?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Zein nanoparticles as a carrier system for BMP6-derived peptide were prepared by liquid–liquid phase separation procedure and characterized with SEM, DLS, FTIR and thermogravimetric methods. After peptide encapsulation, nanoparticle size increased from 236.3 ± 92.2 nm to 379.4 ± 116.8 nm. The encapsulation efficiency of peptide was 72.6% and the release of peptide from Zein nanoparticles was partly sustained in trypsin containing phosphate buffered saline (pH 7.4) for up to 14 days. Peptide-loaded nanoparticles showed similar cell viability compared with blank ones. ALP activity of C2C12 cells treated with peptide-loaded nanoparticles (500 µg/mL) was evaluated 7, 14, 21 and 28 days after culture. In peptide-loaded nanoparticles, ALP activity was significantly higher (p < .05) compared with other groups at day 14. Alizarin Red S staining showed, C2C12 cells behind peptide-loaded nanoparticles had significantly (p < .05) higher calcium deposition at day 21. The results of RT-qPCR show that the BMP-6 peptide activated expression of RUNX2 as a transcription factor. In turn, RUNX2 regulates SPP1 and BGLAP gene expression, as osteogenic marker genes. The results confirm that the peptide-loaded Zein nanoparticles, as osteoinductive material, may be used to repair small area of bone defects, with low load bearing.
Introduction
Bone tissue engineering is a new and developing technique that reduces limitations of bone grafts and improves the healing process of bone fractures and defects [Citation1]. Using this method, the combined use of scaffolds and growth factors initiates migration and recruitment of osteoprogenitor cells, followed by their proliferation, differentiation and matrix formation [Citation2,Citation3]. The most important growth factors in bone formation and regeneration are bone morphogenetic proteins (BMPs), members of the cytokine group [Citation4]. BMPs are divided into BMP-2/-4, BMP-5/-6/-7/-8/-8b, BMP-9/-10 and BMP-12/-13/-14 subgroups. The sequences of the members of these subgroups are more than 50% similar [Citation5]. The high osteoinductive capacity of BMPs was one of the main reasons for their extensive usage in clinical therapies for bone tissue engineering [Citation6]. BMP-2, 6 and 9 exhibited the greatest ability for inducing both early and late osteogenic markers, as well as matrix mineralization in osteoblastic progenitor cell lines such as C2C12 [Citation7]. BMPs have a short half-life and a large amount is needed for marked osteoinductive effects [Citation8]. On the other hand, high doses of BMPs may cause adverse effects such as ectopic bone formation [Citation9], cyst-like bone, soft tissue swelling [Citation10], organogenesis, apoptosis and immunogenicity [Citation4]. Short peptides derived from knuckle epitope (type II receptor binding site) of BMPs are an appropriate substituent for native BMP proteins [Citation11]. These peptides can be synthesized in laboratories and are relatively more stable during the modification process. Delivery systems such as nanoparticles are useful for controlled release and bioactivity maintenance of peptides [Citation12] and also may decrease undesirable effects of BMP molecules [Citation13]. The strategy of drug conjugation to the nanocarrier is highly important for a controlled drug delivery. A drug may be encapsulated into the nanocarrier or covalently attached or absorbed to its surface [Citation14]. Nanomaterial fabrication with natural polymers has several advantages, such as high biocompatibility, biodegradability and low toxicity [Citation15]. Nanocarriers from hydrophobic plant proteins, in contrast to hydrophilic animal proteins, are more desirable in sustained drug release [Citation16,Citation17], since they are less expensive and there is a lower risk of zoonotic disease transmission than animal proteins [Citation18]. Zein is a corn storage protein and classified generally recognized as safe (GRAS) by the Food and Drug Administration (FDA). Zein contains a high proportion of hydrophobic amino acids, like leucine, proline and alanine, and is hence, insoluble in water [Citation19]. Zein nanoparticles have been used to encapsulate several drugs, including ivermectin, 5-fluorouracil (5-FU) [Citation20] and terpinen-4-ol (T4OL); they are candidates for sustained drug release [Citation21]. In this study, Zein nanoparticles were loaded with BMP6-derived peptide (BMP6Pep) using the encapsulation method. Peptide encapsulation efficiency and release kinetics were estimated and in vitro activity was followed by determining the osteoinduction ability of the peptide in C2C12 cells.
