ABSTRACT
The rising need for repeated booster vaccinations against SARS-CoV-2 infections raises the question of whether chronic immunosuppressive chemotherapies influence the efficacy of vaccination. Here, we present the case of a 70-year-old post-thymoma surgery patient who received Vepesid (etoposide, Xediton Pharmaceuticals Inc) chemotherapy for six months before vaccination with Comirnaty (Pfizer-BioNTech COVID-19 mRNA Vaccine). The first two vaccinations elicited only minimal increases of IgG antibodies specific against the receptor-binding domain (RBD) on the spike protein (S1), while the third vaccination was effective in providing high, slowly subsiding antibody titers over a 7-month period. The patient also developed a cellular immune response after the third vaccination. Also, measuring of anti-polyethylene glycol (PEG) IgM titers before and after vaccinations showed no immunogenicity for PEG. Later, a single dose of Sinopharm (China National Pharmaceutical Group) inactivated virus-type vaccine was administered, which also modestly increased the level of IgG. A symptomless COVID-19 infection, however, greatly increased the serum level of anti-RBD IgG, which later subsided. This case confirms that an effective immune response can be achieved with a series of COVID-19 vaccinations despite cytostatic treatment in an old thymus cancer surviving patient in the absence of adverse reactions.
Introduction
Recent studies have shown that the COVID-19 vaccine-induced antibody levels and immune protection against SARS-COV-2 wanes with time.Citation1,Citation2 To overcome this difficulty repeated vaccinations became necessary. Thus, the efficacies of different SARS-CoV-2 vaccination regimens attract intense public and scientific attention and scrutiny.Citation3 While all the currently available COVID-19 vaccines are known to be effective against COVID-19 in healthy people with normal immune systems, it has been reported that immunocompromised individuals, who are at increased risk for infectious diseases in many different ways, may develop a delayed or less robust antibody response, and are therefore in double jeopardy during the pandemic.Citation4–9
Chemotherapy against cancer with cytotoxic drugs represents a risk for immune suppression, but the diversity of cancer and its chemotherapy as well as the age and immune status of patients make it very difficult, if not impossible at all, to make generalized recommendations regarding COVID-19 vaccinations. Decisions have to be made individually, considering the vaccine options, patient conditions, and potential outcomes, as learned from relevant case studies. Therefore, there is a need for information on the immune responses of immune suppressed patients to COVID-19 vaccinations, a gap in the vaccine literature that motivated the present case report. The following is an account of the immune response of an elderly male patient to multiple COVID-19 vaccinations. He underwent thymus cancer surgery followed by an immune suppressive chemotherapy. In particular, he was treated with Vepesid in seven 3-day infusion cycles, four of which coincided with two consecutive vaccinations with Comirnaty (Pfizer-BioNTech’s BNT162b2, an mRNA type vaccine), and later with Sinopharm (BBIBP-CorV, an inactivated virus vaccine).Citation10,Citation11
Since Vepesid is a nucleic acid purine base-crosslinker alkylating agent with known bone marrow toxicity,Citation12–14 its possible interference with mRNA transcription and adequate immune response to the vaccine was a major concern for this patient. To explore this risk, we performed serial measurements of SARS-CoV-2 anti-RBD IgG levels following each vaccination over 1.5 year. Furthermore, since both Vepesid and Comirnaty can rarely cause hypersensitivity (anaphylactic) reactions,Citation12–16 another concern for the patient was a potential increase in the risk for such reactions, because of Comirnaty-induced rise of anti-PEG antibody levels, a likely contributing factor to the anaphylaxis to this vaccine.Citation16–19 Thus, we have also measured anti-PEG IgM levels in the patient’s blood, as well as neutrophil and lymphocyte counts, complement levels, and nonspecific cellular immune response to T cell stimulation after vaccinations, taken as measures of innate immune response to the vaccines under chemotherapy.
