Abstract
The precipitation of grain boundary (GB) κ-carbides critically influences the damage-tolerance ability of high-Mn high-Al lightweight steels, particularly in harsh environments (cryogenic and H environments). The formation and growth behavior of these carbides thus need to be understood. In this work, we use atom probe tomography and four-dimensional scanning transmission electron microscopy to study the formation mechanisms of GB κ-carbides in a Fe-28Mn-8Al-1.3C (wt.%) steel that is aged at 550°C, a temperature at which GB κ-carbide formation is often believed to be delayed or avoided. We observe that the formation of GB κ-carbides results from spinodal decomposition of grain interior (GI) κ-carbides and their further interaction with GB planes rather than from heterogeneous GB nucleation, as has been formerly proposed. However, the subsequent growth of GB κ-carbides shows different kinetics in comparison to GI κ-carbides. The underlying reason and its implications for future microstructure design of such steels are discussed.
IMPACT STATEMENT
We originally report that grain boundary κ-carbides can result from spinodal decomposition rather than conventional heterogeneous nucleation and refute the possibility of avoiding their precipitation by controlling aging parameters.
Austenitic high-Mn and high-Al lightweight steels, with a typical composition of Fe-(18–30)Mn-(7–12)Al-(0.7–2)C (in wt.%), belong to the group of advanced high-strength steels with potential applications in cryogenic, automotive and aircraft parts [Citation1–4]. The addition of Al enables a mass density reduction in these steels by 10–18% for the addition of 7–12 wt.% Al, respectively [Citation5]. Moreover, these steels possess higher specific energy absorption and strain hardening capabilities compared to other weight-reduced steels [Citation5] as well as a high tensile strength-ductility combination above ∼80,000 MPa% [Citation6].
The mechanical properties of these steels are highly tunable via the homogenous precipitation of nanosized κ-carbides throughout the matrix during age hardening. For instance, the yield strength can be increased from 540 to 880 MPa by age hardening at 550°C while maintaining a high ductility of 45% [Citation7]. However, the excellent damage tolerance of these steels becomes vulnerable by the formation of coarse heterogeneous grain boundary (GB) κ-carbides and more so when discontinuous precipitation occurs due to long-time and/or high-temperature age hardening [Citation8–11]. Discontinuous precipitation leads to the precipitation of a lamellar structure consisting of a solute-lean matrix, γ0, and solute-rich micro-sized carbides, κ0 [Citation9]. The growth of this lamellar structure is associated with GB migration [Citation12,Citation13]. The presence of large GB κ-carbides deteriorates the room-temperature tensile properties due to the brittle nature of κ-carbides [Citation14], which is not an issue for the smaller coherent, shearable grain interior (GI) κ-carbides [Citation15].
To suppress the formation of GB κ-carbides, a lower aging temperature (e.g. 450–550°C [Citation8,Citation9,Citation16–20]) has been proposed to favor precipitation of GI κ-carbides [Citation8,Citation14]. However, in a recent work [Citation7], atom probe tomography (APT) and four-dimensional scanning transmission electron microscopy (4D-STEM) results revealed the precipitation of nano-sized (GB) κ-carbides in a Fe-28Mn-8Al-1.3C steel heat treated at 550°C for 1 and 16 h. These precipitates were not detected by conventional scanning electron microscopy (SEM) techniques. While the 16-hour-aged steel exhibited a good room-temperature tensile property with a combination of 1 GPa tensile strength and 42% ductility, tests under harsh environments revealed significant ductility loss. Tensile samples tested after H-charging lost 77% of their ductility, while samples tested at cryogenic temperatures exhibit no macroscopic plastic deformation [Citation7]. The investigation of the fracture surface and cracking behavior attributed this deterioration to the precipitation of nano-sized GB κ-carbides and the decohesion of their associated hetero-interfaces under harsh conditions. These results strongly threaten the application potential of these steels and render them viable only in their relatively soft solution-treated state. Hence, the understanding of the formation of GB κ-carbides pertaining to both formation and growth should be revisited to aid future microstructure design, which is the main focus of this study.