Materials and methods
Synthesis of BMP6Pep
The BMP6Pep (H-YVPKPCCAPTKLNAISVLYF-OH) with 90% purity was synthesized by Mimotopes Peptide Company (Mulgrave, Australia). Liquid chromatography and mass spectroscopy analysis confirmed peptide purity.
Preparation of Zein nanoparticles
Zein nanoparticles were prepared by liquid–liquid phase separation chromatography. One hundred milligrams of Zein purified powder (Sigma-Aldrich, St. Louis, MO, CAS Number: 9010-66-6) was dissolved in 10 mL aqueous ethanol solution (70% (v/v)). Twenty-five millilitres of MilliQ water was added to a beaker and stirred vigorously (10,000 rpm). The Zein solution was added to the stirring water using a fine dropper slowly (1 mL/min). Subsequently, the resulting suspension was placed on a magnetic stirrer (1000 rpm) for another 3 h to evaporate the ethanol. Large particles were removed by centrifugation (Sigma, St. Louis, MO) at 2000×g for 5 min. The remaining suspension was then freeze-dried (Martin Christ, Osterode am Harz, Germany) at –20 °C overnight. To prepare peptide-loaded Zein nanoparticles, 10 mg BMP6Pep was dissolved in the hydroalcoholic phase and the nanoparticles were prepared using the same method as described above.
Peptide encapsulation efficiency and peptide release studies
For encapsulation efficiency, the nanoparticulate suspension was centrifuged at 16,000×g for 20 min at 4 °C. The peptide encapsulated nanoparticles were collected and washed three times with PBS. The supernatant and the washing solutions were collected together to determine the peptide encapsulation efficiency using Gradient HPLC (KNAUER) with UV detector (K2600) and C18 column (Eurosil Bioselect 300-C, 125 × 4.6 mm). The mobile phase (solvent A: 0.1% trifluoroacetic acid in 100% water and solvent B: 0.1% trifluoroacetic acid in 90% acetonitrile (aqueous)) consisted of 10% B for 1.0 min and then 10–66.6% B (linear) over 15.0 min; the flow rate was 1.0 mL/min. The injection volume was 20 μL and detection was carried out at 214 nm. Zein nanoparticle supernatant was used as control. Different concentrations of peptide solution were used to draw a standard curve. Encapsulation efficiency was calculated according to the following equation:
(1)
(1)
In vitro release of peptide from Zein nanoparticles was determined in Trypsin (Sigma, St. Louis, MO) containing PBS solution (pH 7.4) during different time periods (every two days) for 14 days. The peptide-loaded nanoparticles (100 mg) were suspended directly in 2 mL PBS and placed in a shaker incubator at a speed of 150 rpm at 37 °C for different time periods. At the end of each time point, the PBS solution was collected and replaced with a fresh solution. The amount of peptide released in the medium was monitored using gradient HPLC as above described.
Characterization
Particle size detection and zeta potential measurements
Size and ζ-potential of particles were measured by dynamic light scattering (DLS) using a Zetasizer Nano (Malvern Instruments Ltd, Malvern, UK). Nanoparticle lyophilized powder (1 mg) was dispersed in PBS (pH: 7.4), probe sonicated and centrifuged at 2000×g for 5 min. Supernatant was diluted with PBS with appropriate dilution to avoid multiple particle effects. All measurements were repeated three times.
Scanning electron microscopy imaging
The surface morphology of nanoparticles was analysed by scanning electron microscopy (SEM) (Carl Zeiss, Oberkochen, Germany, LEO 1430 VP). Briefly, 1 mg of nanoparticle powder was dispersed in 0.5 mL of deionized water and centrifuged at 2000×g for 5 min. The nanoparticles in the supernatant were vacuum-dried on glass and coated with gold. An accelerating voltage of 15 kV was used for imaging. Nanoparticle size was measured by using SEM images processed with the ImageJ software (version 1.46r, NIH, Bethesda, MD).
FTIR spectrum
For IR analysis, nanoparticle powder was first dispersed in KBr (Merck, Kenilworth, NJ, infrared grade) and then converted into pellets. The FT-IR spectrum of Zein nanoparticles was recorded by a FTIR spectrometer (alpha FTIR, Bruker, Billerica, MA) from 4000 to 400 cm−1.
TGA analysis
The thermogravimetric analysis (TGA) of nanoparticles and peptide-loaded nanoparticles was carried out in a thermogravimetric analyzer (LINSEIS TGA/DSC Instruments, Robbinsville, NJ, STA PT1000). The temperature range was from 50 to 500 °C with a heating rate of 10 °C/min and under a nitrogen stream.