Patient history
The 70-years-old male patient underwent open chest surgery to remove a thymic carcinoma (Masaoka stage IVa), followed by chemotherapy with Vepesid, 170 mg/500 ml NaCl/day, seven 3-day cycles with 3 weeks breaks, during the period of September 9, 2020 to June 23, 2021. In 2021, during the last 4 cycles, the patient received BNT162b2 mRNA vaccinations 3-times. Then, at a later time, the patient received a single dose of the Sinopharm vaccine. None of the vaccinations caused any early or late side effects. The blood withdrawal was approved by the Research Ethics Committee of the Hungarian Medical Research Council (52685–6/2022/EÜIG).
Antibody tests
SARS-CoV-2 neutralization Ab specific against the receptor-binding region of viral spike protein (anti-S) was measured as described in Refs.Citation46,Citation47 using a kit provided by TECOmedical AG (Sissach, Switzerland, Catalog No. TE 1076).
Materials and methods
Measurement of humoral and cellular immune response
We used two IgG antibody tests to assess the specific humoral immune response against the receptor-binding domain (RBD) on the S1 subunit of the spike protein of SARS-CoV-2. One, a chemiluminescent microparticle immunoassay offered by the Central Microbiology Laboratory of Synlab Hungary (https://www.synlab.hu). It utilized the SARS-CoV-2 IgG II Quant kit (Abbott, Wiesbaden, Germany), had the RBD of S1 as antigen and a cutoff value of 50 U/ml).Citation20,Citation21 The second assay was a SARS-CoV-2 antibody kit provided by TECOmedical AG (Sissach, Switzerland, Catalog No. TE 1076). It is a competitive ELISA wherein the human angiotensin-converting enzyme 2 (ACE-2) is attached to the solid phase as antigen and peroxidase-conjugated RBD, present in the liquid phase, competes with the RBD-specific antibodies for binding to the ACE-2 antigen.Citation3,Citation22 This assays’ cutoff was 20 U/mL. Both anti-RBD antibody assays are considered to quantitate the virus neutralizing antibody levels in blood.Citation2,Citation4 Cellular immune response was evaluated as a service by the Diagnostic Laboratory of Labmagister Kft (Budapest, Hungary, www.labmagister.hu) using Quantiferon® SARS-CoV-2 Starter Set (Qiagen, Hilden, anti-RBD antibody assays Germany). The IFNγ-detecting whole-blood assay involves 16 h incubation of blood at 37°C in 2 test tubes to which two kinds of S-protein-derived peptide mixtures are adhered; one stimulates C2,4D4+, while the other CD8+ T cells holding memories for SARS-CoV-2 S protein antigens.Citation23,Citation24 All assays were run according to the manufacturer’s instructions.
Blood cells analysis
Neutrophil granulocyte (G) and lymphocyte (Ly) counts were determined in an automatic Hematology Blood Analyzer at the Central Laboratory of Semmelweis University.
Complement measurements
Complement C3 and C4 were measured by turbidimetry (Beckman Coulter, Brea, CA, USA). Factors H (FH) and C1q were measured by ELISA, and Factor B (FB) and Factor I (FI) by radial immune diffusion, using specific polyclonal antibodies, as described earlier.Citation25 Complement activation via the classical pathway was measured by the sheep hemolysis assay based on Mayer’s method, and the alternative pathway using a commercially available kit (Wieslab, KOMPL AP330, Svar Life Science, Malmö, Sweden), according to the manufacturer’s instructions.Citation25
Measurement of anti-PEG IgM in blood
The anti-PEG IgM titers in blood were determined using a sandwich ELISA, as described previously.Citation17
Results
Antibody response
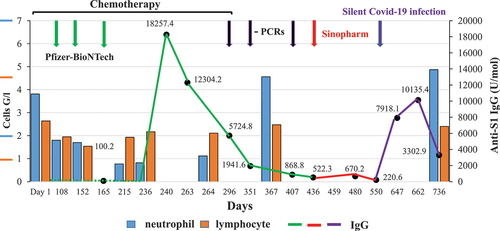
shows the timing of vaccinations, the time course of neutralizing IgG antibody changes and neutrophil and lymphocyte counts, as well as the dates of negative PCR tests over a year, starting from the first vaccination. The two anti-RBD IgG assays gave similar indication of no, or very low anti-RBD antibody levels after the first two vaccinations with Comirnaty, and then a robust increase after the 3rd injection of this vaccine, indicating an effective immune response. Accordingly, the 3 SARS-CoV-2 PCR tests were negative (black arrows in ), indicating that the patient was not infected with COVID-19 during the first year of investigation. The antibody titers then gradually subsided to minimal levels over 5–7 months after the 3rd Comirnaty booster, but the pandemic was still present, so the patients received a 4th vaccination this time with Sinopharm, which only modestly increased the neutralizing antibody level despite normal levels of neutrophils and lymphocytes. Then, unexpectedly, 7 months after the last vaccine, follow-up tests showed high levels of anti-RBD IgG, although the patient did not detect any symptoms of SARS-CoV-2 infection. This rise then subsided in 3 months.