The steel investigated in this work has a composition of Fe-28Mn-8Al-1.3C (wt.%) [Citation21]. The material was cold rolled, solution treated at 1000°C for 1 h, and lastly aged at 550°C for 16 h. This temperature has been considered the optimum aging temperature due to the suppressed formation of large-sized GB κ-carbides [Citation8,Citation9,Citation16–20]. At this temperature, the peak tensile strength and the peak hardness were reached after two and four months, respectively [Citation9]. Site-specific APT tips were prepared using a focused ion beam (FIB)/scanning electron microscopy (SEM) dual beam instrument (FEI Helios Nano-Lab 600i). Key steps in the preparation of GB-containing APT tips are shown in Supplementary Figure 1 in the Supplementary Materials. The APT experiments were performed in voltage mode in a LEAP 5000XS device operated at 70 K using a pulse fraction of 15%, a pulse repetition rate of 200 kHz and a detection rate of 0.5% ions per pulse. The acquired data was reconstructed and processed using the IVAS 6.3 software. We conducted 4D-STEM experiments to analyze the distribution of κ-carbides in a JEM-2200FS TEM (JEOL) operated at 200 kV, utilizing the TemCam-XF416 pixelated complementary metal–oxide-semiconductor (CMOS) detector for data acquisition. Further information regarding sample preparation and data collection can be found elsewhere [Citation7].
Figure shows the APT and TEM results of the solution-treated sample. The three-dimensional (3D) atomic map of C and B is shown in Figure (a). While B is only present as an impurity element, it serves to highlight the position of the GB due to its high tendency toward grain boundary segregation [Citation22]. In this sample, we observe no elemental clustering, as confirmed by the frequency distribution analysis [Citation23] shown in Figure (b). In this analysis, the observed distribution of elemental concentration in blocks of 100 atoms is compared with the binomial distribution representing ideal randomness. The near-perfect match between the observed and binomial distributions indicates the absence of clustering. A TEM selected area diffraction pattern (SADP) captured along the [001] zone axis is shown in Figure (c). We observe the existence of faint 100 superlattice reflections, indicating the onset of long-range ordering at this stage. This result matches well with other recent studies [Citation24,Citation25], which revealed the existence of ordered clusters with a size of 2–3 nm after quenching from the solution treatment temperature without any detectable chemical partitioning [Citation25].
Figure 1. Precipitation state in solution-treated condition. (a) 3D atomic map of C and B in a GB-containing APT sample. (b) Comparison of observed frequency distribution of elemental concentrations with the binomial frequency distribution reveals the absence of chemical clustering in the sample. (c) Diffraction pattern along the [001] zone axis.
![Figure 1. Precipitation state in solution-treated condition. (a) 3D atomic map of C and B in a GB-containing APT sample. (b) Comparison of observed frequency distribution of elemental concentrations with the binomial frequency distribution reveals the absence of chemical clustering in the sample. (c) Diffraction pattern along the [001] zone axis.](/cms/asset/9c689973-58b4-4ea7-b593-cdeb42934fa7/tmrl_a_2284321_f0001_oc.jpg)
Figure shows the APT and 4D-STEM results of samples age-hardened at 550°C for 16 h. The APT 3D atomic map of C and the impurity element B (Figure (a)) indicates the GB position as viewed from an edge-on perspective. Figure (b) shows the 3D concentration map of Al + C from the same point of view. This map is useful for the visualization [Citation26] of the Al and C-enriched κ-carbides, with an ideal chemical formula of (Fe, Mn)3AlC [Citation9,Citation27]. Hence, the regions with a higher Al + C concentration, green to yellow regions, represent κ-carbides, while the Al + C lean matrix is identified by blue color. In both grains