Abstract
Abnormal expression of microRNAs (miRNAs) contributes to tumour growth and invasion. MiR-326 expression often down-regulates in several kinds of cancer and low expression of miR-326 is linked with poor prognosis in cancer patients. In the present study, we aimed to explore the modulatory mechanism of miR-326 in hepatocellular carcinoma (HCC). miR-326 expression was significantly decreased in HCC cell lines and tissues. miR-326 decreased HCC cell growth by affecting cell-cycle progression and by promoting apoptosis. In addition, miR-326 inhibited HCC cell invasion by decreasing the EMT phenotype. We found that miR-326 functioned as a tumour suppressor by repressing its down-stream target PDK1. C-myc contributed to miR-326 down-regulation through binding at its promoter and inhibited its expression. Based on these results, we conducted a therapeutic experiment by using gold nano-particles (AuNPs) carrying miR-326. Restoration of miR-326 reduced tumour growth in vivo. Our findings suggest that miR-326 may be a candidate prognostic biomarker and a target for new therapies in HCC patients.
Introduction
Hepatocellular carcinoma (HCC) ranks the third leading cause of cancer-related deaths worldwide [Citation1,Citation2]. Lots of HCC patients have stepped in the late clinical stage when they were diagnosed. Some patient may undergo surgical resection. However, there is a high frequency of recurrence and metastasis after the surgery [Citation3,Citation4]. Thus, it is urgent in need to develop new strategies for early diagnosis and treatment for HCC.
In a previous study, miR-326 expression was found down-regulated in HCC tissues. MiR-326 low expression was negatively correlated with HCC patient survival rate and time and miR-36 could be applied for HCC diagnostic biomarkers [Citation5]. However, the biological role of miR-326 in HCC cells is still poorly understood.
Phosphoinositide-dependent protein kinase 1 (PDK1) plays a significant role in several kinds of cancer [Citation6], including in HCC [Citation7]. Previous studies reveal that PDK1 is oncogenic and this is dependent on activation of phosphoinositide 3-kinase (PI3K) pathway. Inhibition PDK1 is a valid therapeutic target in cancer treatment [Citation8].
In the study, we explored the role of miR-326 and PDK1 on HCC cell growth and invasion.
Materials and methods
Collection of patient samples
The HCC patient samples, together with the matched-normal tissue samples (formalin-fixed and embedded in paraffin), were obtained from Gaozhou People's Hospital. Written informed consent was obtained from these patients. All experimental protocols were approved by the Clinical Research Ethics Committees of Gaozhou People's Hospital.
Culture of HCC cell line
HCC cell lines (HepG-2, Huh7, Bel7402 and Hep3B) and normal liver epithelial cell line LO2 were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China) and maintained in our lab. All cell lines were cultured at 37 °C in a humidified 5% CO2 atmosphere in DMEM medium supplemented with 10% foetal bovine serum.
MTT and colony formation assays
To perform MTT assay, HCC cells were seeded in 96-well plates, then the MTT (concentration with 5 mg/ml) was added into each well. After incubation, DMSO was added to each well and the absorbance was read at 490 nm.
To carry out colony formation assay, HCC cells were seeded in 6-well plates and were arrowed to grow for 2 weeks. Cells were then stained with haematoxylin solution and were counted under microscope.
Apoptosis and cell invasion assay
The apoptosis assay was carried out as previously described [Citation9]. To perform cell invasion assay, cells were plated in the serum-free medium in the upper chamber with Matrigel-coated membrane. The lower chamber contained medium with 10% FBS. The cells were incubated for 36 h at 37 °C. The cells did not invade the membrane were removed and the cells on the lower surface of the membrane were stained with crystal violet and counted under a microscope.
Cell transfection
The miRNA control, miR-326, anti-miR-ctrl, and anti-miR-326 were purchased from Genechem (Shanghai, China). pcDNA-3.1-PDK1 and pcDNA-3.1-c-myc were purchased from Addgene (Cambridge, MA). Oligonucleotide transfection was carried out with using lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions.
Quantitative real-time PCR assay
Total RNA from HCC cells was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The RNA was reverse transcribed to cDNA using a Reverse Transcription Kit (Takara, Mountain View, CA). Real-time PCR analyses were conducted with Power SYBR Green (Takara, Mountain View, CA). miR-326 expression level was detected with the TaqMan stem-loop qRT-PCR method by using a mirVana miRNA Detection Kit and gene-specific primers, and normalized to U6.
Lentivirus production and transduction
The pLV-has-miR-326 plasmid and the negative control pLV-miRNA-vector were purchased from Biosettia Inc. (San Diego, CA). The viruses were packaged by using 293 T cells according to standard protocol and then were filtered [Citation10]. The packaged lentiviruses were named LV-miR-326 and LV-control accordingly. The cells were infected with virus particles plus 8 μg/ml Polybrene.