Figure 1. As a function of time, X axis, neutrophil blood cell (blue), lymphocyte (orange) counts of the patient are depicted on the left Y axis. As a function of time (X axis) neutralizing antibody levels of the patient (values at black dots, anti-RBD IgG, right Y axis) are shown during and after Vepesid cytostatic treatment. Anti-COVID vaccinations with Pfizer-BioNTech (on days 125, 146, 224) and Sinopharm (on day 370) are shown on the top with green and red arrows, respectively. Green line connects IgG levels in response to Pfizer’s-, red line for Sinopharm vaccinations, whereas blue line after silent Covid-19 infection. Also, the times of negative PCR tests are shown (black arrows). The figure clearly indicates that the first two Pfizer vaccinations did not elicit increase in IgG, whereas the 3rd Pfizer vaccination resulted in very high level of IgG response despite of low level of neutrophil and lymphocytes. Sinopharm elicited only modest increase (despite the normal levels of neutrophils and lymphocytes, in contrast a silent Covid-19 infection again elicited a great increase in IgG level, indicating proper activation of the immune system.
Cellular immune response
T-cell readiness for antiviral response was evaluated using Qiagen’s Quantiferon kit, 4 and 8 months after the last (3rd) vaccine. Two different SARS-CoV-2 spike peptide cocktails stimulating CD4+ and CD8+ T cells (“Epitope 1 and 2”) were incubated with the patient’s peripheral blood for 16 h, and IFN-alpha production was measured as an index of T cell activation.Citation23,Citation24 Both cocktails led to IFN-alpha rises over the cutoff value (CV), on 07.01.07.2021: E1 0.045 IU/mL and E2 0,162 IU/mL (CV: 0,017) and on 22.11.2012, E1 0.12 and E2 0,18 IU/mL (CV: 0,027) indicating effective T cell activation. The antigen composition of Epitopes 1 and 2 is not specified by the manufacturer.
Blood cell changes
The data show substantial granulopenia with less expressed lymphopenia during cytostatic treatment, the latter also seen at by the end of the study period that could not be linked to drug treatment. Thus, in agreement with the known cytotoxic effects of Vepesid, the patient displayed clear signs of myelosuppression and minor, borderline immune suppression, although, in lack of data, we would refrain from making conclusions on the patient’s immune functions.
Complement proteins
In addition to the humoral and cellular immune responses, we also measured complement protein levels in the patient’s blood as an index of innate immune competence after the 3rd cytostatic cycle, which was 1 month after the first vaccination (01/12/2021). As shown in , aside from a minor rise of factor H, all parameters remained in the normal range.
Table 1. Complement proteins 1 month after the first vaccination with Comirnaty.
shows that the anti-PEG IgM antibody levels remained in the normal range after three vaccinations with Comirnaty.
Table 2. Anti-PEG IgM levels after three vaccinations with Comirnaty.