observed, stacks of κ-carbides are found in two perpendicular directions, as indicated by the black dashed lines (Figure (b)). This pattern is a typical behavior of GI κ-carbides since they form homogenously by spinodal decomposition along <001> directions [Citation9,Citation28,Citation29]. The observed precipitates do not show the well-defined cuboid shape reported in similar steels heat treated at higher aging temperatures and/or for longer times, e.g. 24 h at 600°C in [Citation15]. We observe that some of the GI κ-carbides stacks (indicated by red arrows) terminate close to the GB, leaving solute-lean regions in the GB vicinity, as indicated by white-dotted ellipses. Frequently, large GB κ-carbides, associated with stacks indicated by black arrows, exist near these solute-lean regions. These GB κ-carbides have a larger average size of ∼13 nm compared with ∼6 nm for GI κ-carbides. GB κ-carbides in the left grain are viewed in Figure (c) through a section in the 3D Al + C concentration map parallel to the GB. We observe that GB κ-carbides exhibit a periodic arrangement in the GB plane, very similar to that of GI κ-carbides. Two sets of perpendicular nano-sized GB κ-carbides stacks can be observed, as highlighted by the black dashed lines. Such behavior indicates the occurrence of spinodal decomposition in the GB plane as well. 4D-STEM experiments were carried out to investigate the orientation relationship between GB κ-carbides and the matrix (Figure (d)). The virtual dark field image, shown in Figure (d1), reveals the precipitation state at and close to a high-angle GB. In this image, the left grain (G1) is imaged along the [001] zone axis. The GB is marked by a blue dotted line based on the change in diffraction pattern across the interface. Figure (d2 and d3) show the diffraction patterns obtained from a near-GB matrix region and a GB κ-carbide region, as indicated by the red and blue arrows, respectively. The diffraction patterns reveal the GB κ-carbides have the same orientation as the surrounding matrix. Hence, at this stage, GB κ-carbides grow into the matrix via the migration of the coherent interface rather than the migration of the incoherent interface, i.e. the GB. This confirms that the discontinuous precipitation process has not been initiated yet. The difference between the growth directions in both cases is illustrated in Figure (e).
Figure 2. Precipitation state in samples age-hardened at 550°C for 16 h. (a) 3D atomic map of C and B to highlight GB position. (b, c) κ-carbides visualization via 3D concentration maps of Al + C from a GB edge-on and GB face-on perspectives, respectively. (d) 4D-STEM observations of the GB region imaged along the [001] zone axis. (d1) Virtual dark field image. (d2, d3) diffraction patterns from the matrix and a GB κ-carbides, respectively. (e) The direction of GB κ-carbides growth observed in this work (top) compared with the growth direction during discontinuous precipitation (bottom).
![Figure 2. Precipitation state in samples age-hardened at 550°C for 16 h. (a) 3D atomic map of C and B to highlight GB position. (b, c) κ-carbides visualization via 3D concentration maps of Al + C from a GB edge-on and GB face-on perspectives, respectively. (d) 4D-STEM observations of the GB region imaged along the [001] zone axis. (d1) Virtual dark field image. (d2, d3) diffraction patterns from the matrix and a GB κ-carbides, respectively. (e) The direction of GB κ-carbides growth observed in this work (top) compared with the growth direction during discontinuous precipitation (bottom).](/cms/asset/11584a7d-1c4f-46f4-b9ab-10541d9803ac/tmrl_a_2284321_f0002_oc.jpg)
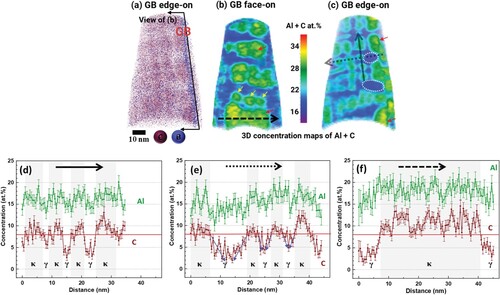
