ABSTRACT
Mechanical alloying has been repeatedly demonstrated as an effective means to create unique structural materials, but it is comparatively rare that functional alloys are produced. Multi-phase functional alloy production is demonstrated with an oxide-dispersed Ni–Cu catalyst. These catalyst particles are designed to form porosity and catalyze carbon nanofiber deposition in situ, which results in a six-fold increase in deposition rate over traditional preparation methods. This technique serves as a template for many other systems possible with MA.
GRAPHICAL ABSTRACT
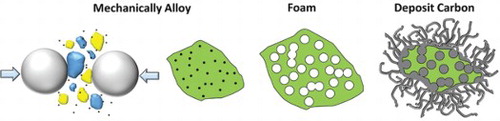
IMPACT STATEMENT
Solid state foaming and catalysis are combined using mechanical alloying. A six-fold increase in catalytic activity is achieved using a simple process that can be applied to other systems.
Introduction
Microstructural control through mechanical alloying (MA) is routinely used to create useful properties in diverse metallic and composite systems [Citation1–3]. Structural properties and applications have been thoroughly studied for decades [Citation4], but functional applications are relatively rare. Thermoelectric materials [Citation5–7], chemically active materials [Citation8,Citation9] (especially for hydrogen storage [Citation10,Citation11]), and reactive milling for in situ control and creation of energetic materials [Citation12,Citation13], such as carbides [Citation14,Citation15], are prominent examples. The diversity of these studies illustrates the unique capability and broad reach of nonequilibrium and nanoscale alloy development.
Catalysis is another important discipline that MA may provide unique strategies in. Applications may include direct chemical conversion r the production of other nanomaterials, such as depositing carbon nanofibers (CNFs) [Citation16]. CNFs are related to more well-known carbon nanotubes (CNTs), but they may exhibit a wider range of fiber diameter, crystallinity, and morphology. Recent CNF research has focused on functional applications and composite properties [Citation17], but there are still central questions regarding the mechanisms of their formation. In a recent review of catalytic deposition methods [Citation18], Lu et al. note that promising results have been realized using bimetallic and multimetallic catalysts for higher deposition rates and longer lifetime, but significant work remains to achieve cost-effective production. Ni-Cu alloys are among the most promising for this purpose [Citation19–23].
In a recent report [Citation24], MA was used to create Ni 30 at% Cu feedstock (known as Ni 400 alloy or Monel) for carbon nanofiber (CNF) catalysis with enhanced catalytic activity while also reducing process complexity over chemical precipitation methods. Because MA resulted in particle dimensions of 10 s of microns, surface break-up to form the nanoscale particles needed for CNF deposition is a rate-limiting step. If surface area or microstructural factors can be enhanced, it is expected that deposition can be accelerated. To that end, solid state foaming by oxide reduction has also been recently demonstrated to produce microscale and nanoscale porosity and ultrafine grain size in this alloy [Citation25]. By combining the capability of MA to produce nanostructured functional alloys and fine dispersions of insoluble phases, we demonstrate that catalysis and solid state foaming may be united in an oxide-dispersed Ni-Cu alloy powder. The individual particles can be foamed and then catalyze the deposition of CNFs in a single, continuous process. Even in rudimentary form at laboratory scale, this technique is capable of producing a catalyst for kilogram-per-hour carbon nanofiber production. This approach represents a significant departure from current processes, but more importantly, it exemplifies the broader possibilities in multifunctional materials through MA.