In vitro activity of BMP6Pep loaded nanoparticles
Cell culture
C2C12 cells (Pasteur Institute of Iran, Tehran, Iran, C521) were trypsinized (0.05% trypsin–EDTA, Bio-Idea), centrifuged and resuspended in a complete culture medium that contained Dulbecco’s modified Eagle’s medium (Bio-Idea, Houston, TX, Glutamax high glucose), 10% heat-inactivated foetal bovine serum (Gibco, Carlsbad, CA) and 1% antibiotics (Bio-Idea, Houston, TX, 100× penicillin/streptomycin solution). Aliquots of 100 µl containing 3 × 104 cells were then cultured in each well of a 48 well plate. After 24 h, one group of cells was cultured in a medium alone, a second group was cultured in the same medium plus 500 µg/mL BMP6Pep encapsulated Zein nanoparticles and the third group was cultured with a medium plus blank Zein nanoparticles.
MTT assay
Cell proliferation was measured using the MTT assay kit (Bio-Idea, Houston, TX) at 2 and 3 days. For MTT assay, different concentrations of nanoparticles (500, 750, 1000 and 1250 µg/mL) were used. The test was performed using manufacturers recommended protocol. Briefly, the medium was removed and replaced with 200 µl ready to use DMEM and then 20 µl of MTT solution was added to each well. This was incubated for 4 h (5% CO2, 37 °C, 95% humidity). The solution was then removed and 100 µl dimethylsulphoxide (DMSO, 99.5%; Sigma, St. Louis, MO) was added to dissolve the formazan products. The plate was incubated for 15 min at room temperature with gentle shaking. A 100 µl of solution was transferred to an ELISA plate and the absorbance was read by an ELISA plate reader (Biotek, Winooski, VT) at 490 nm with a reference wavelength of 630 nm.
ALP assay
Nanoparticle treated cells (500 µg/mL), were studied for alkaline phosphatase activity during different time periods (1, 2, 3 and 4 weeks). After medium removal, cells were rinsed with PBS and homogenized in ice cold RIPA buffer (150 mM NaCl, 0.1% Triton X-100, 50 mM Tris–HCl pH 8.0 and PMSF) for 30 min at 4 °C. Then the homogenized mixture was centrifuged at 16,000×g for 20 min at 4 °C. The supernatant was transferred to a fresh tube and stored in –20 °C until further analysis. ALP activity of the supernatant was determined using the colorimetric ALP activity assay. Briefly, 50 µl of supernatant was added to 150 µl assay buffer containing diethanolamin (1 M), magnesium chloride (0.5 mM) and p-nitrophenylphosphate (10 mM) and incubated at 37 °C for 30 min. Absorbance was recorded at 405 nm after addition of 2 M NaOH and 0.2 mM EDTA for reaction inhibition.
Alizarin Red staining
Calcium deposition in C2C12 cells was evaluated with Alizarin Red S (ARS) assay. Twenty one days after cell seeding, the medium was removed and cells were washed with PBS. Afterwards, cells were fixed in 4% formaldehyde at room temperature for 15 min. The fixative was removed and the cells washed three times with distilled H2O. One millilitre of ARS 2% (w/v) solution (Bio-Idea, Houston, TX) was added to each well and the plate incubated at room temperature with gentle shaking. After 30 min the dye was removed and the cells washed five times with distilled H2O. To quantify the ARS, after the cells were washed with 10% cetylpyridinium chloride (for dye extraction) the optical density was read at 490 nm with an ELISA plate reader (Biotek, Winooski, VT).