Discussion
Due to a lack of data, there is uncertainty even to this day whether or not vaccinations against SARS-CoV-2 infections are effective in chronic myelosuppressive chemotherapy patients, especially in older age, in which immunosencece may also be present. Thus, the goal of this study was to present a case of successful immunization with a series of Comirnaty and an additional Sinopharm vaccine despite myelosuppressive chemotherapy of an elderly cancer patient.
The laboratories providing tests against anti-RBD IgG used different kits to measure these antibodies, which had different assay principles, as detailed in the Methods section. Both tests measure the level of neutralization antibodies, which represent only a small portion of all clones stimulated after SARS-CoV-2 infection or by vaccination by the whole S1 protein.Citation26 Although there were proportional differences in the readings even after their conversions to binding antibody unit (BAU) per milliliter, using the WHO international standard for SARS-CoV-2 immunoglobulin,Citation27 they consistently showed very low values of immunogenicity after the first and second vaccinations nearly 3 weeks later. This is not a typical immune response after Comirnaty injection, and the lack of immunogenicity may be attributed to the chemotherapy whose myelosuppressive effect was evident from the reduced neutrophil counts all through the chemotherapy. The typical response, seen in most vaccine recipients, is a gradual rise of anti-RBD antibodies within 1–3 weeks after the first, and an even faster rise after the second vaccination. Finally, this antibody shows a constant, slow decline over several months, faster in elderly people compared to young adults.Citation1 According to Khoury et al.,Citation2 the neutralizing titers in vaccinated subjects can decay over the first 3–4 months after vaccination, which is not different from what we observed in our myelosuppressed patients after the third vaccination. Likewise, our finding that only the third vaccination could overcome the effect of chemotherapy (manifested in up to 60 and 30% decreases of neutrophil and lymphocyte counts) and induce a robust seroconversion with high levels of plasma anti-RBD IgG is in keeping with the results of Kamar et al.,Citation7 showing that only the third vaccination could restore the immunogenicity of Comirnaty in transplant patients undergoing immunosuppressive chemotherapy. It should be noted about the latter study that the agents administered to the patient were dedicated immune-suppressive drugs (glucocorticoids, calcineurin inhibitors, mycophenolic acid, rapamycin inhibitors, and belatacept),Citation7 while the patient in our study was treated with an alkylating drug with known myelosuppressive, rather than immune suppressive side effects. Thus, the similarity of delay in seroconversion suggests that the impacts of myelo- and immune suppression on Comirnaty’s immunogenicity may not be fundamentally different.
The cellular immune response assay performed 3 and 7 months after the third vaccination showed positivity, which is consistent with the patient’s protection against manifest disease after infection. However, the interpretation of this result as an indicator of protection against infection is questionable until more studies become available addressing the test’s clinical predictive value in individual infections by the various mutants. Although preliminary studies showed a correlation of test positivity with infection strength and booster numbers,Citation23,Citation28 in our present understanding the test was developed on principles applicable to TB bacteria, which may not be applicable to a highly variable corona virus. Notably, due to lack of information on the stimulating peptides in the proprietary “Epitope 1 and 2” cocktails in the Quantiferon® test it may be difficult to determine if the unique MHC molecules on antigen presenting cells will also present the S peptides of virus variants to the uniquely MHC-restricted T-cells. T-cell response in COVID-19 infection is an early step in defending the body.Citation23 This evasion may contribute to the increasing infectivity of successive variants. On the other hand, nonspecific stimulation of T cells (cytokine storm) by the virus is a clinical characteristic of some end-stage COVID-19 illnesses,Citation29–32 raising the possibility that the Quantiferon® test might actually serve to evaluate the lymphocytes’ proneness to produce cytokine storm in severe COVID-19 cases.
