disease. Immunization of BALB/c mice with Salmonella LAVs strains carrying a fusion construct based on the SPI-2-T3SS effector SseJ has been seen to cause heterologous antigens to translocate into antigen-presenting cells, thereby inducing a potent CD4 + T cell response.Citation180 Lee and colleaguesCitation181 reported that the SPI-2-T3SS translocon subunit SseB was only modestly protective in C57BL/6 mice, but that co-administration of flagellin markedly improved vaccine efficacy in comparison with immunization with SseB or flagellin alone, possibly owing to flagellin-induced boosting of the SseB-specific CD4 + T cell response. The group of JneidCitation182 found that four oral administrations of the SPI-1-T3SS component SipD adjuvanted with cholera toxin induced 72% protection against a lethal dose of S. Typhimurium, thus identifying for the first time an SPI-1-T3SS component as a potential candidate for protein-based iNTS vaccines. Kurtz and colleaguesCitation183 demonstrated that immunization with the SPI-2-T3SS peptide SseI was able to reduce mortality upon S. Typhimurium SL1344 challenge. Protection was attributed to a substantial SseI-specific CD4 + T cell response, whereas antibody-mediated and CD8 + T cell responses proved to be less marked.Citation183,Citation184
Given the rarity of protective T cell epitopes,Citation185 the importance of CD4 + T cell immunity in the defense against Salmonella infections,Citation186 and the fact that sera from Malawian HIV-infected subjects contain a high proportion of NTS-specific non-bactericidal anti-LPS IgG,Citation79,Citation80 T cell epitopes such as SseI have been regarded as promising iNTS vaccine candidates. However, the pseudogenization of the sseI gene observed in D2358054 makes it unfeasible to include SseI epitopes as the sole components of iNTS protein-based vaccines for use in sSA.
A summary of the iNTS vaccines that are currently at the most advanced stage of development is presented in .
Figure 1. iNTS vaccine candidates currently at the most advanced stage of development.
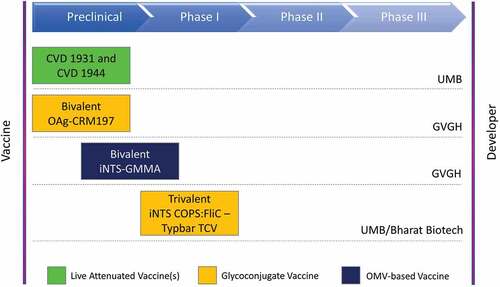
6. Conclusions
iNTS continues to constitute a significant cause of bacterial bloodstream infections in Africa. The management of iNTS infections in resource-limited settings, such as sSA, is hampered by the nonspecificity of the symptoms of iNTS disease, by the frequency of concurrent life-threatening conditions, by a poor healthcare system and by the lack of accurate diagnostic tools. The emergence of MDR iNTS strains, and particularly the ST313 S. Typhimurium clone, further complicates this scenario by limiting the use of previously recommended antibiotics. All these reasons make the development of iNTS vaccines for use in African populations a priority for global health policy-makers.
WGS has offered new solutions for monitoring Salmonella resistance patterns and has proved useful in pinpointing key strain-specific mutations that can be exploited for vaccine development. Identifying host risk factors associated with an increased likelihood of NTS bacteremia in Africa is also of the utmost importance in vaccine research, as it helps us to understand the modalities of anti-iNTS immunity in sSA, which, owing to the presence of such comorbidities as HIV and malaria, may be different from those observed in the high-income countries. In this context, GWAS studies are gaining growing interest, since they have uncovered complex genetic traits associated with susceptibility to a wide range of conditions, including those which are endemic in Africa, such as malaria,Citation187 HIVCitation187 and iNTS disease.Citation111
Together with immuno-epidemiological investigations, these studies have shed light on the importance of NTS-specific cell-mediated and humoral immunity in controlling invasive Salmonella infections. Recent evidence has shown that antibodies directed against the LPS OAg are associated with a reduced risk of NTS bacteremia in healthy Malawian children.Citation45,Citation123 However, anti-LPS IgG are present in high concentrations in some HIV-infected individuals, and an excess of these antibodies has shown a lack of complement-mediated Salmonella killing in vitro,Citation79,Citation80 highlighting the need for further research into the identification of a serological correlate of iNTS protection.
Given the contribution of the main acquired risk factors (HIV, malaria and malnutrition) to the development of invasive non-typhoidal salmonellosis in sSA, it is likely that public health interventions aimed at reducing these risk factors can lower the burden of iNTS disease in Africa. Indeed, as shown by the successful ART program implemented in 2005 in Blantyre, a decline in iNTS disease can be achieved by reducing the incidence of HIV.Citation75 Similarly, the strong epidemiological association between malaria and iNTS diseaseCitation8 suggests that implementing strategies to control the transmission of malaria parasites (such as the very recent roll-out of the anti-malaria vaccine RTS,S (GlaxoSmithKline) in Malawi, Ghana and Kenya) might yield similar results. Moreover, reducing malnutrition would also contribute enormously to indirectly reducing the incidence of iNTS disease. All these efforts are of the greatest importance, as the iNTS vaccines that are at the most advanced stage of development are currently in the preclinical or early clinical phase, and thus will not be available for human use in the very near future.
The introduction of a trivalent typhoid-iNTS conjugate vaccine (iNTS COPS:FliC conjugates coupled with Typbar-TCVCitation159), which is currently in clinical phase 1, constitutes an attractive approach, as this formulation could prevent both typhoid fever and invasive non-typhoidal salmonellosis. Given the high incidence of typhoid fever and iNTS disease in sSA, the use a trivalent formulation capable of covering S. Typhi, S. Typhimurium, and S. Enteritidis may be able to lower the overall burden of invasive Salmonella infections in Africa. Moreover, an economically sustainable GMMA-based bivalent vaccine against the African invasive S. Typhimurium and S. Enteriditis pathovars will enter clinical phase 1 in the coming months. The GMMA method allows high-yield (roughly 100,000,000 doses of vaccine per year even in low-capacity facilitiesCitation169) and low-cost vaccine production, hence constituting an ideal platform for low-income countries.
The introduction of iNTS vaccination in sSA would be accelerated by research to define a clear correlate of protection, the implementation of controlled human challenge models and the possibility to perform large-scale immunogenicity, efficacy and safety trials at multiple African sites afflicted by iNTS. Global research and development funding and broad governmental support are necessary in order to proceed in this direction.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- World Health Organization. WHO estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007–2015. Accessed 2019 Apr 4]. https://apps.who.int/iris/bitstream/handle/10665/199350/9789241565165_eng.pdf
- Ryan MP, O’Dwyer J, Adley CC. Evaluation of the complex nomenclature of the clinically and veterinary significant pathogen salmonella. Biomed Res Int. 2017;2017:3782182. doi:10.1155/2017/3782182.
- Khan CM. The dynamic interactions between salmonella and the microbiota, within the challenging niche of the gastrointestinal tract. Int Sch Res Notices. 2014;2014:846049. doi:10.1155/2014/846049.
- Gordon MA, Kankwatira AM, Mwafulirwa G, Walsh AL, Hopkins MJ, Parry CM, Faragher EB, Zijlstra EE, Heyderman RS, Molyneux ME. Invasive non-typhoid salmonellae establish systemic intracellular infection in HIV-infected adults: an emerging disease pathogenesis. Clin Infect Dis. 2010;50(7):953–62. doi:10.1086/651080.
- Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive salmonella infections. Clin Microbiol Rev. 2015;28(4):901–37. doi:10.1128/CMR.00002-15.
- Stanaway JD, Parisi A, Sarkar K, Blacker BF, Reiner RC, Hay SI, Nixon MR, Dolecek C, James SL, Mokdad AH; GBD 2017 Non-Typhoidal Salmonella Invasive Disease Collaborators. The global burden of non-typhoidal salmonella invasive disease: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2019;19(12):1312–24. doi:10.1016/S1473-3099(19)30418-9.
- Msefula CL, Olgemoeller F, Jambo N, Segula D, Van Tan T, Nyirenda TS, Nedi W, Kennedy N, Graham M, Henrion MYR, et al. Ascertaining the burden of invasive Salmonella disease in hospitalised febrile children aged under four years in Blantyre, Malawi. PLoS Negl Trop Dis. 2019;13(7):e0007539. doi:10.1371/journal.pntd.0007539.