Real-time PCR (RT-qPCR) analysis
Real-time PCR was conducted to evaluate the expression of three osteogenic genes including RUNX2, BGLAP and SPP1. Expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an endogenous control. The RNA was extracted using TRIzol Reagent (ThermoFisher, Waltham, MA, CFX 96), after a time period of seven days and 14 days. Culture medium was removed and 500 µl TRIzol Reagent was added to each well and after homogenization, RNA was isolated by following the protocol supplied by the manufacturer. RNA quantity was determined using a NanoDrop instrument (Thermo-Onec, Wilmington, DE). Complimentary DNA (cDNA) was synthesized according to Revert Aid First Strand cDNA Synthesis kit (ThermoFisher, Waltham, MA) protocol. The primer sequences of the genes were obtained from NCBI online primer design as follows: RUNX2 gene primers; 5′-CAGCCACC TTTACCTACACC-3′ (forward) and 5′-GACTCATCCATTCTGCCGCTA-3′ (reverse), SPP1 gene primers; 5′-TCCAATGAAAGCCATGACCAC-3′ (forward) and 5′-ATCAGACTCATCCGAATGGTG-3′ (reverse), BGLAP gene primers; 5′-CTGACAAAGCCTTCATGTCCA-3′ (forward) 5′-AGCCATACTGGT CTGATAGCTC-3′ (reverse). RT-qPCR (Bio-Rad PCR machine, Hercules, CA) was carried out as follows: 35 cycles of PCR consisting of 95 °C for 30 s, 58 °C for 30 s and 72 °C for 30 s. The initial denaturation step was 5 min at 95 °C and final extension step was 72 °C for 5 min. PCR components included 10 μL of SYBR Green master mix (Invitrogen, Carlsbad, CA), 1 μL of each forward and reverse primers and 1 μL of cDNA templates in a final reaction volume of 25 µl. Quantification of gene expression was based on the CT value for each sample compared to GAPDH, calculated as the average of three replicate measurements for each sample analysed.
Statistical analysis
One-way ANOVA was used to determine the differences between control and nanoparticle treated samples. The Kolmogorov–Smirnov test was used as normal probability distribution. Data are reported as mean ± SD at a significance level of p < .05.
Results and discussion
Peptide encapsulation efficiency and peptide release studies
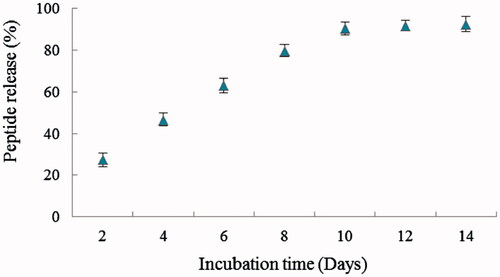
Retention time of BMP6Pep was ∼15 min and the surface area of HPLC chromatogram was used for peptide concentration determination. The encapsulation efficiency of BMP6Pep in Zein was determined to be ∼72.6%. Therefore, peptide concentration in 500, 750, 1000 and 1200 µg/mL of BMP6Pep loaded nanoparticle was ∼36, ∼54, ∼73 and ∼91 µg/mL, respectively. shows in vitro release of BMP6Pep from Zein nanoparticles. Except for the first time period, the peptide release was sustained up to 14 days, without any burst effects. Fast release of peptide, two days after incubation, may be due to BMP6Pep absorption to the high surface area of Zein nanoparticles [Citation22]. Release speed of active components from nanoparticles may be affected by a combination of several factors such as size and physicochemical property of nanoparticles, the characteristic and concentration of active component and the condition of release medium (pH, temperature and ionic force) [Citation23].
Nanoparticle characterization
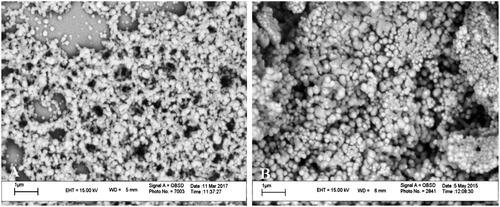
shows the particle size distribution and zeta potential of blank and peptide-loaded Zein nanoparticles. Average size in blank nanoparticles (236.3 ± 92.2 nm, PdI: 0.151) was smaller than peptide-loaded ones (379.4 ± 116.8 nm, PdI: 0.096). As mentioned before with Podaralla and Perumal, Zein nanoparticles in pH 7.4 had a negative surface charge [Citation24] and in peptide-loaded nanoparticles, there was no significant change in the surface charge (–10.2 ± 3.96 and –12 ± 5.59 for blank and peptide-loaded nanoparticles, respectively). SEM analyses were performed in blank and peptide-loaded nanoparticles (). The particles were sphere shaped and had a smooth surface. The average diameter of nanoparticles that was calculated using the imagej software was ∼187 nm and ∼209 nm for blank and peptide-loaded nanoparticles, respectively. The size distribution was widely scattered and smaller average particle diameter in SEM images compared to DLS analysis may be due to nanoparticle agglomeration in liquid medium [Citation25].
Figure 2. Representative particle size distribution and zeta potential of blank Zein nanoparticles (A, C) and peptide-loaded Zein nanoparticles (B, D), respectively.