Figure shows the reconstruction of another age-hardened sample lifted out of a different high-angle GB. Figure (a) provides the 3D atomic map of C and B. Figure (b and c) show sections in the 3D concentration map of Al + C from GB face-on and edge-on perspectives, respectively. We observe that the GB carbides have different sizes between ∼7 nm and ∼35 nm (Figure (b)). The largest GB carbide in this specimen, indicated by the black dashed arrow, has a dimension of 12 × 21 × 35 nm3. Larger precipitates, indicated by red arrows, appear to expand over the positions of multiple stacks of κ-carbides when compared with the smaller precipitates indicated by yellow arrows. The location of the largest GB κ-carbide next to the smallest GB κ-carbides suggests the dissolution of the latter and growth of the former via Ostwald ripening. The driving force behind this process is the discrepancy in chemical potential between larger and smaller κ-carbides. The higher surface energy, or equivalently, the higher chemical potential, prompts the migration of atoms from smaller κ-carbides to larger ones. Evidence of such κ-carbide dissolution is also demonstrated in Figure (c), which shows distinct near-GB solute-lean areas (as indicated by white-dotted ellipses) adjacent to larger κ-carbides. Figure (d and e) show the one-dimensional (1D) concentration profiles for C and Al across GI κ-carbides along directions parallel and perpendicular to GB, respectively, as indicated by the solid and dotted black lines in Figure (c). We observe that the C content in the solute-lean matrix decreases as the distance from κ-carbides increases. This means that larger inter-particle spacings are associated with a lower minimum carbon content as shown by the blue arrows in Figure (e). This is due to the elastic distortion resulting from the difference in lattice parameter between the κ-carbides and the matrix [Citation30]. This elastic strain is largest at the interface between the precipitate and the matrix, which leads to a concentration gradient across the interface. The distorted matrix near the carbide interface tends to dissolve more C to minimize the elastic strain. For closely precipitated carbides, the whole interparticle spacing is affected by the strain field from both carbides and a higher average concentration of C in the adjoining γ-matrix is favorable for stress release. As the distance from the carbides increases, less C is necessary to minimize the elastic strain in the matrix. On the other hand, C concentration in κ-carbides is lower at the interface. This phenomenon makes it difficult to define the interface between κ-carbides and the surrounding C-rich matrix. When different chemical compositions and age-hardening stages are also considered, different C concentrations could be used to define κ-carbides. Previously, C concentrations of 9 at.% [Citation15] and 7 at.% [Citation31] have been used. In Figure (d–f), we use C concentrations of 8 at.% to identify κ-carbides, as indicated by the horizontal red line. This value leads to a good match between the sizes of κ-carbides measured from 1D concentration profiles and Al + C 3D concentration maps. Figure (f) shows the 1D concentration profile for C and Al across a large GB precipitate, as indicated by the black dashed arrow in Figure (b). We observe that the C content is not constant across the precipitate but shows a periodicity similar to that observed across multiple smaller, unconnected precipitates. This implies the coalescence of multiple GB κ-carbides, where the relatively solute-lean regions resemble former interparticle spacing. We note that the APT results in Figures and provide localized information. This allowed the investigation of GB κ-carbides at different stages of growth (different sizes) even within one sample.
Figure 3. Analysis of an age-hardened GB-containing APT sample. (a) 3D atomic map of C and B to highlight the GB position. (b, c) κ-carbides visualization via sections in the 3D concentration map of Al + C from GB face-on and GB edge-on perspectives, respectively. (d–f) 1D concentration profiles, (d) parallel to the GB, (e) perpendicular to the GB and (f) in the GB plane, as indicated by black solid, dotted and dashed lines, respectively. The red horizontal line at 8 at.% signifies the C concentration used to identify κ-carbides.