The normal complement levels are consistent with limited immune suppression not affecting innate immune responses, and the anti-PEG IgM measurements also represent an evaluation of innate immunity. However, the rationale of the latter assay needs more commenting, as it may seem to be an irrelevant sidetrack in our study. In fact, the anti-PEG IgM measurements in our patient was done as part of a different project aimed to establish the risk of allergic people to develop anaphylactic reaction to Comirnaty, which contains PEG.Citation15,Citation16 High blood levels of such antibodies have been implicated in the anaphylactic reactions to numerous PEGylated drugs,Citation17–19,Citation33,Citation34 Furthermore there is a suspicion that the increased occurrence of such reactions after mRNA vaccinesCitation35–40 may be due, at least in part, to the presence of high levels of anti-PEG antibodies.Citation15,Citation16,Citation41 In fact, a recent study on 55 people receiving Comirnaty showed the common presence of anti-PEG IgG and IgM, which were boosted by vaccination by a mean of 2–3-fold, up to 13–17-fold in some individuals.Citation42 This implies the possible sensitization by PEG-containing vaccines of some individuals to PEGylated medicines and vaccines. This may be a contributing factor to the increased rate of allergic reactions after repeated vaccinations.Citation35–40
The relevance of anti-PEG IgM determinations in the immunosuppressed patient in our study lies in the fact that these antibodies are mostly induced via T-cell independent (Class 2 or 3) immunogenicity,Citation43–46 i.e., not via the same T-cell dependent mechanism as the anti-COVID antibodies are triggered by the mRNA vaccines. For these reasons, it could not be a priori excluded that although the classic immunogenicity is suppressed in our patient, the T-cell independent anti-PEG antibody formation remains intact, and we see anti-PEG antibody formation or boosting, making our patient oversensitive to potential additional vaccinations needed for anti-COVID protection. Fortunately, the anti-PEG IgM titers remained low in the patient, although we do not know whether this was due to immune suppression or an inherently weak immunogenicity for such antibodies. The message from these measurements and considerations is that anti-PEG antibody levels should be monitored in patients who undergo repeated vaccinations with PEG-(or polysorbate)-containing vaccines in order to avoid possible allergic reactions, and also to exclude the small risk that the anti-PEG antibodies bind and neutralize the vaccine.
At the end of 2021, the COVID-19 pandemic was still ongoing, therefore the patients received – at this time – a single dose of Sinopharm vaccine in order to avoid the potential unwanted effects of Comirnaty, and because by that time the original virus had undergone mutations potentially increasing the immune evasion potential of these variantsCitation47 to the very specific response to Comirnaty. We have found that Sinopharm elicited only a modest 30% increase in IgG level – which, however, cannot be considered a sufficient immunogenic stimulus. - This then subsided after 3 months. The IgG values at these times also indicated that the patient was not infected with Covid-19. Importantly to note, at a later time when the patient was infected with COVID-19 t virus, it was symptomless. This may be due to the adequate immune response, indicated by the high serum level if IgG elicited by vaccinations ().
In summary, there have been lengthy discussions in the literature regarding the protection of immunocompromised and/or immunosencece patient against coronavirus especially, because cancer is a major co-morbidity in COVID-19 related deaths. The present case provides a proof for effective immunization against COVID-19 even in an elderly patient under chemotherapy, which may apply to other vaccines and other virus infections as well.
Ethics statement
Written informed consent has been obtained from the individual to take part in the research prior to the commencement of the study, according to the local institutional ethics committee (TUKEB 15576/2020) to meet national and international guidelines for research on humans.
Acknowledgments
The authors are grateful to Tamás Mészáros, Gergely Kozma, Petra Berényi, Réka Facskó for their excellent technical contributions and Marieluise Wippermann (TECOMedical, Sissach, Switzerland) for providing the competitive anti-RBD IgG assay kit.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Additional information
Funding
References
- Doria-Rose N, Suthar MS, Makowski M, O’Connell S, McDermott AB, Flach B, Ledgerwood JE, Mascola JR, Graham BS, Lin BC, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for covid-19. N Engl J Med. 2021;384(23):2259–6. doi:10.1056/NEJMc2103916.
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–11. doi:10.1038/s41591-021-01377-8.
- Rose R, Neumann F, Grobe O, Lorentz T, Fickenscher H, Krumbholz A. Humoral immune response after different SARS-CoV-2 vaccination regimens. BMC Med. 2022;20:31. doi:10.1186/s12916-021-02231-x.