- Park SE, Pak GD, Aaby P, Adu-Sarkodie Y, Ali M, Aseffa A, Biggs HM, Bjerregaard-Andersen M, Breiman RF, Crump JA, et al. The relationship between invasive nontyphoidal salmonella disease, other bacterial bloodstream infections, and Malaria in Sub-Saharan Africa. Clin Infect Dis. 2016;62(Suppl 1):S23–S31. doi:10.1093/cid/civ893.
- Uche IV, MacLennan CA, Saul A, Baker S. A systematic review of the incidence, risk factors and case fatality rates of Invasive Nontyphoidal Salmonella (iNTS) Disease in Africa (1966 to 2014). PLOS Negl Trop Dis. 2017;11(1):e0005118. doi:10.1371/journal.pntd.0005118.
- Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet. 2012;379(9835):2489–99. doi:10.1016/S0140-6736(11)61752-2.
- Kingsley RA, Msefula CL, Thomson NR, Kariuki S, Holt KE, Gordon MA, Harris D, Clarke L, Whitehead S, Sangal V, et al. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res. 2009;19(12):2279–87. doi:10.1101/gr.091017.109.
- Okoro CK, Kingsley RA, Connor TR, Harris SR, Parry CM, Al-Mashhadani MN, Kariuki S, Msefula CL, Gordon MA, de Pinna E, et al. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat Genet. 2012;44(11):1215–21. doi:10.1038/ng.2423.
- Feasey NA, Hadfield J, Keddy KH, Dallman TJ, Jacobs J, Deng X, Wigley P, Barquist L, Langridge GC, Feltwell T, et al. Distinct Salmonella Enteritidis lineages associated with enterocolitis in high-income settings and invasive disease in low-income settings. Nat Genet. 2016;48(10):1211–17. doi:10.1038/ng.3644.
- Kurtz JR, Goggins JA, McLachlan JB. Salmonella infection: interplay between the bacteria and host immune system. Immunol Lett. 2017;190:42–50. doi:10.1016/j.imlet.2017.07.006.
- Gordon MA. Invasive nontyphoidal Salmonella disease: epidemiology, pathogenesis and diagnosis. Curr Opin Infect Dis. 2011;24(5):484–89. doi:10.1097/QCO.0b013e32834a9980.
- Kariuki S, Revathi G, Gakuya F, Yamo V, Muyodi J, Hart CA. Lack of clonal relationship between non-typhi Salmonella strain types from humans and those isolated from animals living in close contact. FEMS Immunol Med Microbiol. 2002;33(3):165–71. doi:10.1111/j.1574-695X.2002.tb00587.x.
- Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Muyodi J, Githinji JW, Kagendo D, Munyalo A, Hart CA. Invasive multidrug-resistant non-typhoidal Salmonella infections in Africa: zoonotic or anthroponotic transmission? J Med Microbiol. 2006;55(Pt 5):585–91. doi:10.1099/jmm.0.46375-0.
- Post AS, Diallo SN, Guiraud I, Lompo P, Tahita MC, Maltha J, Van Puyvelde S, Mattheus W, Ley B, Thriemer K, et al. Supporting evidence for a human reservoir of invasive non-Typhoidal Salmonella from household samples in Burkina Faso. PLoS Negl Trop Dis. 2019;13(10):e0007782. doi:10.1371/journal.pntd.0007782.
- Gilchrist JJ, MacLennan CA, Hill AV. Genetic susceptibility to invasive Salmonella disease. Nat Rev Immunol. 2015;15(7):452–63. doi:10.1038/nri3858.
- Gordon MA, Graham SM, Walsh AL, Wilson L, Phiri A, Molyneux E, Zijlstra EE, Heyderman RS, Hart CA, Molyneux ME. Epidemics of invasive Salmonella enterica serovar enteritidis and S. enterica Serovar typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis. 2008;46(7):963–69. doi:10.1086/529146.
- Feasey NA, Masesa C, Jassi C, Faragher EB, Mallewa J, Mallewa M, MacLennan CA, Msefula C, Heyderman RS, Gordon MA. Three epidemics of invasive multidrug-resistant Salmonella Bloodstream infection in Blantyre, Malawi, 1998–2014. Clin Infect Dis. 2015;61(Suppl 4):S363–S371. doi:10.1093/cid/civ691.
- Akullian A, Montgomery JM, John-Stewart G, Miller SI, Hayden HS, Radey MC, Hager KR, Verani JR, Ochieng JB, Juma J, et al. Multi-drug resistant non-typhoidal Salmonella associated with invasive disease in western Kenya. PLoS Negl Trop Dis. 2018;12(1):e0006156. doi:10.1371/journal.pntd.0006156.
- Chu C, Chiu CH. Evolution of the virulence plasmids of non-typhoid Salmonella and its association with antimicrobial resistance. Microbes Infect. 2006;8(7):1931–36. doi:10.1016/j.micinf.2005.12.026.
- Rodríguez I, Rodicio MR, Guerra B, Hopkins KL. Potential international spread of multidrug-resistant invasive Salmonella enterica serovar enteritidis. Emerg Infect Dis. 2012;18(7):1173–76. doi:10.3201/eid1807.120063.
- García V, García P, Rodríguez I, Rodicio R, Rodicio MR. The role of IS26 in evolution of a derivative of the virulence plasmid of Salmonella enterica serovar Enteritidis which confers multiple drug resistance. Infect Genet Evol. 2016;45:246–49. doi:10.1016/j.meegid.2016.09.008.
- Moran NA, Plague GR. Genomic changes following host restriction in bacteria. Curr Opin Genet Dev. 2004;14(6):627–33. doi:10.1016/j.gde.2004.09.003.
- García V, Mandomando I, Ruiz J, Herrera-León S, Alonso PL, Rodicio MR. Salmonella enterica serovars typhimurium and enteritidis causing mixed infections in febrile children in Mozambique. Infect Drug Resist. 2018;11:195–204. doi:10.2147/IDR.S147243.
- Msefula CL, Kingsley RA, Gordon MA, Molyneux E, Molyneux ME, MacLennan CA, Dougan G, Heyderman RS, Mantis NJ. Genotypic homogeneity of multidrug resistant S. Typhimurium infecting distinct adult and childhood susceptibility groups in Blantyre, Malawi. PLoS One. 2012;7(7):e42085. doi:10.1371/journal.pone.0042085.
- Kariuki S, Okoro C, Kiiru J, Njoroge S, Omuse G, Langridge G, Kingsley RA, Dougan G, Revathi G. Ceftriaxone-resistant Salmonella enterica serotype typhimurium sequence type 313 from Kenyan patients is associated with the blaCTX-M-15 gene on a novel IncHI2 plasmid. Antimicrob Agents Chemother. 2015;59(6):3133–39. doi:10.1128/AAC.00078-15.
- Oneko M, Kariuki S, Muturi-Kioi V, Otieno K, Otieno VO, Williamson JM, Folster J, Parsons MB, Slutsker L, Mahon BE, et al. Emergence of community-acquired, multidrug-resistant invasive nontyphoidal salmonella disease in Rural Western Kenya, 2009–2013. Clin Infect Dis. 2015;61(Suppl 4):S310–S316. doi:10.1093/cid/civ674.
- Van Puyvelde S, Pickard D, Vandelannoote K, Heinz E, Barbé B, de Block T, Clare S, Coomber EL, Harcourt K, Sridhar S, et al. An African Salmonella Typhimurium ST313 sublineage with extensive drug-resistance and signatures of host adaptation. Nat Commun. 2019;10(1):4280. doi:10.1038/s41467-019-11844-z.
- Lunguya O, Lejon V, Phoba MF, Bertrand S, Vanhoof R, Glupczynski Y, Verhaegen J, Muyembe-Tamfum JJ, Jacobs J. Antimicrobial resistance in invasive non-typhoid Salmonella from the Democratic Republic of the Congo: emergence of decreased fluoroquinolone susceptibility and extended-spectrum beta lactamases. PLoS Negl Trop Dis. 2013;7(3):e2103. doi:10.1371/journal.pntd.0002103.