Experimental
Nickel–copper and nickel–copper oxide (CuO) powders were prepared using a modified Spex 8000M cryogenic high-energy ball mill with a ball-to-powder mass ratio of 10:1. Each milling run consisted of 5 g of powder in a 7:3 ratio of Ni:Cu or Ni:CuO, or a 70:28:2 ratio of Ni:Cu:CuO. Herein these alloys will be referenced as 0% oxide, 30% oxide, and 2% oxide, respectively. The starting powders used to make the alloys were nickel (99.8%, <1 µm), copper (99%, <75 µm) and copper (II) oxide (98%, <10 µm). All alloys were milled for 1 hr under constant liquid nitrogen cooling (−196°C). All powders were handled and stored in a glove box under argon (< 1 ppm O2, < 1 ppm H2O). Reactions were performed in a 50 mm diameter, single zone tube furnace (Across International). Ethylene (chemically pure) and forming gas (5% H2 in Ar) were controlled by MKS G-series mass flow controllers to achieve a 4:1 ratio (C2H4:H2) during reactions. Nitrogen (99.9999%) was used to purge the furnace before and after reactions were performed. Reaction was conducted at 450, 500, and 550°C for 1 hr, and reaction durations were varied between 5 and 120 min at 550°C. Catalytic activity was measured by percent mass gain as a result of carbon deposition (i.e. g carbon/g catalyst ×100). Reaction rates were determined by averaging three or more samples processed under the same conditions. The deposition products and microstructural development of the particles were assessed by focused ion beam (FIB) sectioning and scanning electron microscopy (SEM) using a Zeiss Auriga 60 Crossbeam SEM/FIB.
To assess changes in porosity at the reaction temperatures, the as-milled powders were compacted uniaxially into 3 mm pellets at a pressure of 2 GPa. Three compacts of each composition, approximately 50 mg each, were measured before and after annealing at 450, 500, 550°C for 1 h in a reducing atmosphere (5% H2 in Ar). Volumetric density was determined using the measured mass (± 0.0001 g) and volume (± 0.01 mm for diameter and height). The change in porosity was determined by subtracting the final relative density from the initial (as-compacted) density to isolate porosity resulting from the foaming process. Error bars for foaming and deposition results represent one standard deviation from the mean.
Results and discussion
In Figure (A), the 0% and 30% oxide samples show similar deposition rates at each temperature, but the 2% oxide sample shows a marked increase at 550°C, where the deposition rate exceeds the others by a factor greater than 1.5. The other two alloys also outperform ‘traditional’ Ni–Cu catalysts [Citation20] having a maximum efficiency of about 1600%/hr (i.e. 1.2 g carbon after 1.5 hr using 50 mg catalyst) under similar conditions. During a 1 hr deposition, the same alloy created by MA (i.e. 0% oxide) is more than 3.5 times more efficient (∼6000%/hr), and 2% oxide is more than six times more efficient (∼10,000%/hr). Specifically, 5 mg of catalyst produces as much as 500 mg of carbon in an hour (∼74,000% volume increase).
Figure 1. (A) Carbon deposition quantities for each sample after 1 hr under 4:1 C2H4:H2 at listed temperatures, and (B) increase in porosity after 1 hr under 5% H2 (bal. Ar) at listed temperatures.
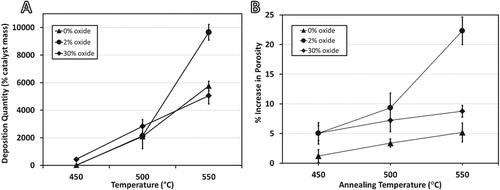
The increase in catalytic efficiency is mirrored by the development of porosity, as shown in Figure (B), where the 2% oxide alloy is the only one to show appreciable porosity change. The ∼5% porosity at 550°C in ‘pure’ Ni–Cu alloy is attributed to native oxide mixed within it, and in the 30% oxide alloy, the higher percentage of oxide may hinder pore expansion because of the concurrent pinning and strengthening effect it has on the matrix.
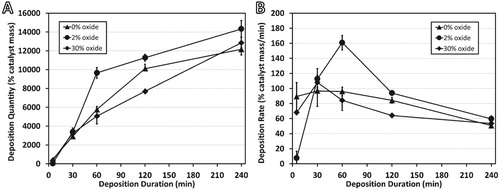
The deposition kinetics are shown in Figure . The total deposition quantity, shown in Figure (A), reveals similar performance from all three alloys up to 30 min of reaction. After 60 min, the 2% oxide alloy is significantly more active and maintains a higher activity until the maximum time of 240 min. It is notable that the reaction rates of all three alloys eventually reach similar levels, as shown in Figure (B). This indicates the factors leading to the enhanced deposition are temporal, and in this particular system, it is expected that the catalyst rapidly disintegrates, thus allowing many catalytic particles to become active simultaneously. Many smaller particles can increase the deposition rate, but they also deactivate more quickly [Citation26]. In all samples, deposition will be a balance between surface break-up and catalyst deactivation. The 0% oxide samples are the most consistent, indicating that these processes are happening steadily as the particles slowly disintegrate.