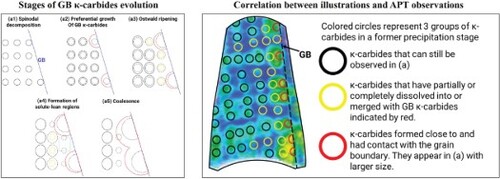
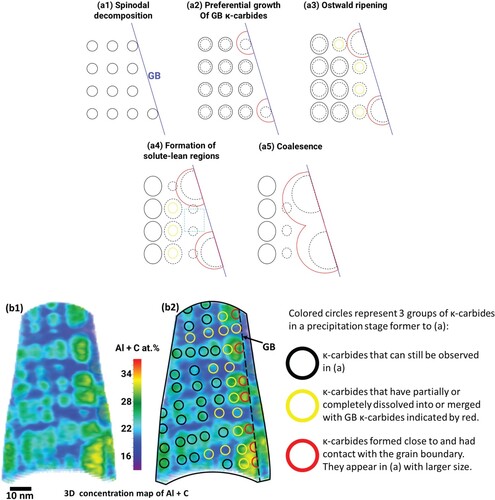
Based on these observations, we illustrate the behavior of the formation of GB κ-carbides in the steel investigated in Figure (a) and summarize it as follows: (a) Phase separation: Spinodal decomposition produces a precursor state preceding the formation of κ-carbides continuously in the matrix, Figure (a1). This process takes place during quenching from the single-phase region. Naturally, some of these precursors/κ-carbides will occur next to (or at) GBs. During their growth, these κ-carbides contact the GBs to form GB κ-carbides, which have one incoherent interface (i.e. the GB) with the neighboring grain, in contrast to the coherent interface with the parent grain from which they stem. This finding contradicts the previous understanding of GB κ-carbide formation at higher aging temperatures or longer aging times, which has been attributed to heterogeneous GB nucleation as the predominant mechanism [Citation8,Citation12]. Unlike heterogeneous nucleation, there is no energy barrier to spinodal decomposition. Hence, while GB κ-carbides could still nucleate heterogeneously, they would be disfavored at temperatures in the spinodal range. (b) GB κ-carbide growth and coalescence: In this stage, GB κ-carbides grow into the parent grain rather than the neighboring grain. This is probably driven by the lower energy burden associated with the increase in the area of coherent interfaces with the parent grain compared with incoherent interfaces with the neighboring grain. The growth kinetics of GB κ-carbides are probably driven by solute diffusion along GBs, which can be several orders of magnitude faster than bulk diffusion [Citation32]. This difference in diffusion leads to a size advantage for GB κ-carbides over GI κ-carbides, Figure (a2). Due to the variance in the size of κ-carbides, competitive Ostwald ripening is initiated (Figure (a3)), leading to the dissolution of smaller GB κ-carbides and the nearest GI κ-carbides in favor of the growth of larger GB κ-carbides. This process leaves solute-lean regions in the vicinity of GB, as highlighted by the teal square in Figure (a4). In Figure (b), we establish the connection between our illustrations and observations by overlaying a section in the 3D Al + C concentration map, Figure (b1), with an array of circles that resemble κ-carbides at an earlier stage of precipitation, as shown in Figure (b2). κ-carbides formed as a result of spinodal decomposition are distributed periodically, as indicated by colored circles. The red-colored group benefited from GB diffusion, leading to faster growth kinetics. Their larger size compared with neighboring carbides allowed them to consume the yellow group and grow to the sizes observed in Figure (b1). As observed, some of these growing precipitates will cross paths and coalesce (Figure (a5)). The presence of coarse intergranular carbides on GBs eventually leads to the onset of GB migration and discontinuous precipitation. This has been observed for GB Cr23C6 carbides in a Ni-based Alloy 690, whose orientation relationship with the matrix is the same as that for GB κ-carbides [Citation33].
Figure 4. Illustrations of the formation and growth mechanism of grain boundary (GB) κ-carbides. (a) A sketch of different stages of the formation and growth of κ-carbides. Each stage is redrawn in dashed lines in the following stage. Black circles: κ-carbides, red circles: GB κ-carbides, yellow circles: dissolving κ-carbides. Teal square: solute-lean region. (b) Associating illustrations with APT observations. (b1) A section in the 3D concentration map of the age-hardened sample in Figure . (b2) Overlayed image of (b1) with a schematic of κ-carbides in an aging step prior to (b1).
To conclude, the purpose of this work was to study the early precipitation stage of GB κ-carbide in a high-Mn, high-Al lightweight steel aged at 550 ˚C, in order to provide insights for high-strength and damage-tolerant microstructure design in this type of steel. We find that, at this aging temperature, GB κ-carbides form through spinodal decomposition similar to GI κ-carbides. Formation of GB κ-carbides via a heterogeneous nucleation process is still expected at aging temperatures higher than the spinodal range. Despite forming by the same spinodal decomposition process, the growth of GB precipitates exhibits higher kinetics in comparison to GI κ-carbides, which was proposed to be due to the faster solute diffusion along the GB plane. Our study thus suggests that it would be a very challenging task to completely eliminate the existence of GB κ-carbides in this type of steel subjected to age hardening, which could be an issue for their application in harsh environments with a high damage-tolerance requirement.