- Kagambèga A, Lienemann T, Frye JG, Barro N, Haukka K. Whole genome sequencing of multidrug-resistant Salmonella enterica serovar Typhimurium isolated from humans and poultry in Burkina Faso. Trop Med Health. 2018;46:4. doi:10.1186/s41182-018-0086-9.
- Harrois D, Breurec S, Seck A, Delauné A, Le Hello S, Pardos de la Gándara M, Sontag L, Perrier-Gros-Claude JD, Sire JM, Garin B, et al. Prevalence and characterization of extended-spectrum beta-lactamase-producing clinical Salmonella enterica isolates in Dakar, Senegal, from 1999 to 2009. Clin Microbiol Infect. 2014;20(2):O109–O116. doi:10.1111/1469-0691.12339.
- Akinyemi KO, Iwalokun BA, Oyefolu AO, Fakorede CO. Occurrence of extended-spectrum and AmpC beta-lactamases in multiple drug resistant Salmonella isolates from clinical samples in Lagos, Nigeria. Infect Drug Resist. 2017;10:19–25. doi:10.2147/IDR.S123646.
- Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, Langley JM, Wanke C, Warren CA, Cheng AC, et al. 2017 infectious diseases society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65(12):e45–e80. doi:10.1093/cid/cix669.
- Cuypers WL, Jacobs J, Wong V, Klemm EJ, Deborggraeve S, Van Puyvelde S. Fluoroquinolone resistance in Salmonella: insights by whole-genome sequencing. Microb Genom. 2018;4:7. doi:10.1099/mgen.0.000195.
- Tadesse G, Tessema TS, Beyene G, Aseffa A, Chabalgoity JA. Molecular epidemiology of fluoroquinolone resistant Salmonella in Africa: a systematic review and meta-analysis. PLoS One. 2018;13(2):e0192575. doi:10.1371/journal.pone.0192575.
- Owen SV, Wenner N, Canals R, Makumi A, Hammarlöf DL, Gordon MA, Aertsen A, Feasey NA, Hinton JC. Characterization of the prophage repertoire of African Salmonella Typhimurium ST313 reveals high levels of spontaneous induction of novel phage BTP1. Front Microbiol. 2017;8:235. doi:10.3389/fmicb.2017.00235.
- Ashton PM, Owen SV, Kaindama L, Rowe WPM, Lane CR, Larkin L, Nair S, Jenkins C, de Pinna EM, Feasey NA, et al. Public health surveillance in the UK revolutionises our understanding of the invasive Salmonella Typhimurium epidemic in Africa. Genome Med. 2017;9(1):92. doi:10.1186/s13073-017-0480-7.
- Herrero-Fresno A, Wallrodt I, Leekitcharoenphon P, Olsen JE, Aarestrup FM, Hendriksen RS, Bengoechea JA. The role of the st313-td gene in virulence of Salmonella Typhimurium ST313. PLoS One. 2014;9(1):e84566. doi:10.1371/journal.pone.0084566.
- Kintz E, Davies MR, Hammarlöf DL, Canals R, Hinton JC, van der Woude MW. A BTP1 prophage gene present in invasive non-typhoidal Salmonella determines composition and length of the O-antigen of the lipopolysaccharide. Mol Microbiol. 2015;96(2):263–75. doi:10.1111/mmi.12933.
- Onsare RS, Micoli F, Lanzilao L, Alfini R, Okoro CK, Muigai AW, Revathi G, Saul A, Kariuki S, MacLennan CA, et al. Relationship between antibody susceptibility and lipopolysaccharide O-antigen characteristics of invasive and gastrointestinal nontyphoidal Salmonellae isolates from Kenya. PLoS Negl Trop Dis. 2015;9(3):e0003573. doi:10.1371/journal.pntd.0003573.
- Marshall JM, Gunn JS, Bäumler AJ. The O-antigen capsule of salmonella enterica serovar typhimurium facilitates serum resistance and surface expression of FliC. Infect Immun. 2015;83(10):3946–59. doi:10.1128/IAI.00634-15.
- MacLennan CA, Gondwe EN, Msefula CL, Kingsley RA, Thomson NR, White SA, Goodall M, Pickard DJ, Graham SM, Dougan G, et al. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J Clin Invest. 2008;118(4):1553–62. doi:10.1172/JCI33998.
- Thomson NR, Clayton DJ, Windhorst D, Vernikos G, Davidson S, Churcher C, Quail MA, Stevens M, Jones MA, Watson M, et al. Comparative genome analysis of Salmonella Enteritidis PT4 and Salmonella Gallinarum 287/91 provides insights into evolutionary and host adaptation pathways. Genome Res. 2008;18(10):1624–37. doi:10.1101/gr.077404.108.
- Parkhill J, Dougan G, James KD, Thomson NR, Pickard D, Wain J, Churcher C, Mungall KL, Bentley SD, Holden MT, et al. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature. 2001;413(6858):848–52. doi:10.1038/35101607.
- McClelland M, Sanderson KE, Clifton SW, Latreille P, Porwollik S, Sabo A, Meyer R, Bieri T, Ozersky P, McLellan M, et al. Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat Genet. 2004;36(12):1268–74. doi:10.1038/ng1470.
- Liu WQ, Feng Y, Wang Y, Zou QH, Chen F, Guo JT, Peng YH, Jin Y, Li YG, Hu SN, et al. Salmonella paratyphi C: genetic divergence from Salmonella choleraesuis and pathogenic convergence with Salmonella typhi. PLoS One. 2009;4(2):e4510. doi:10.1371/journal.pone.0004510.
- McClelland M, Sanderson KE, Spieth J, Clifton SW, Latreille P, Courtney L, Porwollik S, Ali J, Dante M, Du F, et al. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature. 2001;413(6858):852–56. doi:10.1038/35101614.
- Canals R, Hammarlöf DL, Kröger C, Owen SV, Fong WY, Lacharme-Lora L, Zhu X, Wenner N, Carden SE, Honeycutt J, et al. Adding function to the genome of African Salmonella Typhimurium ST313 strain D23580. PLoS Biol. 2019;17(1):e3000059. doi:10.1371/journal.pbio.3000059.
- Kingsley RA, Kay S, Connor T, Barquist L, Sait L, Holt KE, Sivaraman K, Wileman T, Goulding D, Clare S, et al. Genome and transcriptome adaptation accompanying emergence of the definitive type 2 host-restricted Salmonella enterica serovar Typhimurium pathovar. Mbio. 2013;4(5):e00565–e00513. doi:10.1128/mBio.00565-13.
- Brink T, Leiss V, Siegert P, Jehle D, Ebner JK, Schwan C, Shymanets A, Wiese S, Nürnberg B, Hensel M, et al. Salmonella Typhimurium effector SseI inhibits chemotaxis and increases host cell survival by deamidation of heterotrimeric Gi proteins. PLoS Pathog. 2018;14(8):e1007248. doi:10.1371/journal.ppat.1007248.
- Carden SE, Walker GT, Honeycutt J, Lugo K, Pham T, Jacobson A, Bouley D, Idoyaga J, Tsolis RM, Monack D. Pseudogenization of the secreted effector gene ssei confers rapid systemic dissemination of S. Typhimurium ST313 within migratory dendritic cells. Cell Host Microbe. 2017;21(2):182–94. doi:10.1016/j.chom.2017.01.009.
- Porwollik S, Santiviago CA, Cheng P, Florea L, Jackson S, McClelland M. Differences in gene content between Salmonella enterica serovar enteritidis isolates and comparison to closely related serovars Gallinarum and Dublin. J Bacteriol. 2005;187(18):6545–55. doi:10.1128/JB.187.18.6545-6555.2005.
- Feng Y, Johnston RN, Liu G-R, Liu S-L, Chakravortty D. Genomic comparison between Salmonella Gallinarum and Pullorum: differential pseudogene formation under common host restriction. PLoS One. 2013;8(3):e59427. doi:10.1371/journal.pone.0059427.
- Klemm EJ, Gkrania-Klotsas E, Hadfield J, Forbester JL, Harris SR, Hale C, Heath JN, Wileman T, Clare S, Kane L, et al. Emergence of host-adapted Salmonella Enteritidis through rapid evolution in an immunocompromised host. Nat Microbiol. 2016;1:15023. doi:10.1038/nmicrobiol.2015.23.