- Ng DL, Goldgof GM, Shy BR, Levine AG, Balcerek J, Bapat SP, Prostko J, Rodgers M, Coller K, Pearce S, et al. SARS-CoV-2 seroprevalence and neutralizing activity in donor and patient blood. Nat Commun. 2020;11(1):4698. doi:10.1038/s41467-020-18468-8.
- Eyre DW, Lumley SF, O’Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, Marsden BD, Cox S, James T, et al. Stringent thresholds in SARS-CoV-2 IgG assays lead to under-detection of mild infections. BMC Infect Dis. 2021;21(1):187. doi:10.1186/s12879-021-05878-2.
- Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernan MA, Lipsitch M, Reis B, Balicer RD. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–23. doi:10.1056/NEJMoa2101765.
- Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–62. doi:10.1056/NEJMc2108861.
- Narasimhan M, Mahimainathan L, Clark AE, Usmani A, Cao J, Araj E, Torres F, Sarode R, Kaza V, Lacelle C, et al. Serological response in lung transplant recipients after two doses of SARS-CoV-2 mRNA vaccines. Vaccines (Basel). 2021;9(7). doi:10.3390/vaccines9070708.
- Benotmane I, Gautier-Vargas G, Cognard N, Olagne J, Heibel F, Braun-Parvez L, Martzloff J, Perrin P, Moulin B, Fafi-Kremer S, et al. Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021;99(6):1487–89. doi:10.1016/j.kint.2021.03.014.
- Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, Tan W, Wu G, Xu M, Lou Z, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi:10.1016/S1473-3099(20)30831-8.
- Saeed U, Uppal SR, Piracha ZZ, Uppal R. SARS-CoV-2 spike antibody levels trend among Sinopharm vaccinated people. Iran J Public Health. 2021;50(7):1486–87. doi:10.18502/ijph.v50i7.6640.
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78. doi:10.1016/j.ejphar.2014.07.025.
- LLC WCC. 2022. Cisplatin: package insert. https://www.accessdatafdagov/drugsatfda_docs/label/2019/018057s089lblpdf.
- Tchounwou PB, Dasari S, Noubissi FK, Ray P, Kumar S. Advances in our understanding of the molecular mechanisms of action of Cisplatin in cancer therapy. J Exp Pharmacol. 2021;13:303–28. doi:10.2147/JEP.S267383.
- Dezsi L, Meszaros T, Kozma TG, Velkei M, Olah C, Szabo M, Patko Z, Fulop GF, Hennies M, Szebeni M, et al. A naturally hypersensitive porcine model may help understanding the mechanism of COVID-19 vaccine-induced (pseudo)allergic reactions: complement activation as a possible contributing factor. Geroscience. 2022;44:597–618. doi:10.1007/s11357-021-00495-y.
- Szebeni J, Storm G, Ljubimova JY, Castells M, Phillips EJ, Turjeman K, Barenholz Y, Dobrovolskaia MA, Crommelin DJA. Applying lessons learned from nanomedicines to understand rare hypersensitivity reactions to mRNA-based SARS-CoV-2 vaccines. Nat Nanotechnol. 2022;17:337–46. doi:10.1038/s41565-022-01071-x.
- Kozma TG, Meszaros T, Berenyi P, Facsko R, Patko Z, Olah C, Nagy A, Fulop G, Glatter KA, Radovits T, et al. Role of anti-polyethylene glycol (PEG) antibodies in the allergic reactions and immunogenicity of PEG-containing Covid-19. medrxiv.Org/cgi/content/short/2022.10.03.22280227v1. doi:10.1101/2022.10.03.22280227
- Senti ME, de Jongh Ca, Dijkxhoorn K, Verhoef JJF, Szebeni J, Storm G. Anti-PEG antibodies compromise the integrity of PEGylated lipid-based nanoparticles via complement. J Controlled Release. 2021;341:475–86. doi:10.1016/j.jconrel.2021.11.042.