Supplemental Material
Download MS Word (887.2 KB)Acknowledgment
ME acknowledges the funding of DAAD and MoHE of Egypt to his PhD studies through GERLS program.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gutierrez-Urrutia I, Raabe D. Influence of Al content and precipitation state on the mechanical behavior of austenitic high-Mn low-density steels. Scr. Mater. 2013;68:343–347. doi:10.1016/j.scriptamat.2012.08.038
- Zuazo I, Hallstedt B, Lindahl B, et al. Low-density steels: complex metallurgy for automotive applications. JOM. 2014;66:1747–1758. doi:10.1007/s11837-014-1084-y
- Gutierrez-Urrutia I. Low density Fe-Mn-Al-C steels: phase structures, mechanisms and properties. ISIJ Int. 2021;61:16–25. doi:10.2355/isijinternational.ISIJINT-2020-467
- Frommeyer G, Brüx U. Microstructures and mechanical properties of high-strength Fe-Mn-Al-C light-weight TRIPLEX steels. Steel Res Int. 2006;77:627–633. doi:10.1002/srin.200606440
- Raabe D, Springer H, Gutierrez-Urrutia I, et al. Alloy design, combinatorial synthesis, and microstructure–property relations for low-density Fe-Mn-Al-C austenitic steels. JOM. 2014;66:1845–1856. doi:10.1007/s11837-014-1032-x
- Yoo JD, Park KT. Microband-induced plasticity in a high Mn-Al-C light steel. Mater Sci Eng A. 2008;496:417–424. doi:10.1016/j.msea.2008.05.042
- Elkot MN, Sun B, Zhou X, et al. Hydrogen-assisted decohesion associated with nanosized grain boundary κ-carbides in a high-Mn lightweight steel. Acta Mater. 2022;241:118392. doi:10.1016/j.actamat.2022.118392
- Acselrad O, Kalashnikov IS, Silva EM, et al. Diagram of phase transformations in the austenite of hardened alloy Fe-28% Mn-8.5% Al-1% C-1.25% Si as a result of aging due to isothermal heating. Met Sci Heat Treat. 2006;48:543–553. doi:10.1007/s11041-006-0133-8
- Choo WK, Kim JH, Yoon JC. Microstructural change in austenitic Fe-30.0wt%Mn-7.8wt%Al-1.3wt%C initiated by spinodal decomposition and its influence on mechanical properties. Acta Mater. 1997;45:4877–4885. doi:10.1016/S1359-6454(97)00201-2
- Yao M. κ-carbide in a high-Mn light-weight steel: precipitation, off-stoichiometry and deformation. Aachen: RWTH Aachen; 2017.
- Banis A, Gomez A, Bliznuk V, et al. Microstructure evolution and mechanical behavior of Fe–Mn–Al–C low-density steel upon aging. Mater Sci Eng A. 2023;875:145109. doi:10.1016/j.msea.2023.145109
- Chen S, Rana R, Haldar A, et al. Current state of Fe-Mn-Al-C low density steels. Prog Mater Sci. 2017;89:345–391. doi:10.1016/j.pmatsci.2017.05.002
- Williams DB, Butler EP. Grain boundary discontinuous precipitation reactions. Int Met Rev. 1981;26:153–180. doi:10.1179/imr.1981.26.1.153
- Cheng WC, Cheng CY, Hsu CW, et al. Phase transformation of the L12 phase to kappa-carbide after spinodal decomposition and ordering in an Fe-C-Mn-Al austenitic steel. Mater Sci Eng A. 2015;642:128–135. doi:10.1016/j.msea.2015.06.096
- Yao MJ, Welsch E, Ponge D, et al. Strengthening and strain hardening mechanisms in a precipitation-hardened high-Mn lightweight steel. Acta Mater. 2017;140:258–273. doi:10.1016/j.actamat.2017.08.049
- Tjong SC. Electron microscope observations of phase decompositions in an austenitic Fe-8.7Al-29.7Mn-1.04C alloy. Mater Charact 1990;24:275–292. doi:10.1016/1044-5803(90)90055-O