Figure 2 (A) Total carbon deposition and (B) carbon deposition rates measured for samples at reaction times shown. All reactions performed at 550°C under 4:1 C2H4:H2.
Although supported catalysts, especially those produced from thin films, may be treated through annealing or other processes to control particle size [Citation27], surface break-up preempts CNF formation in coarse-grained materials, and the CNF deposition process can be designed to prevent sintering in metallic powders [Citation28]. Break-up has been studied in Ni [Citation29,Citation30], and it is generally attributed to carbon accumulation at crystalline defects and subsequent stress build-up and fracturing of grains from the surface. Reports of CNF deposition on ‘traditional,’ reticulated Ni foam [Citation31–33] follow this basic mechanism, but the catalyst here is distinctly different in form and structure.
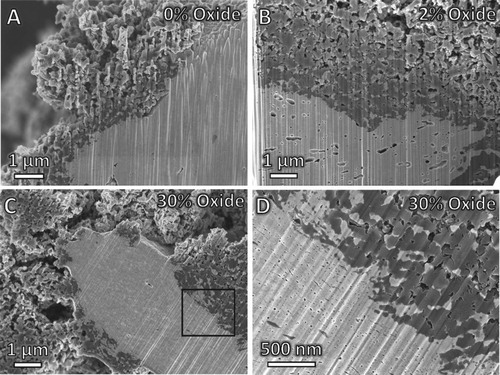
Figure shows that CNF deposition results in disintegration of the catalyst particles after only 5 min of reaction time, where the variation in porosity, the break-up of the particles, and the formation of fibrous structures can be seen. The 0% oxide (Figure (A)) and 30% oxide (Figure (C)) samples do not have prominent porosity, though there are nanoscale pores visible at higher magnification in the 30% oxide sample (Figure (D)). Microscale porosity is distributed throughout the 2% oxide sample (Figure (B)).
Figure 3. Carbon deposition after 5 min on Ni–Cu catalysts containing (A) 0% oxide, (B) 2% oxide, and (C) 30% oxide. (D) magnified view of boxed area in (C). Reactions conducted at 550°C with 4:1 C2H4:H2.
The kinetics and microstructures shown in Figures and , respectively, indicate an increase in deposition rate commensurate with an increase in porosity. Even if all porosity is not readily accessible, as the reaction progresses, the disintegration of the catalyst particles continually exposes more pores. Porosity is expected to develop beyond the 5 min snapshot provided here, but the rapid increase in carbon volume and disintegration of particles makes it untenable to study the interface.
The presence of a surfactant is known to reduce particle size during milling [Citation34], which would increase specific surface area. In prior work [Citation24], the deposition process on MA Ni–Cu catalyst was found to be independent of particle size in similar microscale powders. The 30% oxide sample would have the smallest particle size due to oxide particles coating metallic surfaces so that fracture is favored over welding. Since, it does not have the highest deposition rate, particle size alone is not a primary factor in deposition rate.
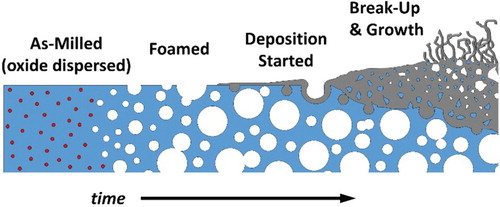
Because surface break-up is required, factors contributing to it will control the deposition rate. Microstructurally, the pure Ni–Cu alloy [Citation24] and the 2% oxide [Citation25] have been found to possess nanoscale and ultra-fine grains after milling, but only the 2% oxide alloy retains them at the reaction temperature. Also, the 2% oxide alloy forms significant porosity, thereby enhancing available surface area for reaction and break-up. The increased reactivity, then, is consistent with a defect-mediated growth mechanism due to enhanced diffusivity in nanocrystalline materials [Citation35] that is aided by in-situ pore formation. Therefore, this multifunctional alloy system allows one-step processing to be used to form and utilize a complex microstructure for efficient catalysis as shown schematically in Figure .