- Cummings LA, Barrett SL, Wilkerson WD, Fellnerova I, Cookson BT. FliC-specific CD4+ T cell responses are restricted by bacterial regulation of antigen expression. J Immunol. 2005;174(12):7929–38. doi:10.4049/jimmunol.174.12.7929.
- Yim L, Sasías S, Martínez A, Betancor L, Estevez V, Scavone P, Bielli A, Sirok A, Chabalgoity JA, Bäumler AJ. Repression of flagella is a common trait in field isolates of Salmonella enterica serovar Dublin and is associated with invasive human infections. Infect Immun. 2014;82(4):1465–76. doi:10.1128/IAI.01336-13.
- Winter SE, Raffatellu M, Wilson RP, Rüssmann H, Bäumler AJ. The Salmonella enterica serotype Typhi regulator TviA reduces interleukin-8 production in intestinal epithelial cells by repressing flagellin secretion. Cell Microbiol. 2008;10(1):247–61. doi:10.1111/j.1462-5822.2007.01037.x.
- Ramachandran G, Perkins DJ, Schmidlein PJ, Tulapurkar ME, Tennant SM, Baker S. Invasive Salmonella Typhimurium ST313 with naturally attenuated flagellin elicits reduced inflammation and replicates within macrophages. PLoS Negl Trop Dis. 2015;9(1):e3394. doi:10.1371/journal.pntd.0003394.
- Carden S, Okoro C, Dougan G, Monack D. Non-typhoidal Salmonella Typhimurium ST313 isolates that cause bacteremia in humans stimulate less inflammasome activation than ST19 isolates associated with gastroenteritis. Pathog Dis. 2015;73:4. doi:10.1093/femspd/ftu023.
- Lokken KL, Stull-Lane AR, Poels K, Tsolis RM, Raffatellu M. Malaria parasite-mediated alteration of macrophage function and increased iron availability predispose to disseminated nontyphoidal salmonella infection. Infect Immun. 2018;86(9):9. doi:10.1128/IAI.00301-18.
- Hammarlöf DL, Kröger C, Owen SV, Canals R, Lacharme-Lora L, Wenner N, Schager AE, Wells TJ, Henderson IR, Wigley P, et al. Role of a single noncoding nucleotide in the evolution of an epidemic African clade of Salmonella. Proc Natl Acad Sci U S A. 2018;115(11):E2614–e2623. doi:10.1073/pnas.1714718115.
- Faucher SP, Porwollik S, Dozois CM, McClelland M, Daigle F. Transcriptome of Salmonella enterica serovar Typhi within macrophages revealed through the selective capture of transcribed sequences. Proc Natl Acad Sci U S A. 2006;103(6):1906–11. doi:10.1073/pnas.0509183103.
- Valenzuela LM, Hidalgo AA, Rodríguez L, Urrutia IM, Ortega AP, Villagra NA, Paredes-Sabja D, Calderón IL, Gil F, Saavedra CP, et al. Pseudogenization of sopA and sopE2 is functionally linked and contributes to virulence of Salmonella enterica serovar Typhi. Infect Genet Evol. 2015;33:131–42. doi:10.1016/j.meegid.2015.04.021.
- Okoro CK, Barquist L, Connor TR, Harris SR, Clare S, Stevens MP, Arends MJ, Hale C, Kane L, Pickard DJ, et al. Signatures of adaptation in human invasive Salmonella Typhimurium ST313 populations from sub-Saharan Africa. PLoS Negl Trop Dis. 2015;9(3):e0003611. doi:10.1371/journal.pntd.0003611.
- Kingsley RA, Humphries AD, Weening EH, De Zoete MR, Winter S, Papaconstantinopoulou A, Dougan G, Bäumler AJ. Molecular and phenotypic analysis of the CS54 island of Salmonella enterica serotype typhimurium: identification of intestinal colonization and persistence determinants. Infect Immun. 2003;71(2):629–40. doi:10.1128/iai.71.2.629-640.2003.
- Bogomolnaya LM, Andrews KD, Talamantes M, Maple A, Ragoza Y, Vazquez-Torres A, Andrews-Polymenis H, Swanson M. The ABC-type efflux pump MacAB protects Salmonella enterica serovar typhimurium from oxidative stress. Mbio. 2013;4(6):e00630–e00613. doi:10.1128/mBio.00630-13.
- Feasey NA, Archer BN, Heyderman RS, Sooka A, Dennis B, Gordon MA, Keddy KH. Typhoid fever and invasive nontyphoidal salmonellosis, Malawi and South Africa. Emerg Infect Dis. 2010;16:1448–51. doi:10.3201/eid1609.100125.
- Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. Global burden of invasive nontyphoidal Salmonella disease, 2010(1). Emerg Infect Dis. 2015;21:6. doi:10.3201/eid2106.140999.
- Balasubramanian R, Im J, Lee J-S, Jeon HJ, Mogeni OD, Kim JH, Rakotozandrindrainy R, Baker S, Marks F. The global burden and epidemiology of invasive non-typhoidal Salmonella infections. Hum Vaccin Immunother. 2019;15(6):1421–26. doi:10.1080/21645515.2018.1504717.
- Keddy KH, Musekiwa A, Sooka A, Karstaedt A, Nana T, Seetharam S, Nchabaleng M, Lekalakala R, Angulo FJ, Klugman KP; for GERMS-SA. Clinical and microbiological features of invasive nontyphoidal Salmonella associated with HIV-infected patients, Gauteng Province, South Africa. Medicine (Baltimore). 2017;96(13):e6448. doi:10.1097/MD.0000000000006448.
- Bachou H, Tylleskär T, Kaddu-Mulindwa DH, Tumwine JK. Bacteraemia among severely malnourished children infected and uninfected with the human immunodeficiency virus-1 in Kampala, Uganda. BMC Infect Dis. 2006;6(1):160. doi:10.1186/1471-2334-6-160.
- Keddy KH, Takuva S, Musekiwa A, Puren AJ, Sooka A, Karstaedt A, Klugman KP, Angulo FJ, Vermund SH. An association between decreasing incidence of invasive nontyphoidal salmonellosis and increased use of antiretroviral therapy, Gauteng Province, South Africa, 2003–2013. PLoS One. 2017;12:e0173091. doi:10.1371/journal.pone.0173091.
- Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14(4):421–28. doi:10.1038/nm1743.
- Bailer RT, Holloway A, Sun J, Margolick JB, Martin M, Kostman J, Montaner LJ. IL-13 and IFN-gamma secretion by activated T cells in HIV-1 infection associated with viral suppression and a lack of disease progression.. J Immunol. 1999;162:7534–42.
- Clerici M, Shearer GM. A TH1–>TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14(3):107–11. doi:10.1016/0167-5699(93)90208-3.
- MacLennan CA, Gilchrist JJ, Gordon MA, Cunningham AF, Cobbold M, Goodall M, Kingsley RA, van Oosterhout JJ, Msefula CL, Mandala WL, et al. Dysregulated humoral immunity to nontyphoidal Salmonella in HIV-infected African adults. Science. 2010;328(5977):508–12. doi:10.1126/science.1180346.
- Goh YS, Necchi F, O’Shaughnessy CM, Micoli F, Gavini M, Young SP, Msefula CL, Gondwe EN, Mandala WL, Gordon MA, et al. Bactericidal immunity to Salmonella in Africans and mechanisms causing its failure in HIV infection. PLoS Negl Trop Dis. 2016;10(4):e0004604. doi:10.1371/journal.pntd.0004604.
- Siggins MK, O’Shaughnessy CM, Pravin J, Cunningham AF, Henderson IR, Drayson MT, MacLennan CA. Differential timing of antibody-mediated phagocytosis and cell-free killing of invasive African Salmonella allows immune evasion. Eur J Immunol. 2014;44(4):1093–98. doi:10.1002/eji.201343529.
- Mooney JP, Butler B 2, Lokken K 1, Xavier MN, Chau JY, Schaltenberg N, Dandekar S, George MD, Santos RL, Luckhart S, et al. The mucosal inflammatory response to non-typhoidal Salmonella in the intestine is blunted by IL-10 during concurrent malaria parasite infection. Mucosal Immunol. 2014;7(6):1302–11. doi:10.1038/mi.2014.18.