- Shi D, Beasock D, Fessler A, Szebeni J, Ljubimova JY, Afonin KA, Dobrovolskaia MA. To PEGylate or not to PEGylate: immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv Drug Deliv Rev. 2022;180:114079. doi:10.1016/j.addr.2021.114079.
- Noda K, Matsuda K, Yagishita S, Maeda K, Akiyama Y, Terada-Hirashima J, Matsushita H, Iwata S, Yamashita K, Atarashi Y, et al. A novel highly quantitative and reproducible assay for the detection of anti-SARS-CoV-2 IgG and IgM antibodies. Sci Rep. 2021;11(1):5198. doi:10.1038/s41598-021-84387-3.
- Abbot. SARS-COV-2 immunoassays; 2022. https://wwwcorelaboratoryabbott/int/en/offerings/segments/infectious-disease/sars-cov-2-.
- Neumann F, Rose R, Rompke J, Grobe O, Lorentz T, Fickenscher H, Krumbholz A. Development of SARS-CoV-2 specific IgG and virus-neutralizing antibodies after infection with variants of concern or vaccination. Vaccines (Basel). 2021;9(7). doi:10.3390/vaccines9070700.
- Martinez-Gallo M, Esperalba J, Pujol-Borrell R, Sanda V, Arrese-Munoz I, Fernandez-NavalC, Anton A, Cardona V, Labrador-Horrillo M, Pumarola T, et al. Commercialized kits to assess T-cell responses against SARS-CoV-2 S peptides. A pilot study in health care workers. Med Clin (Barc). 2021;159(3): 116–123.doi:10.1016/j.medcli.2021.09.013.
- Qiagen. Quantiferon RUO; 2022. https://wwwqiagencom/us/products/diagnostics-and-clinical-research/infectious-disease/quantiferon-sars-cov-2-ruo/.
- Sinkovits G, Mezo B, Reti M, Muller V, Ivanyi Z, Gal J, Gopcsa L, Remenyi P, Szathmary B, Lakatos B, et al. Complement overactivation and consumption predicts in-hospital mortality in SARS-CoV-2 infection. Front Immunol. 2021;12:663187. doi:10.3389/fimmu.2021.663187.
- Andreano E, Nicastri E, Paciello I, Pileri P, Manganaro N, Piccini G, Manenti A, Pantano E, Kabanova A, Troisi M, et al. Extremely potent human monoclonal antibodies from COVID-19 convalescent patients. Cell. 2021;184(7):1821–35 e16. doi:10.1016/j.cell.2021.02.035.
- Perkmann T, Perkmann-Nagele N, Koller T, Mucher P, Radakovics A, Marculescu R, Wolzt M, Wagner OF, Binder CJ, Haslacher H. Anti-spike protein assays to determine SARS-CoV-2 antibody levels: a head-to-head comparison of five quantitative assays. Microbiol Spectr. 2021;9(1):e0024721. doi:10.1128/Spectrum.00247-21.
- Van Praet JT, Vandecasteele S, De Roo A, De Vriese AS, Reynders M. Humoral and cellular immunogenicity of the BNT162b2 messenger RNA coronavirus disease 2019 vaccine in nursing home residents. Clin Infect Dis. 2021;73(11):2145–47. doi:10.1093/cid/ciab300.
- Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2020;93:250–56. doi:10.1002/jmv.26232.
- Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020;133:155151. doi:10.1016/j.cyto.2020.155151.
- Kozma GT, Meszaros T, Bakos T, Hennies M, Bencze D, Uzonyi B, Gyorffy B, Cedrone E, Dobrovolskaia MA, Jozsi M, et al. Mini-factor H modulates complement-dependent IL-6 and IL-10 release in an immune cell culture (PBMC) model: potential benefits against cytokine storm. Front Immunol. 2021;12:642860. doi:10.3389/fimmu.2021.642860.
- Jiang Y, Rubin L, Peng T, Liu L, Xing X, Lazarovici P, Zheng W. Cytokine storm in COVID-19: from viral infection to immune responses, diagnosis and therapy. Int J Biol Sci. 2022;18(2):459–72. doi:10.7150/ijbs.59272.