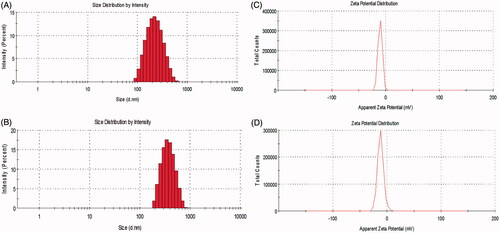
Figure 3. Electron microscopy images of blank Zein nanoparticles (A) and peptide-loaded Zein nanoparticles (B).
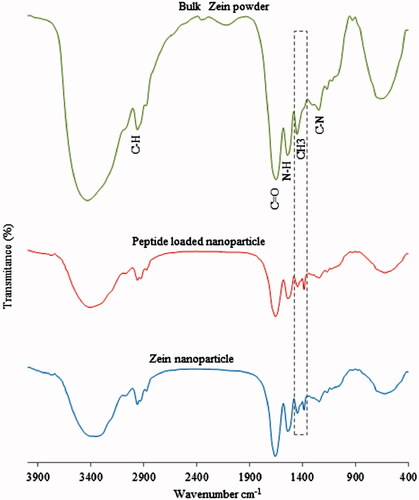
To investigate the interactions of Zein and BMP6Pep in the nanoparticle formation process, the nanoparticles were characterized using FTIR spectroscopy (). The FTIR spectra of the Zein nanoparticles and bulk powder exhibited amides I and II peaks at 1656 and 1537 cm−1 that correspond to carbonyl C=O stretching vibration and both C–N stretching and C–N–H in plane bending [Citation26]. In the FTIR spectrum of the peptide-loaded Zein nanoparticles, the amide I peak related to the α-helical structure of Zein was maintained at the same position indicating that the helical structure of Zein was preserved after encapsulation. The amide I and II bands were weaker in peptide encapsulated nanoparticles and this suggests that the interactions between the peptide and Zein consisted of hydrogen bond and hydrophobic interactions [Citation25]. The 1451 cm−1 peak in bulk material, replaced with 1447 cm−1 and 1384 cm−1 peaks in Zein nanoparticles corresponds to the CH3 bend. Peak intensity in peptide encapsulated nanoparticles changed obviously in 1447 cm−1 and 1384 cm−1 positions that confirmed hydrophobic interaction between the peptide and the CH3 group.
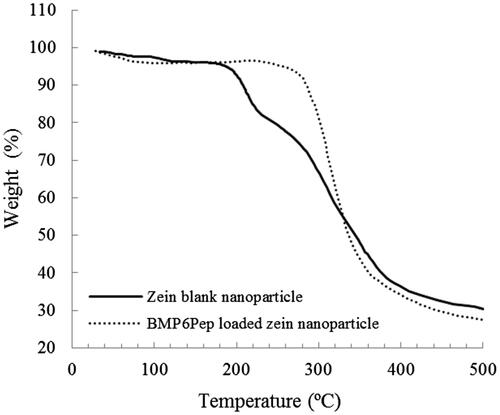
The thermal gravimetric analysis (TGA) was used to investigate the thermal stability of polymeric materials by measuring loss of mass which is caused by volatile compound formation after thermal degradation as a function of temperature increases [Citation27]. Thermogravimetric curves of Zein nanoparticles and BMP6Pep loaded nanoparticles are shown in . In both samples, TGA plots correspond to the thermal behaviour of freeze-dried proteins [Citation28] and have two weight loss steps. The first decrease on mass occurred before 220 °C and is due to the vaporization of water and ethanol from the material. A second weight loss occurs between 200 °C and 500 °C, and is associated with the progressive deamination, decarboxylation and depolymerization arising from the breaking of polypeptide bonds [Citation29,Citation30]. The first step in blank and peptide-loaded nanoparticle occurred in 125 °C and 170 °C, respectively, and both had ∼3% weight loss. The second step in Zein blank and peptide-loaded nanoparticles had ∼65.56% and ∼71.82% weight loss, respectively. Although, BMP6Pep nanoparticles had more thermal resistance in first phase but afterwards, they lose more weight.