- Huang H, Lamikanra AA, Alkaitis MS, Thézénas ML, Ramaprasad A, Moussa E, Roberts DJ, Casals-Pascual C, Craig AG. Interleukin-10 regulates hepcidin in Plasmodium falciparum malaria. PLoS One. 2014;9(2):e88408. doi:10.1371/journal.pone.0088408.
- Nix RN, Altschuler SE, Henson PM, Detweiler CS, Isberg RR. Hemophagocytic macrophages harbor Salmonella enterica during persistent infection. PLoS Pathogens. 2007;3(12):e193. doi:10.1371/journal.ppat.0030193.
- Roux CM, Butler BP, Chau JY, Paixao TA, Cheung KW, Santos RL, Luckhart S, Tsolis RM. Both hemolytic anemia and malaria parasite-specific factors increase susceptibility to Nontyphoidal Salmonella enterica serovar typhimurium infection in mice. Infect Immun. 2010;78(4):1520–27. doi:10.1128/IAI.00887-09.
- Nairz M, Schroll A, Sonnweber T, Weiss G. The struggle for iron – a metal at the host-pathogen interface. Cell Microbiol. 2010;12(12):1691–702. doi:10.1111/j.1462-5822.2010.01529.x.
- Cunnington AJ, de Souza JB, Walther M, Riley EM. Malaria impairs resistance to Salmonella through heme- and heme oxygenase–dependent dysfunctional granulocyte mobilization. Nat Med. 2012;18(1):120–27. doi:10.1038/nm.2601.
- Cunnington AJ, Njie M, Correa S, Takem EN, Riley EM, Walther M. Prolonged neutrophil dysfunction after Plasmodium falciparum malaria is related to hemolysis and heme oxygenase-1 induction. J Immunol. 2012;189(11):5336–46. doi:10.4049/jimmunol.1201028.
- Nyirenda TS, Nyirenda JT, Tembo DL, Storm J, Dube Q, Msefula CL, Jambo KC, Mwandumba HC, Heyderman RS, Gordon MA, et al. Loss of humoral and cellular immunity to invasive nontyphoidal Salmonella during current or convalescent Plasmodium falciparum infection in Malawian children. Clin Vaccine Immunol. 2017;24(7):7. doi:10.1128/CVI.00057-17.
- Martinsen TC, Bergh K, Waldum HL. Gastric juice: a barrier against infectious diseases. Basic Clin Pharmacol Toxicol. 2005;96(2):94–102. doi:10.1111/j.1742-7843.2005.pto960202.x.
- Jose DG, Shelton M, Tauro GP, Belbin R, Hosking CS. Deficiency of immunological and phagocytic function in aboriginal children with protein-calorie malnutrition. Med J Aust. 1975;2(18):699–705. doi:10.5694/j.1326-5377.1975.tb106221.x.
- Vásquez-Garibay E, Campollo-Rivas O, Romero-Velarde E, Méndez-Estrada C, García-Iglesias T, Alvizo-Mora JG, Vizmanos-Lamotte B. Effect of renutrition on natural and cell-mediated immune response in infants with severe malnutrition. J Pediatr Gastroenterol Nutr. 2002;34(3):296–301. doi:10.1097/00005176-200203000-00015.
- Nájera O, González C, Toledo G, López L, Ortiz R. Flow cytometry study of lymphocyte subsets in malnourished and well-nourished children with bacterial infections. Clin Diagn Lab Immunol. 2004;11(3):577–80. doi:10.1128/CDLI.11.3.577-580.2004.
- Nájera O, González C, Toledo G, López L, Cortés E, Betancourt M, Ortiz R. CD45RA and CD45RO isoforms in infected malnourished and infected well-nourished children. Clin Exp Immunol. 2001;126(3):461–65. doi:10.1046/j.1365-2249.2001.01694.x.
- Nájera O, González C, Cortés E, Toledo G, Ortiz R. Effector T lymphocytes in well-nourished and malnourished infected children. Clin Exp Immunol. 2007;148(3):501–06. doi:10.1111/j.1365-2249.2007.03369.x.
- Gilchrist JJ, MacLennan CA. Invasive nontyphoidal Salmonella disease in Africa. EcoSal Plus. 2019;8(2):2. doi:10.1128/ecosalplus.ESP-0007-2018.
- Roos D. Chronic granulomatous disease. Br Med Bull. 2016;118(1):50–63. doi:10.1093/bmb/ldw009.
- International Union of Immunological Societies Expert Committee on Primary Immunodeficiencies; Notarangelo LD, Fischer A, Geha RS, Casanova J-L, Chapel H, Conley ME, Cunningham-Rundles C, Etzioni A, Hammartröm L, Nonoyama S, et al. Primary immunodeficiencies: 2009 update. J Allergy Clin Immunol. 2009;124(6):1161–78. doi:10.1016/j.jaci.2009.10.013.
- MacLennan CA. Antibodies and protection against invasive salmonella disease. Front Immunol. 2014 Dec 22;5:635. doi:10.3389/fimmu.2014.00635.
- Humbert JR, Winsor EL, Githens JM, Schmitz JB. Neutrophil dysfunctions in sickle cell disease. Biomed Pharmacother. 1990;44(3):153–58. doi:10.1016/0753-3322(90)90002-q.
- Evans C, Orf K, Horvath E, Levin M, De La Fuente J, Chakravorty S, Cunnington AJ. Impairment of neutrophil oxidative burst in children with sickle cell disease is associated with heme oxygenase-1. Haematologica. 2015;100(12):1508–16. doi:10.3324/haematol.2015.128777.
- Falcão RP, Donadi EA. [Infection and immunity in sickle cell disease]. AMB Rev Assoc Med Bras. 1989;35:70–74.
- Sanhadji K, Chout R, Gessain A, Sasco AJ, Yoyo M, Mezard F, de The G, Touraine JL. Cell-mediated immunity in patients with sickle cell anaemia. Thymus. 1988;12:203–13.
- Hand WL, King NL. Deficiency of serum bactericidal activity against Salmonella typhimurium in sickle cell anaemia. Clin Exp Immunol. 1977;30:262–70.
- Williams TN, Uyoga S, Macharia A, Ndila C, McAuley CF, Opi DH, Mwarumba S, Makani J, Komba A, Ndiritu MN, et al. Bacteraemia in Kenyan children with sickle-cell anaemia: a retrospective cohort and case-control study. Lancet. 2009;374(9698):1364–70. doi:10.1016/S0140-6736(09)61374-X.
- Makani J, Mgaya J, Balandya E, Msami K, Soka D, Cox SE, Komba AN, Rwezaula S, Meda E, Muturi D, et al. Bacteraemia in sickle cell anaemia is associated with low haemoglobin: a report of 890 admissions to a tertiary hospital in Tanzania. Br J Haematol. 2015;171(2):273–76. doi:10.1111/bjh.13553.
- Douamba S, Nagalo K, Tamini L, Traoré I, Kam M, Kouéta F, Yé D. [Major sickle cell syndromes and infections associated with this condition in children in Burkina Faso]. Pan Afr Med J. 2017;26:7. doi:10.11604/pamj.2017.26.7.9971.
- Alima Yanda AN, Nansseu JR, Mbassi Awa HD, Tatah SA, Seungue J, Eposse C, Koki PO. Burden and spectrum of bacterial infections among sickle cell disease children living in Cameroon. BMC Infect Dis. 2017;17(1):211. doi:10.1186/s12879-017-2317-9.
- Soothill G, Darboe S, Bah G, Bolarinde L, Cunnington A, Anderson ST. Invasive bacterial infections in Gambians with sickle cell anemia in an era of widespread pneumococcal and hemophilus influenzae type b vaccination. Medicine (Baltimore). 2016;95(49):e5512. doi:10.1097/MD.0000000000005512.
- Odey F, Okomo U, Oyo-Ita A. Vaccines for preventing invasive salmonella infections in people with sickle cell disease. Cochrane Database Syst Rev. 2009;(4)CD006975. doi:10.1002/14651858.CD006975.pub2.