Luciferase reporter assay
In the luciferase reporter assay, PDK1 luciferase reporter constructs with wide-type or mutated miR-326 binding sites were transfected with the pRL-SV40 Renilla luciferase vector into HCC cells. After transfection, we performed Luciferase assays by using the dual luciferase reporter assay system (Promega, Madison, MI).
Western blot assay
The western blot assay was carried out as previously described [Citation9]. The primary antibodies cyclin D1, CDK4, c-myc, MMP-2, and MMP-9 were purchased from Santa Cruz Technology (Santa Cruz, CA). The primary antibodies PDK1 and GAPDH were purchased from Abcam (Cambridge, UK). The levels of protein were detected with enhanced chemiluminescence reagents (Pierce, Rockford, IL).
Chromatin immunoprecipitation (CHIP) assay
We performed the CHIP assay according to the manufacturer's instructions by a ChIP assay kit (Millipore, catalog: 17–371). Briefly, the cells were fixed with 1% formaldehyde to covalently crosslink proteins to DNA followed by harvesting chromatin from the cells. Subsequently, the cross-linked DNA (sheared to 200–1000 base pairs in length) that linked with sonication were processed to an immmmunoselection process. Then the PCR assay was performed to measure enrichment of DNA fragments in the putative c-myc-binding sites in the miR-326 promoter.
AuNPs-miR-326 preparation
The AuNPs was prepared as previously described method [Citation11]. For AuNP-miR-326 preparation, thiolated miR-326 was added to 10 nM solution of AuNPs at a ratio of 1 nmol RNA per 500 μL AuNP solution supplemented with 0.1% Tween-20. Subsequently, the solution was incubated at room temperature (RT) for 10 min, followed by aged with gradual additions of NaCl over 4 h to bring the final NaCl concentration to 0.5 M. The AuNPs were separated from free RNA strands via centrifugation, supernatant removal, and addition of phosphate buffered saline (PBS) at 4 °C. The concentration of miR-326 in AuNP-miR-326 solution was determined following release of the miRNA from the NPs with dithiothreitol (DTT) using previously described procedures [Citation12].
In vivo tumour formation assay
About 2 × 106 HCC cells (LV-ctrl or LV-miR-326) were subcutaneously inoculated into the flank of 5-week-old BALB/c-nu/nu mice. Five weeks later, the mice were sacrificed and the tumours were harvested and weighed. All animal studies were permitted by the Animal Care Committee of Gaozhou People’s Hospital. Animal maintenance and experimental procedures were carried out in accordance with the US National Institute of Health Guidelines for Use of Experimental Animals.
Statistical analysis
Data are expressed as mean ± SD as indicated. Fisher’s exact test was used to identify differences between categorical variables. One-way ANOVA or two-tailed Student’s t-test was used for comparisons between groups. p < .05 was considered statistically significant.
Results
miR-326 expression was down-regulated in HCC cell lines and tissues
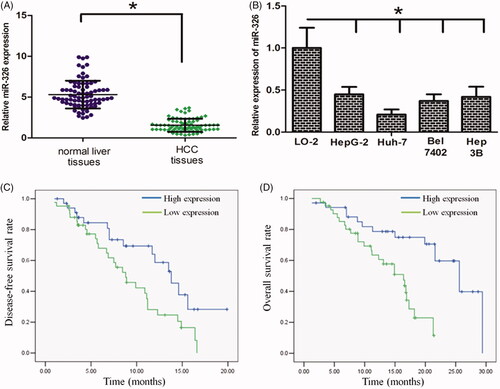
With qRT-PCR, it was observed that miR-326 expression was significantly down-regulated in HCC cell lines when compared with the normal human liver cell LO2 (, p < .05). In addition, we revealed that miR-326 expression was also significantly down-regulated in HCC tissues when compared with that in their normal counterparts (, p < .05).
Figure 1. miR-326 is down-regulated in HCC tissues and cell lines. (A) miR-326 expression was significantly decreased in the primary HCC tissues. (B) miR-326 expression was lower in HCC cells when compared with that in normal liver cell line LO-2. (C) and (D) Low-level expression of miR-326 was associated with a shorter overall survival and disease-free survival time of HCC patients (The above and below curves represent high- and low expression of miR-326, respectively). *p Value<.05.
miR-326 down-regulation predicted unfavourable prognosis in HCC
We next explored the association between miR-326 expression and HCC patient prognosis. We reported the associations between miR-326 expression and patients’ clinicopathological features in . The parameters such as gender (p = .751), age (p = .579) and HBV infection (p = .813) were not significantly associated with miR-326 expression. However, low miR-326 expression was significantly correlated with tumour size (p = .004), lymph node metastasis (p = .003), and late clinical stage (p = .022). In the Kaplan–Meier analysis assay, we uncovered that low-level miR-326 expression was correlated with a shorter overall survival time and disease-free time in patients with HCC (, p < .05).