In vitro activity of BMP6Pep loaded nanoparticles
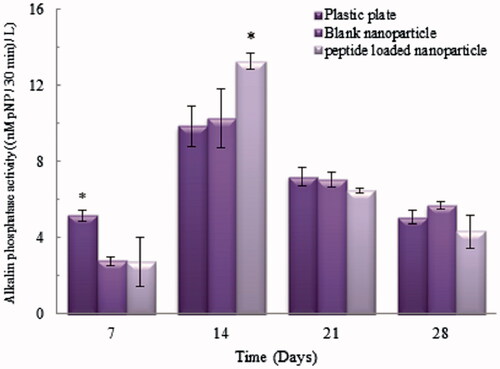
In vitro cytotoxicity of nanoparticles was assessed using MTT assay and plastic plate dishes were used as control. The effects of various doses (500, 750, 1000 and 1250 µg/mL) of Zein blank and peptide-loaded nanoparticles were studied on C2C12 cells. In all concentrations of nanoparticles, over 90% of cells were viable after incubation for 48 h and 96 h. Furthermore, peptide-loaded nanoparticles showed significantly higher cell viability than blank ones at the concentration of 1250 µg/mL after 96 h incubation (p < .05). These results demonstrated that peptide-loaded nanoparticles had good cytocompatibility. The binding of the BMP6Pep to BMP receptors increased in a concentration-dependent manner and 40 μg/mL of peptide is essential for the binding affinity of BMP receptors [Citation31]; therefore, the 500 µg/mL concentration of nanoparticles that contained ∼36 µg/mL peptide was used for osteoinduction analysis. shows ALP activity of C2C12 cells 7, 14, 21 and 28 days after culture. ALP activity showed increased levels at day 14 in all groups but then returned to the baseline level after 21 days. In peptide-loaded nanoparticles, ALP activity was significantly higher (p < .05) compared with other groups at day 14. It is clear that presence of peptide-loaded nanoparticles elevates ALPase activity as osteogenic marker [Citation32].
Figure 6. ALP activity in C2C12 cells at 7, 14, 21 and 28 days incubation. *Statistically significant difference (p < .05) between ALPase activity for a given time point. Error bars correspond to SD for n = 3.
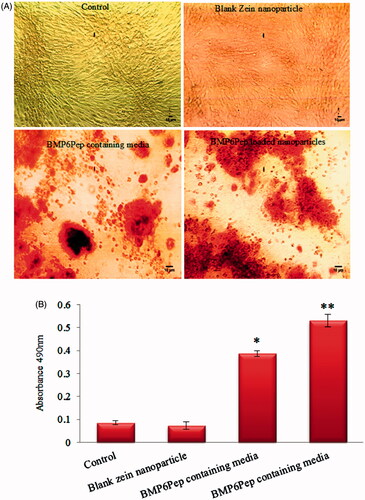
Optical microscope images after staining with ARS () showed that C2C12 cells behind peptide-loaded nanoparticles and BMP6Pep containing media had significantly (p < .05) higher calcium deposition (). Previously, Choi et al. demonstrated that peptide derived from BMP6 knuckle epitope induces osteogenic differentiation of human mesenchymal stem cells with similar potency to BMP-6, as measured by ALP activity and calcium deposition [Citation31].
Figure 7. Optical microscope images of different samples after staining with Alizarin Red S on day 21 (A). The amount of Alizarin Red S that stained the mineralized matrix was quantified using the spectrophotometric method (B). Mineralization occurred in all groups, but C2C12 cell behind peptide-loaded nanoparticles has significantly higher calcium deposition. *,**Statistically significant difference (p < .05) and (p < .001) respectively for calcium deposition. Error bars correspond to SD for n = 3.