Figure 4. Schematic representation of the multifunctional alloy created through mechanical alloying. A single alloy is designed to serve multiple purposes through in-situ transformation.
The nearest metal–oxide materials to those used here are Ni–MgO and Ni–Cu–MgO [Citation22,Citation23] where MgO is a catalyst support, not a distributed phase. This represents the first report of in-situ pore development to enhance catalytic activity for CNF deposition, but CNFs have also been used to enhance the surface area and otherwise add functionality to ‘traditional’ metal foams. For example, work on the immobilization of a washcoat of CNFs on a preserved bulk Ni foam with pores that are 100s of microns has been reported [Citation31,Citation32]. CNFs deposited on these foams were found to be strongly anchored as long as catalyst disintegration was minimized.
The primary application of this powder is accelerated CNF deposition, but the same processing strategy can be applied for diverse functional purposes. For instance, nanostructured catalytic powder may be consolidated to create large-scale metallic foams by traditional solid state foaming methods, as recently reviewed [Citation36], and used for electrodes, filtration, and other functional designs. The CNFs produced also have diverse applications, especially as free-standing nanofibrous aerogel materials [Citation37]. These centimeter-scale aerogels require rapid deposition to be efficiently produced within three-dimensional molds. Smaller catalyst quantities are beneficial to achieve a nearly pure carbon structure (i.e. less than 0.01 vol% catalyst), or if the catalyst is beneficial to the function of the material, a high catalyst load may be used.
This work demonstrates that MA can be used to produce materials that provide multiple functionalities during processing and application. Although other strategies may be employed to achieve similar ends (e.g. using catalytic nanoparticles), the simplicity and versatility of this approach may be useful in other aspects as well. The enabling feature of this technique is that nonequilibrium and nanoscale distributions can be efficiently achieved, and the evolution of those features during later processing can be controlled to create unique properties. This principle is well known in other applications of MA, especially in thermal stabilization by phase formation or segregation [Citation38], but here we suggest there are diverse opportunities to tailor functional properties by similar processing, especially in catalytic applications.
Conclusion
A Ni 30 at% Cu catalyst was prepared by MA with 0, 2, or 30% CuO additions. By judicious selection of oxide content and processing, the catalyst particles could be made porous, with a commensurate increase in CNF deposition rate. All alloys performed better than comparable catalyst prepared by ‘traditional’ co-precipitation methods, with the 2% oxide sample reaching a peak deposition rate of 10,000% mass gain (g carbon/g catalyst) per hr. This represents an increase in efficiency of more than six times that of a co-precipitated alloy, and more than 1.5 times higher than the 0% and 30% MA alloys. The increased efficiency in all cases is attributed to efficient surface break-up facilitated by a refined microstructure, but the 2% oxide sample is further enhanced by the formation of porosity (∼20%) during deposition. CNFs have multiple uses as a stand-alone material and when applied to metallic surfaces, but the use of MA to create multifunctional alloys can provide a unique method to meet even more diverse applications.