- Gilchrist JJ, Rautanen A, Fairfax BP, Mills TC, Naranbhai V, Trochet H, Pirinen M, Muthumbi E, Mwarumba S, Njuguna P, et al. Risk of nontyphoidal Salmonella bacteraemia in African children is modified by STAT4. Nat Commun. 2018;9(1):1014. doi:10.1038/s41467-017-02398-z.
- Spees AM, Kingsbury DD, Wangdi T, Xavier MN, Tsolis RM, Bäumler AJ, Morrison RP. Neutrophils are a source of gamma interferon during acute Salmonella enterica serovar Typhimurium colitis. Infect Immun. 2014;82(4):1692–97. doi:10.1128/IAI.01508-13.
- Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med. 2000;192(2):227–36. doi:10.1084/jem.192.2.227.
- Fierer J. Polymorphonuclear leukocytes and innate immunity to Salmonella infections in mice. Microb Infect. 2001;3(14–15):1233–37. doi:10.1016/s1286-4579(01)01483-6.
- Cheminay C, Chakravortty D, Hensel M. Role of neutrophils in murine salmonellosis. Infect Immun. 2004;72(1):468–77. doi:10.1128/iai.72.1.468-477.2004.
- Conlan JW. Neutrophils prevent extracellular colonization of the liver microvasculature by Salmonella typhimurium. Infect Immun. 1996;64(3):1043–47. doi:10.1128/IAI.64.3.1043-1047.1996.
- Muotiala A, Mäkelä PH. Role of gamma interferon in late stages of murine salmonellosis. Infect Immun. 1993;61(10):4248–53. doi:10.1128/IAI.61.10.4248-4253.1993.
- Mittrücker HW, Kaufmann SH. Immune response to infection with Salmonella typhimurium in mice. J Leukoc Biol. 2000;67(4):457–63. doi:10.1002/jlb.67.4.457.
- Hughes EA, Galán JE. Immune response to Salmonella: location, location, location? Immunity. 2002;16(3):325–28. doi:10.1016/s1074-7613(02)00293-5.
- Barr TA, Brown S, Mastroeni P, Gray D. B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c. J Immunol. 2009;183:1005–12. doi:10.4049/jimmunol.0803706.
- Gondwe EN, Molyneux ME, Goodall M, Graham SM, Mastroeni P, Drayson MT, MacLennan CA. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proc Natl Acad Sci U S A. 2010;107(7):3070–75. doi:10.1073/pnas.0910497107.
- Rondini S, Lanzilao L, Necchi F, O’Shaughnessy CM, Micoli F, Saul A, MacLennan CA. Invasive African Salmonella Typhimurium induces bactericidal antibodies against O-antigens. Microb Pathog. 2013;63:19–23. doi:10.1016/j.micpath.2013.05.014.
- Nyirenda TS, Gilchrist JJ, Feasey NA, Glennie SJ, Bar-Zeev N, Gordon MA, MacLennan CA, Mandala WL, Heyderman RS. Sequential acquisition of T cells and antibodies to nontyphoidal Salmonella in Malawian children. J Infect Dis. 2014;210(1):56–64. doi:10.1093/infdis/jiu045.
- Ko HJ, Yang JY, Shim DH, Yang H, Park SM, Curtiss R 3rd, MN K. Innate immunity mediated by MyD88 signal is not essential for induction of lipopolysaccharide-specific B cell responses but is indispensable for protection against Salmonella enterica serovar Typhimurium infection. J Immunol. 2009;182(4):2305–12. doi:10.4049/jimmunol.0801980.
- MacLennan C, Fieschi C, DA L, Picard C, Dorman SE, Sanal O, MacLennan JM, Holland SM, Ottenhoff TH, Casanova JL, et al. Interleukin (IL)-12 and IL-23 are key cytokines for immunity against Salmonella in humans. J Infect Dis. 2004;190(10):1755–57. doi:10.1086/425021.
- Goh YS, Necchi F, Rondini S, O’Shaughnessy CM, Micoli F, Gavini M, Msefula CL, Gondwe EN, Mandala WL, Gordon MA, et al. Bactericidal potential of S. Typhimurium LPS-specific antibodies from HIV-infected African adults. Poster presented at: the 8th International Conference on Typhoid Fever and Other Invasive Salmonelloses; 2013 Mar 1–2;Dhaka, Bangladesh.
- Mooney JP, Lee S-J, Lokken KL, Nanton MR, Nuccio S-P, McSorley SJ, Tsolis RM, Ryan ET. Transient loss of protection afforded by a live attenuated non-typhoidal Salmonella vaccine in mice co-infected with malaria. PLOS Negl Trop Dis. 2015;9(9):e0004027. doi:10.1371/journal.pntd.0004027.
- Prendergast AJ. Malnutrition and vaccination in developing countries. Philos Trans R Soc Lond B Biol Sci. 2015;370(1671):20140141. doi:10.1098/rstb.2014.0141.
- Robbins JB, Pearson HA. Normal response of sickle cell anemia patients to immunization with salmonella vaccines. J Pediatr. 1965;66(5):877–82. doi:10.1016/s0022-3476(65)80062-2.
- Mastroeni P, Rossi O. Immunology, epidemiology and mathematical modelling towards a better understanding of invasive non-typhoidal Salmonella disease and rational vaccination approaches. Expert Rev Vaccines. 2016;15(12):1545–55. doi:10.1080/14760584.2016.1189330.
- MacLennan CA, Martin LB, Micoli F. Vaccines against invasive Salmonella disease. Hum Vaccin Immunother. 2014;10(6):1478–93. doi:10.4161/hv.29054.
- Sahastrabuddhe S 1, Saluja T. Overview of the typhoid conjugate vaccine pipeline: current status and future plans. Clin Infect Dis. 2019;68(Suppl Supplement_1):S22–S26. doi:10.1093/cid/ciy884.
- Hindle Z, Chatfield SN, Phillimore J, Bentley M, Johnson J, Cosgrove CA, Ghaem-Maghami M, Sexton A, Khan M, Brennan FR, et al. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect Immun. 2002;70(7):3457–67. doi:10.1128/iai.70.7.3457-3467.2002.
- Tennant SM, Wang J-Y, Galen JE, Simon R, Pasetti MF, Gat O, Levine MM, Fang FC. Engineering and preclinical evaluation of attenuated nontyphoidal Salmonella strains serving as live oral vaccines and as reagent strains. Infect Immun. 2011;79(10):4175–85. doi:10.1128/IAI.05278-11.
- Tennant SM, Schmidlein P, Simon R, Pasetti MF, Galen JE, Levine MM, Palmer GH. Refined live attenuated Salmonella enterica serovar Typhimurium and Enteritidis vaccines mediate homologous and heterologous serogroup protection in mice. Infect Immun. 2015;83(12):4504–12. doi:10.1128/IAI.00924-15.
- Li P, Liu Q, Luo H, Liang K, Yi J, Luo Y, Hu Y, Han Y, Kong Q. O-serotype conversion in Salmonella Typhimurium induces protective immune responses against invasive non-typhoidal Salmonella infections. Front Immunol. 2017;8:1647. doi:10.3389/fimmu.2017.01647.
- Zhao X, Dai Q, Jia R, Zhu D, Liu M, Wang M, Chen S, Sun K, Yang Q, Wu Y, et al. Two novel Salmonella bivalent vaccines confer dual protection against two salmonella serovars in Mice. Front Cell Infect Microbiol. 2017;7:391. doi:10.3389/fcimb.2017.00391.
- Ramachandran G, Panda A, Higginson EE, Ateh E, Lipsky MM, Sen S, Matson CA, Permala-Booth J, DeTolla LJ, Tennant SM. Virulence of invasive Salmonella Typhimurium ST313 in animal models of infection. PLOS Negl Trop Dis. 2017;11(8):e0005697. doi:10.1371/journal.pntd.0005697.
- Abd El Ghany M, Jansen A, Clare S, Hall L, Pickard D, Kingsley RA, Dougan G. Candidate live, attenuated Salmonella enterica serotype Typhimurium vaccines with reduced fecal shedding are immunogenic and effective oral vaccines. Infect Immun. 2007;75(4):1835–42. doi:10.1128/IAI.01655-06.