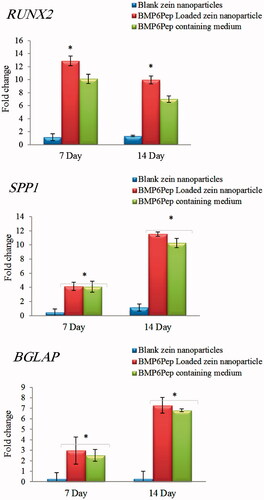
There are three major stages of osteogenesis including proliferation, matrix maturation and mineralization, which are characterized by consecutive expression of distinctive osteoblast markers [Citation33]. In this study, expression of three osteogenic marker genes, RUNX2, SPP1 (osteopontin) and BGLAP (osteocalcin) was evaluated with RT-qPCR. BMPs induce RUNX2 expression in osteoprogenitor cells, e.g. C2C12 cells, and are required for the early and late stages of osteoblast differentiation [Citation34]. Runx2 is frequently described as a master gene for osteoblast differentiation and it regulates the differentiation of mesenchymal progenitor cells to pre-osteoblasts and induces expression of non-collagenous proteins, such as osteocalcin (OCN) and osteopontin (OPN) [Citation35]. In this study, marker gene expression in C2C12 cells was higher in BMP6Pep loaded Zein nanoparticles and BMP6Pep containing media compared to the control group (). RUNX2 gene expression down-regulated at day 14, but SPP1 and BGLAP expression elevated at same time point. The results of RT-qPCR confirmed that the BMP6Pep loaded Zein nanoparticles significantly promote RUNX2 expression compared to blank nanoparticles which revealed that the BMP pathway was activated. When BMP-6 derived peptide interacted with the BMP receptors, the peptide–receptor complex activated the downstream Smad pathway and then regulated gene expression of the osteogenic transcription factors, such as RUNX2 [Citation36]. RUNX2 then regulates SPP1 and BGLAP gene expression in the osteogenesis pathway [Citation37]. Generally, the results showed that the BMP-6 peptide still kept its bioactivity after being encapsulated onto the Zein nanoparticles and in some cases, BMP6Pep loaded nanoparticles had more desirable activity compared with BMP6 peptide containing media.
Conclusions
The peptide-loaded Zein nanoparticles were prepared using the encapsulation method and in vitro release of BMP6Pep in PBS solution revealed a sustained release profile. The FTIR spectra confirmed hydrogen bond and hydrophobic interactions between Zein nanoparticles and BMP6Pep. Size distribution in both the SEM image and DLS analysis was slightly larger in peptide-loaded nanoparticles than that of Zein nanoparticles. This may be due to the encapsulation of BMP6Pep in the nanoparticles. TGA plots correspond to the thermal behaviour of proteins and have two weight loss steps. In vitro activity of the released peptide was evaluated with MTT assay, ALP activity and alizarin red staining. BMP6Pep loaded nanoparticles showed high cell viability, ALP activity and calcium deposition. RT-qPCR results confirmed expression of RUNX2, SPP1 and BGLAP as osteogenic marker genes behind BMP6Pep loaded Zein nanoparticles. Therefore, BMP6Pep loaded nanoparticles due to low cytotoxicity, high encapsulation efficiency and osteoinductivity could be a good candidate as a bone regenerative medicine.
Acknowledgements
The authors acknowledge Nova Teb Research Laboratory (NTRL, Tehran, Iran) for technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Casagrande S, Tiribuzi R, Cassetti E, et al. Biodegradable composite porous poly(dl-lactide-co-glycolide) scaffold supports mesenchymal stem cell differentiation and calcium phosphate deposition. Artif Cells Nanomed Biotechnol. 2017;1–11. doi: https://doi.org/10.1080/21691401.2017.1417866 [Epub ahead of print]
- Gong T, Xie J, Liao J, et al. Nanomaterials and bone regeneration. Bone Res. 2015;3:15029.
- Oryan A, Alidadi S, Moshiri A, et al. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res. 2014;9:1.
- Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16:247–252.
- Senta H, Park H, Bergeron E, et al. Cell responses to bone morphogenetic proteins and peptides derived from them: biomedical applications and limitations. Cytokine Growth Factor Rev. 2009;20:213–222.
- Barradas A, Yuan H, Blitterswijk CA, et al. Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. ECM. 2011;21:407–429.
- Cheng H, Jiang W, Phillips FM, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am. 2003;85:1544–1552.
- Malafaya P, Silva G, Baran E, et al. Drug delivery therapies II: strategies for delivering bone regenerating factors. Curr Opin Solid State Mater Sci. 2002;6:297–312.
- Wong DA, Kumar A, Jatana S, et al. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008;8:1011–1018.
- Zara JN, Siu RK, Zhang X, et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng. 2011;17:1389–1399.
- Saito A, Suzuki Y, Ogata S-i, et al. Activation of osteo-progenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochim Biophys Acta Protein Proteonomics. 2003;1651:60–67.
- Harisa GI, Badran MM, Alanazi FK, et al. An overview of nanosomes delivery mechanisms: trafficking, orders, barriers and cellular effects. Artif Cells Nanomed Biotechnol. 2017;1–11. doi: https://doi.org/10.1080/21691401.2017.1354301 [Epub ahead of print]
- Niu X, Feng Q, Wang M, et al. Preparation and characterization of chitosan microspheres for controlled release of synthetic oligopeptide derived from BMP. J Microencapsul. 2009;26:297–305.