Acknowledgements
The authors are grateful for access to the University of Delaware’s Keck Center for Advanced Microscopy and Microanalysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Mark A. Atwater http://orcid.org/0000-0001-7888-6209
Additional information
Funding
References
- Suryanarayana C. Mechanical alloying and milling. Prog Mater Sci. 2001;46:1–184. doi: 10.1016/S0079-6425(99)00010-9
- Ma E, Zhu T. Towards strength–ductility synergy through the design of heterogeneous nanostructures in metals. Mater Today. 2017;20:323–331. doi: 10.1016/j.mattod.2017.02.003
- Wu X, Zhu Y. Heterogeneous materials: a new class of materials with unprecedented mechanical properties. Mater Res Lett. 2017;5:527–532. doi: 10.1080/21663831.2017.1343208
- Koch CC. Structural nanocrystalline materials: an overview. J Mater Sci. 2007;42:1403–1414. doi: 10.1007/s10853-006-0609-3
- Humphry-Baker SA, Schuh CA. Spontaneous solid-state foaming of nanocrystalline thermoelectric compounds at elevated temperatures. Nano Energy. 2017;36:223–232. doi: 10.1016/j.nanoen.2017.04.018
- Cook B, Chan T, Dezsi G, et al. High-performance three-stage cascade thermoelectric devices with 20% efficiency. J Electron Mater. 2015;44:1936–1942. doi: 10.1007/s11664-014-3600-9
- Wang H, Li J-F, Nan C-W, et al. High-performance ag 0.8 pb 18+ x sbte 20 thermoelectric bulk materials fabricated by mechanical alloying and spark plasma sintering. Appl Phys Lett. 2006;88:092104. doi: 10.1063/1.2181197
- Dai W, Pan Y, Wang N, et al. Nanocrystalline nis particles synthesized by mechanical alloying as a promising oxygen evolution electrocatalyst. Mater Lett. 2018;218:115–118. doi: 10.1016/j.matlet.2018.01.141
- Zaluski L, Zaluska A, Ström-Olsen J. Hydrogen absorption in nanocrystalline mg2ni formed by mechanical alloying. J Alloys Compd. 1995;217:245–249. doi: 10.1016/0925-8388(94)01348-9
- Webb C. A review of catalyst-enhanced magnesium hydride as a hydrogen storage material. J Phys Chem Solids. 2015;84:96–106. doi: 10.1016/j.jpcs.2014.06.014
- Liang G, Huot J, Boily S, et al. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2–tm (tm = Ti, V, Mn, Fe and Ni) systems. J Alloys Compd. 1999;292:247–252. doi: 10.1016/S0925-8388(99)00442-9
- Schoenitz M, Ward T, Dreizin E. Preparation of energetic metastable nano-composite materials by arrested reactive milling. MRS Online Proceedings Library Archive 2003, 800.
- Ward TS, Chen W, Schoenitz M, et al. A study of mechanical alloying processes using reactive milling and discrete element modeling. Acta Mater. 2005;53:2909–2918. doi: 10.1016/j.actamat.2005.03.006
- Manotas-Albor M, Vargas-Uscategui A, Palma R, et al. In situ production of tantalum carbide nanodispersoids in a copper matrix by reactive milling and hot extrusion. J Alloys Compd. 2014;598:126–132. doi: 10.1016/j.jallcom.2014.01.191
- Orthner H, Tomasi R. Reaction sintering of titanium carbide and titanium silicide prepared by high-energy milling. Mater Sci Eng A. 2002;336:202–208. doi: 10.1016/S0921-5093(01)01963-3
- Guevara L, Wanner C, Welsh R, et al. Using mechanical alloying to create bimetallic catalysts for vapor-phase carbon nanofiber synthesis. Fibers. 2015;3:394–410. doi: 10.3390/fib3040394
- Feng L, Xie N, Zhong J. Carbon nanofibers and their composites: a review of synthesizing, properties and applications. Materials. 2014;7:3919–3945. doi: 10.3390/ma7053919
- Lu W, He T, Xu B, et al. Progress in catalytic synthesis of advanced carbon nanofibers. J Mater Chem A. 2017;5:13863–13881. doi: 10.1039/C7TA02007D
- Rodriguez NM. A review of catlytically grown carbon nanofibers. J Mater Res. 1993;8:3233–3250. doi: 10.1557/JMR.1993.3233