- Wang Y, Li J, Xiong K, Chen Z, Zheng C, Tan Y, Cong Y, Gerlach RG. Elimination of persistent vaccine bacteria of Salmonella enterica serovar Typhimurium in the guts of immunized mice by inducible expression of truncated YncE. PLoS One. 2017;12(6):e0179649. doi:10.1371/journal.pone.0179649.
- Van Immerseel F 1, Methner U, Rychlik I, Nagy B, Velge P, Martin G, Foster N, Ducatelle R, Barrow PA. Vaccination and early protection against non-host-specific Salmonella serotypes in poultry: exploitation of innate immunity and microbial activity. Epidemiol Infect. 2005;133(6):959–78. doi:10.1017/S0950268805004711.
- Shakya M, Colin-Jones R, Theiss-Nyland K, Voysey M, Pant D, Smith N, Liu X, Tonks S, Mazur O, Farooq YG, et al. Phase 3 efficacy analysis of a typhoid conjugate vaccine trial in Nepal. N Engl J Med. 2019;381(23):2209–18. doi:10.1056/NEJMoa1905047.
- Capeding MR, Teshome S, Saluja T, Syed KA, Kim DR, Park JY, Yang JS, Kim YH, Park J, Jo S-K. Safety and immunogenicity of a Vi-DT typhoid conjugate vaccine: phase I trial in healthy filipino adults and children. Vaccine. 2018;36(26):3794–801. doi:10.1016/j.vaccine.2018.05.038.
- Capeding MR, Alberto E, Sil A, Saluja T, Teshome S, Kim DR, Park JY, Yang JS, Chinaworapong S, Park J, et al. Immunogenicity, safety and reactogenicity of a Phase II trial of Vi-DT typhoid conjugate vaccine in healthy Filipino infants and toddlers: A preliminary report. Vaccine. 2019;S0264–410X(19)31302–7. doi:10.1016/j.vaccine.2019.09.074.
- Simon R, Tennant SM, Wang JY, Schmidlein PJ, Lees A, Ernst RK, Pasetti MF, Galen JE, Levine MM, Baümler AJ. Salmonella enterica serovar enteritidis core o polysaccharide conjugated to H:g,m Flagellin as a candidate vaccine for protection against invasive infection with S. enteritidis. Infect Immun. 2011;79(10):4240–49. doi:10.1128/IAI.05484-11.
- Carlin NI, Svenson SB, Lindberg AA. Role of monoclonal O-antigen antibody epitope specificity and isotype in protection against experimental mouse typhoid. Microb Pathog. 1987;2(3):171–83. doi:10.1016/0882-4010(87)90019-2.
- Svenson SB, Lindberg AA. Artificial Salmonella vaccines: salmonella typhimurium O-antigen-specific oligosaccharide-protein conjugates elicit protective antibodies in rabbits and mice. Infect Immun. 1981;32(2):490–96. doi:10.1128/IAI.32.2.490-496.1981.
- Rondini S, Micoli F, Lanzilao L, Gavini M, Alfini R, Brandt C, Clare S, Mastroeni P, Saul A, MacLennan CA. Design of glycoconjugate vaccines against invasive African Salmonella enterica serovar Typhimurium. Infect Immun. 2015;83(3):996–1007. doi:10.1128/IAI.03079-14.
- Baliban SM, Yang M, Ramachandran G, Curtis B, Shridhar S, Laufer RS, Wang JY, Van Druff J, Higginson EE, Hegerle N, et al. Development of a glycoconjugate vaccine to prevent invasive Salmonella Typhimurium infections in sub-Saharan Africa. PLOS Negl Trop Dis. 2017;11(4):e0005493. doi:10.1371/journal.pntd.0005493.
- Fiorino F, Rondini S, Micoli F, Lanzilao L, Alfini R, Mancini F, MacLennan CA, Medaglini D. Immunogenicity of a bivalent adjuvanted glycoconjugate vaccine against Salmonella Typhimurium and Salmonella Enteritidis. Front Immunol. 2017;8:168. doi:10.3389/fimmu.2017.00168.
- Lanzilao L, Stefanetti G, Saul A, MacLennan CA, Micoli F, Rondini S, Mantis NJ. Strain selection for generation of O-antigen-based glycoconjugate vaccines against invasive nontyphoidal Salmonella disease. PLoS One. 2015;10(10):e0139847. doi:10.1371/journal.pone.0139847.
- Alving CR, Beck Z, Matyas GR, Rao M. Liposomal adjuvants for human vaccines. Expert Opin Drug Deliv. 2016;13(6):807–16. doi:10.1517/17425247.2016.1151871.
- Tritama E, Riani C, Rudiansyah I, Hidayat A, Kharisnaeni SA, Retnoningrum DS. Evaluation of alum-based adjuvant on the immunogenicity of salmonella enterica serovar typhi conjugates vaccines. Hum Vaccin Immunother. 2018;14(6):1524–29. doi:10.1080/21645515.2018.1431599.
- Ramachandran G, Tennant SM, Boyd MA, Wang JY, Tulapurkar ME, Pasetti MF, Levine MM, Simon R, Hensel M. Functional activity of antibodies directed towards Flagellin proteins of non-typhoidal Salmonella. PLoS One. 2016;11(3):e0151875. doi:10.1371/journal.pone.0151875.
- Simon R, Wang JY, Boyd MA, Tulapurkar ME, Ramachandran G, Tennant SM, Pasetti M, Galen JE, Levine MM, Chakravortty D. Sustained protection in mice immunized with fractional doses of Salmonella enteritidis core and O polysaccharide-flagellin glycoconjugates. PLoS One. 2013;8(5):e64680. doi:10.1371/journal.pone.0064680.
- Soyer Y, Moreno Switt A, Davis MA, Maurer J, McDonough PL, Schoonmaker-Bopp DJ, Dumas NB, Root T, Warnick LD, Gröhn YT, et al. Salmonella enterica serotype 4,5,12: i:-,an emerging Salmonella serotype that represents multiple distinct clones. J Clin Microbiol. 2009;47(11):3546–56. doi:10.1128/JCM.00546-09.
- Baliban SM, Curtis B, Toema D, Tennant SM, Levine MM, Pasetti MF, Simon R, Darton TC. Immunogenicity and efficacy following sequential parenterally-administered doses of Salmonella enteritidis COPS:FliC glycoconjugates in infant and adult mice. PLOS Negl Trop Dis. 2018;12(5):e0006522. doi:10.1371/journal.pntd.0006522.
- Baliban SM, Curtis B, Amin MN, Levine MM, Pasetti MF, Simon R. Maternal antibodies elicited by immunization with an O- polysaccharide glycoconjugate vaccine protect infant mice against lethal Salmonella Typhimurium infection. Front Immunol. 2019;10:2124. doi:10.3389/fimmu.2019.02124.
- Baliban SM, Allen JC, Curtis B, Amin MN, Lees A, Rao RN, Naidu G, Venkatesan R, Rao DY, Mohan VK, et al. Immunogenicity and induction of functional antibodies in rabbits immunized with a trivalent typhoid-invasive nontyphoidal Salmonella glycoconjugate formulation. Molecules. 2018;23(7):7. doi:10.3390/molecules23071749.
- Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010;74(1):81–94. doi:10.1128/MMBR.00031-09.
- Beernink PT, Vianzon V, Lewis LA, Moe GR, Granoff DM, Pirofski L-A. A meningococcal outer membrane vesicle vaccine with overexpressed mutant FHbp elicits higher protective antibody responses in infant rhesus macaques than a licensed serogroup B vaccine. mBio. 2019;10(3). doi:10.1128/mBio.01231-19.
- Pastor Y, Camacho AI, Zúñiga-Ripa A, Merchán A, Rosas P, Irache JM, Gamazo C. Towards a subunit vaccine from a shigella flexneri ΔtolR mutant. Vaccine. 2018;36(49):7509–19. doi:10.1016/j.vaccine.2018.10.066.
- Liu Q, Liu Q, Yi J, Liang K, Hu B, Zhang X, Curtiss R 3rd, Kong Q. Outer membrane vesicles from flagellin-deficient Salmonella enterica serovar Typhimurium induce cross-reactive immunity and provide cross-protection against heterologous Salmonella challenge. Sci Rep. 2016;6(1):34776. doi:10.1038/srep34776.