- Wilczewska AZ, Niemirowicz K, Markiewicz KH, et al. Nanoparticles as drug delivery systems. Pharmacol Rep. 2012;64:1020–1037.
- Dasi F, Benet M, Crespo J, et al. A drug delivery system for the treatment of periodontitis. Drug Deliv. 2002;6:862–863.
- Lohcharoenkal W, Wang L, Chen YC, et al. Protein nanoparticles as drug delivery carriers for cancer therapy. BioMed Res Int. 2014;2014. doi:https://doi.org/10.1155/2014/180549
- Orecchioni AM, Duclairoir C, Irache JM, et al. Plant protein‐based nanoparticles. New York: John Wiley & Sons; 2007; p. 54.
- Elzoghby AO, Samy WM, Elgindy NA. Protein-based nanocarriers as promising drug and gene delivery systems. J Control Release. 2012;161:38–49.
- Zein PA. Encyclopedia of polymer science and technology, vol. 15; 1971; p. 125–132.
- Podaralla S, Perumal O. Preparation of Zein nanoparticles by pH controlled nanoprecipitation. J Biomed Nanotechnol. 2010;6:312–317.
- Marini VG, Martelli SM, Zornio CF, et al. Biodegradable nanoparticles obtained from zein as a drug delivery system for terpinen-4-ol. Quím Nova. 2014;37:839–843.
- Leo E, Scatturin A, Vighi E, et al. Polymeric nanoparticles as drug controlled release systems: a new formulation strategy for drugs with small or large molecular weight. J Nanosci Nanotechnol. 2006;6:3070–3079.
- da Rosa CG, Maciel MVdOB, de Carvalho SM, et al. Characterization and evaluation of physicochemical and antimicrobial properties of zein nanoparticles loaded with phenolics monoterpenes. Colloid Surface Physicochem Eng Aspect. 2015;481:337–344.
- Cabra V, Arreguin R, Vazquez-Duhalt R, et al. Effect of temperature and pH on the secondary structure and processes of oligomerization of 19 kDa alpha-zein. Biochim Biophys Acta Protein Proteonomics. 2006;1764:1110–1118.
- Zou T, Li Z, Percival SS, et al. Fabrication, characterization, and cytotoxicity evaluation of cranberry procyanidins-zein nanoparticles. Food Hydrocolloids. 2012;27:293–300.
- Aswathy RG, Sivakumar B, Brahatheeswaran D, et al. Biocompatible fluorescent zein nanoparticles for simultaneous bioimaging and drug delivery application. Adv Nat Sci: Nanosci Nanotechnol. 2012;3:025006.
- Coats A, Redfern J. Thermogravimetric analysis. A review. Analyst. 1963;88:906–924.
- Sionkowska A, Skopinska-Wisniewska J, Gawron M, et al. Chemical and thermal cross-linking of collagen and elastin hydrolysates. Int J Biol Macromol. 2010;47:570–577.
- Magoshi J, Nakamura S, Murakami KI. Structure and physical properties of seed proteins. I. Glass transition and crystallization of zein protein from corn. J Appl Polym Sci. 1992;45:2043–2048.
- Torres‐Giner S, Martinez‐Abad A, Lagaron JM. Zein‐based ultrathin fibers containing ceramic nanofillers obtained by electrospinning. II. Mechanical properties, gas barrier, and sustained release capacity of biocide thymol in multilayer polylactide films. J Appl Polymer Sci. 2014;131:778–789.
- Choi YJ, Lee JY, Chung CP, et al. The identification of a receptor-binding peptide derived from bone morphogenetic protein-6 and its role in osteogenesis. Biomater Res. 2014;5:19–24.
- Luu HH, Song WX, Luo X, et al. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25:665–677.
- Huang W, Yang S, Shao J, et al. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci. 2007;12:3068.
- Hu W, Ye Y, Wang J, et al. Bone morphogenetic proteins induce rabbit bone marrow-derived mesenchyme stem cells to differentiate into osteoblasts via BMP signals pathway. Artif Cells Nanomed Biotechnol. 2013;41:249–254.
- Miron R, Zhang Y. Osteoinduction: a review of old concepts with new standards. J Dent Res. 2012;91:736–744.
- Langenbach F, Handschel J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res Ther. 2013;4:117.
- Bruderer M, Richards R, Alini M, et al. Role and regulation of RUNX2 in osteogenesis. Eur Cell Mater. 2014;28:269–286.