- Abu Lila AS, Shimizu T, Ishida T. PEGylation and anti-PEG antibodies. In: Engineering of biomaterials for drug delivery systems: beyond polyethylene glycol. Woodhead Publishing Series in Biomaterials; 2018. p. 51–68. doi:10.1016/B978-0-08-101750-0.00003-9.
- Shimizu T, Abu Lila AS, Awata M, Kubo Y, Mima Y, Hashimoto Y, Ando H, Okuhira K, Ishima Y, Ishida T. A cell assay for detecting anti-PEG immune response against PEG-modified therapeutics. Pharm Res. 2018;35(11):223. doi:10.1007/s11095-018-2505-3.
- CDC. Interim considerations: preparing for the potential management of anaphylaxis at COVID-19 vaccination sites summary. CDC; 2020. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/downloads/anaphylaxis-management.pdf.
- Banerji A, Wickner PG, Saff R, Stone CA Jr., Robinson LB, Long AA, Wolfson AR, Williams P, Khan DA, Phillips E, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2020;9:1423–37. doi:10.1016/j.jaip.2020.12.047.
- Garvey LH, Nasser S. Anaphylaxis to the first COVID-19 vaccine: is polyethylene glycol (PEG) the culprit? Br J Anesthesia. 2020;126:e106–08. doi:10.1016/j.bja.2020.12.020.
- Risma KA, Edwards KM, Hummell DS, Little FF, Norton AE, Stallings A, Wood RA, Milner JD. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J Allergy Clin Immunol. 2021;147:2075–82.e2. doi:10.1016/j.jaci.2021.04.002.
- Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325:780. doi:10.1001/jama.2021.0600.
- Team CC-R, Food, Drug A. Allergic reactions including anaphylaxis after receipt of the first dose of moderna COVID-19 vaccine - United States, December 21, 2020-January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(4):125–29. doi:10.15585/mmwr.mm7004e1.
- Vrieze J. Suspicions grow that nanoparticles in Pfizer’s COVID-19 vaccine trigger rare allergic reactions. Science. 2020. https://www.sciencemag.org/news/2020/12/suspicions-grow-nanoparticles-pfizer-s-covid-19-vaccine-trigger-rare-allergic-reactions.
- Ju Y, Lee SW, Kelly HG, Pilkington EH, Wragg KM, Subbarao K, Nguyen THO, Rowntree LC, Allen LF, Bond K, et al. Anti-PEG antibodies boosted in humans by SARS-CoV-2 lipid nanoparticle mRNA vaccine. medRxiv 2022. doi:10.1101/2022.01.08.22268953.
- Ichihara M, Shimizu T, Imoto A, Hashiguchi Y, Uehara Y, Ishida T, Kiwada H. Anti-PEG IgM response against PEGylated liposomes in mice and rats. Pharmaceutics. 2010;3(1):1–11. doi:10.3390/pharmaceutics3010001.
- Ishida T, Wang X, Shimizu T, Nawata K, Kiwada H. Pegylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. J Control Release. 2007;122(3):349–55. doi:10.1016/j.jconrel.2007.05.015.
- Koide H, Asai T, Hatanaka K, Akai S, Ishii T, Kenjo E, Ishida T, Kiwada H, Tsukada H, Oku N. T cell-independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes. Int J Pharm. 2010;392(1–2):218–23. doi:10.1016/j.ijpharm.2010.03.022.
- Shimizu T, Ishida T, Kiwada H. Transport of PEGylated liposomes from the splenic marginal zone to the follicle in the induction phase of the accelerated blood clearance phenomenon. Immunobiology. 2013;218(5):725–32. doi:10.1016/j.imbio.2012.08.274.
- Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, Planchais C, Porrot F, Robillard N, Puech J, et al. Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization. Nature. 2021;596(7871):276–80. doi:10.1038/s41586-021-03777-9.