- Kim MS, Rodriguez NM, Baker RTK. The interaction of hydrocarbons with copper-nickel and nickel in the formation of carbon filaments. J Catal. 1991;131:60–73. doi: 10.1016/0021-9517(91)90323-V
- Klein KL, Melechko AV, Rack PD, et al. Cu–Ni composition gradient for the catalytic synthesis of vertically aligned carbon nanofibers. Carbon. 2005;43:1857–1863. doi: 10.1016/j.carbon.2005.02.027
- Wang H, Baker RTK. Decomposition of methane over a Ni−Cu−MgO catalyst to produce hydrogen and carbon nanofibers. J Phys Chem B. 2004;108:20273–20277. doi: 10.1021/jp040496x
- Izadi N, Rashidi A, Borghei M, et al. Synthesis of carbon nanofibres over nanoporous Ni–MgO catalyst: influence of the bimetallic Ni–(Cu, CO, MO) MgO catalysts. J Exp Nanosci. 2012;7:160–173. doi: 10.1080/17458080.2010.513019
- Guevara L, Welsh R, Atwater M. Parametric effects of mechanical alloying on carbon nanofiber catalyst production in the Ni-Cu system. Metals. 2018;8:286. doi: 10.3390/met8040286
- Atwater MA, Luckenbaugh TL, Hornbuckle BC, et al. Solid state foaming of nickel, Monel, and copper by the reduction and expansion of NiO and CuO dispersions. Adv Eng Mater. 2018;20:1800302. doi: 10.1002/adem.201800302
- Chen D, Christensen KO, Ochoa-Fernández E, et al. Synthesis of carbon nanofibers: effects of Ni crystal size during methane decomposition. J Catal. 2005;229:82–96. doi: 10.1016/j.jcat.2004.10.017
- Malesevic A, Chen H, Hauffman T, et al. Study of the catalyst evolution during annealing preceding the growth of carbon nanotubes by microwave plasma-enhanced chemical vapour deposition. Nanotechnology. 2007;18:455602. doi: 10.1088/0957-4484/18/45/455602
- Atwater MA, Phillips J, Leseman ZC. The effect of powder sintering on the palladium-catalyzed formation of carbon nanofibers from ethylene–oxygen mixtures. Carbon. 2010;48:1932–1938. doi: 10.1016/j.carbon.2010.01.060
- Toebes ML, Bitter JH, Van Dillen AJ, et al. Impact of the structure and reactivity of nickel particles on the catalytic growth of carbon nanofibers. Catal Today. 2002;76:33–42. doi: 10.1016/S0920-5861(02)00209-2
- Zeng Z, Natesan K. Relationship of carbon crystallization to the metal-dusting mechanism of nickel. Chem Mater. 2003;15:872–878. doi: 10.1021/cm020807l
- Jarrah NA, Ommen J, Lefferts L. Mechanistic aspects of the formation of carbon-nanofibers on the surface of Ni foam: a new microstructured catalyst support. J Catal. 2006;239:460–469. doi: 10.1016/j.jcat.2006.02.021
- Jarrah NA, Li F, van Ommen JG, et al. Immobilization of a layer of carbon nanofibres (CNFs) on Ni foam: a new structured catalyst support. J Mat Chem. 2005;15:1946–1953. doi: 10.1039/b416977h
- Jeong N, Lee J. Growth of filamentous carbon by decomposition of ethanol on nickel foam: influence of synthesis conditions and catalytic nanoparticles on growth yield and mechanism. J Catal. 2008;260:217–226. doi: 10.1016/j.jcat.2008.10.006
- Nouri A, Wen C. Surfactants in mechanical alloying/milling: a catch-22 situation. Cr Rev Sol State. 2014;39:81–108. doi: 10.1080/10408436.2013.808985
- Würschum R, Herth S, Brossmann U. Diffusion in nanocrystalline metals and alloys—a status report. Adv Eng Mater. 2003;5:365–372. doi: 10.1002/adem.200310079
- Atwater MA, Guevara LN, Darling KA, et al. Solid state porous metal production: a review of the capabilities, characteristics, and challenges. Adv Eng Mater. 2018;20:33.
- Atwater MA, Welsh RJ, Edwards DS, et al. Multiscale design of nanofibrous carbon aerogels: synthesis, properties and comparisons with other low-density carbon materials. Carbon. 2017;124:588–598. doi: 10.1016/j.carbon.2017.09.041
- Koch CC, Scattergood RO, Darling KA, et al. Stabilization of nanocrystalline grain sizes by solute additions. J Mater Sci. 2008;43:7264–7272. doi: 10.1007/s10853-008-2870-0