- Springer H, Raabe D. Rapid alloy prototyping: compositional and thermo-mechanical high throughput bulk combinatorial design of structural materials based on the example of 30Mn-1.2C-xAl triplex steels. Acta Mater. 2012;60:4950–4959. doi:10.1016/j.actamat.2012.05.017
- Feng Y, Song R, Pei Z, et al. Effect of aging isothermal time on the microstructure and room-temperature impact toughness of Fe–24.8Mn–7.3Al–1.2C austenitic steel with κ-carbides precipitation. Met Mater Int. 2018;24:1012–1023. doi:10.1007/s12540-018-0112-9
- Bentley AP. Ordering in Fe-Mn-Al-C austenite. J Mater Sci Lett. 1986;5:907–908. doi:10.1007/BF01729270
- Chang KM, Chao CG, Liu TF. Excellent combination of strength and ductility in an Fe-9Al-28Mn-1.8C alloy. Scr Mater. 2010;63:162–165. doi:10.1016/j.scriptamat.2010.03.038
- Ji F, Song W, Ma Y, et al. Recrystallization behavior in a low-density high-Mn high-Al austenitic steel undergone thin strip casting process. Mater Sci Eng A. 2018;733:87–97. doi:10.1016/j.msea.2018.07.023
- Paju M, Viefhaus H, Grabke HJ. Phosphorus segregation in austenite in Fe-P-C, Fe-P-B and Fe-P-C-B alloys. Steel Res. 1988;59:336–343. doi:10.1002/srin.198801524
- Moody MP, Stephenson LT, Ceguerra AV, et al. Quantitative binomial distribution analyses of nanoscale like-solute atom clustering and segregation in atom probe tomography data. Microsc Res Tech. 2008;71:542–550. doi:10.1002/jemt.20582
- Wang Z, Lu W, Zhao H, et al. Formation mechanism of κ-carbides and deformation behavior in Si-alloyed FeMnAlC lightweight steels. Acta Mater. 2020;198:258–270. doi:10.1016/j.actamat.2020.08.003
- Zhang J, Jiang Y, Zheng W, et al. Revisiting the formation mechanism of intragranular κ-carbide in austenite of a Fe-Mn-Al-Cr-C low-density steel. Scr Mater 2021;199:113836. doi:10.1016/j.scriptamat.2021.113836
- Hellman OC, du Rivage JB, Seidman DN. Efficient sampling for three-dimensional atom probe microscopy data. Ultramicroscopy. 2003;95:199–205. doi:10.1016/S0304-3991(02)00317-0
- Yao MJ, Dey P, Seol JB, et al. Combined atom probe tomography and density functional theory investigation of the Al off-stoichiometry of κ-carbides in an austenitic Fe-Mn-Al-C low density steel. Acta Mater. 2016;106:229–238. doi:10.1016/j.actamat.2016.01.007
- Sato K, Tagawa K, Inoue Y. Modulated structure and magnetic properties of age-hardenable Fe-Mn-Al-C alloys. Metall Trans A. 1990;21:5–11. doi:10.1007/BF02656419
- Sato K, Tagawa K, Inoue Y. Spinodal decomposition and mechanical properties of an austenitic Fe-30wt.%Mn-9wt.%Al-0.9wt.%C alloy. Mater Sci Eng A. 1989;111:45–50. doi:10.1016/0921-5093(89)90196-2
- Liebscher CH, Yao M, Dey P, et al. Tetragonal fcc-Fe induced by κ -carbide precipitates: atomic scale insights from correlative electron microscopy, atom probe tomography, and density functional theory. Phys Rev Mater. 2018;2:1–6. doi:10.1103/PhysRevMaterials.2.023804
- Zhi H, Li J, Li W, et al. Simultaneously enhancing strength-ductility synergy and strain hardenability via Si-alloying in medium-Al FeMnAlC lightweight steels. Acta Mater. 2023;245:118611. doi:10.1016/j.actamat.2022.118611
- Aaron HB, Aaronson HI. Growth of grain boundary precipitates in Al-4% Cu by interfacial diffusion. Acta Metall. 1968;16:789–798. doi:10.1016/0001-6160(68)90097-7
- Lim YS, Kim DJ, Hwang SS, et al. M23c6 precipitation behavior and grain boundary serration in Ni-based Alloy 690. Mater Charact 2014;96:28–39. doi:10.1016/j.matchar.2014.07.008