- Liu Q, Tan K, Yuan J, Song K, Li R, Huang X, Liu Q. Flagellin-deficient outer membrane vesicles as adjuvant induce cross-protection of Salmonella Typhimurium outer membrane proteins against infection by heterologous Salmonella serotypes. Int J Med Microbiol. 2018;308(7):796–802. doi:10.1016/j.ijmm.2018.06.001.
- Liu Q, Liu Q, Yi J, Liang K, Liu T, Roland KL, Jiang Y, Kong Q. Outer membrane vesicles derived from Salmonella Typhimurium mutants with truncated LPS induce cross-protective immune responses against infection of Salmonella enterica serovars in the mouse model. Int J Med Microbiol. 2016;306(8):697–706. doi:10.1016/j.ijmm.2016.08.004.
- Kong Q, Six DA, Roland KL, Liu Q, Gu L, Reynolds CM, Wang X, Raetz CR, Curtiss R 3rd. Salmonella synthesizing 1-dephosphorylated [corrected] lipopolysaccharide exhibits low endotoxic activity while retaining its immunogenicity. J Immunol. 2011;187(1):412–23. doi:10.4049/jimmunol.1100339.
- Kong Q, Yang J, Liu Q, Alamuri P, Roland KL, Curtiss R 3rd, McCormick BA. Effect of deletion of genes involved in lipopolysaccharide core and O-antigen synthesis on virulence and immunogenicity of Salmonella enterica serovar typhimurium. Infect Immun. 2011;79(10):4227–39. doi:10.1128/IAI.05398-11.
- Xiao Y, Liu F, Yang J, Zhong M, Zhang E, Li Y, Zhou D, Cao Y, Li W, Yu J, et al. Over-activation of TLR5 signaling by high-dose flagellin induces liver injury in mice. Cell Mol Immunol. 2015;12(6):729–42. doi:10.1038/cmi.2014.110.
- Gerke C, Colucci AM, Giannelli C, Sanzone S, Vitali CG, Sollai L, Rossi O, Martin LB, Auerbach J, Di Cioccio V, et al. Production of a shigella sonnei vaccine based on Generalized Modules for Membrane Antigens (GMMA), 1790GAHB. PLoS One. 2015;10(8):e0134478. doi:10.1371/journal.pone.0134478.
- De Benedetto G, Alfini R, Cescutti P, Caboni M, Lanzilao L, Necchi F, Saul A, MacLennan CA, Rondini S, Micoli F. Characterization of O-antigen delivered by Generalized Modules for Membrane Antigens (GMMA) vaccine candidates against nontyphoidal Salmonella. Vaccine. 2017;35(3):419–26. doi:10.1016/j.vaccine.2016.11.089.
- Koeberling O, Ispasanie E, Hauser J, Rossi O, Pluschke G, Caugant DA, Saul A, MacLennan CA. A broadly-protective vaccine against meningococcal disease in sub-Saharan Africa based on generalized modules for membrane antigens (GMMA). Vaccine. 2014;32(23):2688–95. doi:10.1016/j.vaccine.2014.03.068.
- Micoli F, Rondini S, Alfini R, Lanzilao L, Necchi F, Negrea A, Rossi O, Brandt C, Clare S, Mastroeni P, et al. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc Natl Acad Sci U S A. 2018;115(41):10428–33. doi:10.1073/pnas.1807655115.
- Schager AE, Dominguez-Medina CC, Necchi F, Micoli F, Goh YS, Goodall M, Flores-Langarica A, Bobat S, Cook CNL, Arcuri M, et al. IgG responses to porins and lipopolysaccharide within an outer membrane-based vaccine against nontyphoidal Salmonella develop at discordant rates. mBio. 2018;9(2):2. doi:10.1128/mBio.02379-17.
- Obiero CW, Ndiaye AGW, Sciré AS, Kaunyangi BM, Marchetti E, Gone AM, Schütte LD, Riccucci D, Auerbach J, Saul A, et al. A phase 2a randomized study to evaluate the safety and immunogenicity of the 1790GAHB generalized modules for membrane antigen vaccine against shigella sonnei administered intramuscularly to adults from a shigellosis-endemic country. Front Immunol. 2017;8:1884. doi:10.3389/fimmu.2017.01884.
- Launay O, Ndiaye AGW, Conti V, Loulergue P, Sciré AS, Landre AM, Ferruzzi P, Nedjaai N, Schütte LD, Auerbach J, et al. Booster vaccination with GVGH shigella sonnei 1790GAHB GMMA vaccine compared to single vaccination in unvaccinated healthy European adults: results from a phase 1 clinical trial. Front Immunol. 2019;10:335. doi:10.3389/fimmu.2019.00335.
- Barat S, Willer Y, Rizos K, Claudi B, Mazé A, Schemmer AK, Kirchhoff D, Schmidt A, Burton N, Bumann D. Immunity to intracellular Salmonella depends on surface-associated antigens. PLoS Pathog. 2012;8(10):e1002966. doi:10.1371/journal.ppat.1002966.
- Feodorova VA, Lyapina AM, Zaitsev SS, Khizhnyakova MA, Sayapina LV, Ulianova OV, Ulyanov SS, Motin VL. New promising targets for synthetic omptin-based peptide vaccine against gram-negative pathogens. Vaccines (Basel). 2019;7:2. doi:10.3390/vaccines7020036.
- Samykannu G, Vijayababu P, Antonyraj CB, Perumal P, Narayanan S, Basheer Ahamed SI, Natarajan J. In silico characterization of B cell and T cell epitopes for subunit vaccine design of Salmonella typhi PgtE: a molecular dynamics simulation approach. J Comput Biol. 2019;26(2):105–16. doi:10.1089/cmb.2018.0010.
- Gil-Cruz C, Bobat S, Marshall JL, Kingsley RA, Ross EA, Henderson IR, Leyton DL, Coughlan RE, Khan M, Jensen KT, et al. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc Natl Acad Sci U S A. 2009;106(24):9803–08. doi:10.1073/pnas.0812431106.
- Hegazy WA, Xu X, Metelitsa L, Hensel M, Bäumler AJ. Evaluation of Salmonella enterica type III secretion system effector proteins as carriers for heterologous vaccine antigens. Infect Immun. 2012;80(3):1193–202. doi:10.1128/IAI.06056-11.
- Lee SJ, Benoun J, Sheridan BS, Fogassy Z, Pham O, Pham QM, Puddington L, McSorley SJ. Dual immunization with SseB/flagellin provides enhanced protection against Salmonella infection mediated by circulating memory cells. J Immunol. 2017;199(4):1353–61. doi:10.4049/jimmunol.1601357.
- Jneid B, Moreau K, Plaisance M, Rouaix A, Dano J, Simon S, Ryan ET. Role of T3SS-1 SipD protein in protecting mice against non-typhoidal salmonella typhimurium. PLoS Negl Trop Dis. 2016;10(12):e0005207. doi:10.1371/journal.pntd.0005207.
- Kurtz JR, Petersen HE, Frederick DR, Morici LA, McLachlan JB. Vaccination with a single CD4 T cell peptide epitope from a Salmonella type III-secreted effector protein provides protection against lethal infection. Infect Immun. 2014;82(6):2424–33. doi:10.1128/IAI.00052-14.
- McSorley SJ, Cookson BT, Jenkins MK. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J Immunol. 2000;164(2):986–93. doi:10.4049/jimmunol.164.2.986.
- Sette A, Rappuoli R. Reverse vaccinology: developing vaccines in the era of genomics. Immunity. 2010;33(4):530–41. doi:10.1016/j.immuni.2010.09.017.
- Dougan G, John V, Palmer S, Mastroeni P. Immunity to salmonellosis. Immunol Rev. 2011;240(1):196–210. doi:10.1111/j.1600-065X.2010.00999.x.
- Peprah E, Xu H, Tekola-Ayele F, Royal CD. Genome-wide association studies in Africans and African Americans: expanding the framework of the genomics of human traits and disease. Public Health Genomics. 2015;18(1):40–51. doi:10.1159/000367962.