Table 1 Associations between lncRNA miR-326 expression and patients’ clinicopathological features.
miR-326 inhibited HCC cell growth and invasion
We then asked whether restoration of miR-326 affected HCC cell growth and invasion. We established Huh7 cells stably overexpressing miR-326 or a control by using lentiviral vector-mediated overexpression (LV-miR-326, LV-ctrl). The efficiency of transduction was validated using RT-PCR (Supplementary Figure 1(A)). As determined by the MTT assay, miR-326 significantly decreased cell viability when compared with the LV-ctrl group (). In parallel, the colony formation assay revealed that miR-326 decreased Huh7 cell proliferation (). We further asked whether miR-326 affected cell growth by regulating cell cycle and apoptosis in HCC. The flow cytometry assay revealed a higher proportion of cells in the G1 phase and a lower proportion in the S phase in LV-miR-326 cells, when compared with the control group (). Interestingly, the expression of G1/S phase checkpoint proteins such as cyclin D1, CDK4, and c-myc were significantly decreased in LV-miR-326 cells, as determined by western blot assay (). The flow cytometry assay also revealed that miR-326 induced apoptosis in HCC cells ().
Figure 2. miR-326 inhibition decreased HCC cell proliferation and invasion. (A) The MTT assay revealed that restoration of miR-326 decreased cell proliferation. (B) Colony formation assay demonstrated that oncogenic survival was significantly decreased in LV-miR-326 cells compared with LV-ctrl cells. (C) LV-miR-326 cells displayed a significantly higher frequency of cells at the G1 phase and a lower frequency of cells at S phase. (D) Restoration of miR-326 affected the expression of G1/S phase checkpoint proteins. (E) Restoration of miR-326 increased the proportion of apoptosis in HCC cells. (F) Restoration of miR-326 decreased the HCC cell invasion ability, as revealed by the Boyden assay. (G) The expression levels of MMP-2 and MMP-9 protein were lower in LV-miR-326 cells.
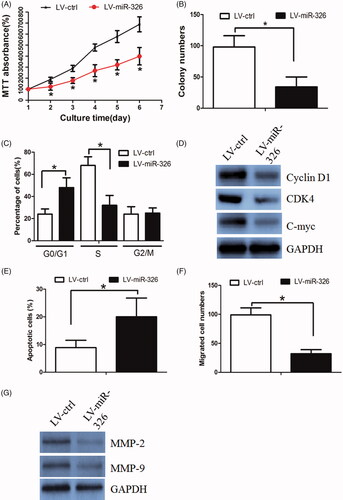
We next perform Boyden assay to examine miR-326’s effect on cell migration. It was found that miR-326 decreased Huh7 cell invasion ability (). The western blot assay revealed that invasion protein markers (including MMP-2 and MMP-9) were markedly decreased in LV-miR-326 cells ().
MiR-326 inactivated PI3K/AKT signalling pathway via regulating PDK1
To find out the underlying mechanism of miR-326 in regulating HCC cell growth and invasion, we identified a serious of pathways that may be regulated by miR-326 with the use of miRPathDB. Among these pathways, we chose the PI3K/AKT signalling pathway for further study (Supplementary Figure 1(B), left panel), as this pathway was a master regulator involved in HCC development. The western blot assayed revealed that miR-326 could suppress the activity of PI3K/AKT signalling pathway (Supplementary Figure 1(B), right panel).
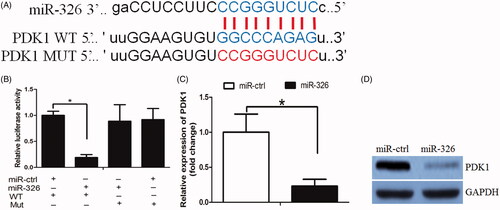
By using bioinformatic analysis (TargetScan and miRanda), it was predicted that PDK1 was one of the miR-326 targets. PDK1 was able to active the PI3K/AKT signalling pathway in cancer [Citation13], we thus chose it for further study. To examine whether miR-326 directly targeted the PDK1 3′-UTR, the PDK1 3′-UTR was subcloned into the luciferase reporter plasmids, including the predicted miR-326 recognition site (WT) or a mutated sequence (MUT) (). There was a significant decrease in luciferase activity in Huh7 cells transfected with the wild-type miR-326 vector, when compared with cells transfected with the mutant vector (). Subsequently, by RT-PCR and western blot we found that miR-326 inhibited PDK1- mRNA and protein levels, respectively (). More important, we observed a negative association between miR-326 and PDK1 expression in HCC tissues ().
Figure 3. miR-326 repressed PDK1 expression in HCC cells. (A) The binding sites of miR-326 on PDK1. (B) The luciferase assay showed that cells transfected with miR-326 had less luciferase activity than those transfected with miR-ctrl. (C) miR-326 repressed PDK1 mRNA expression in HCC cells. (D) miR-326 repressed PDK1 protein expression in HCC cells.
Interestingly, rescue of PDK1 expression could counteract miR-326’s effect on cell growth, invasion and PI3K/AKT signalling pathway (Supplementary Figure 1(C–G)).
The PI3K/AKT/c-myc axis negatively regulated miR-326 expression
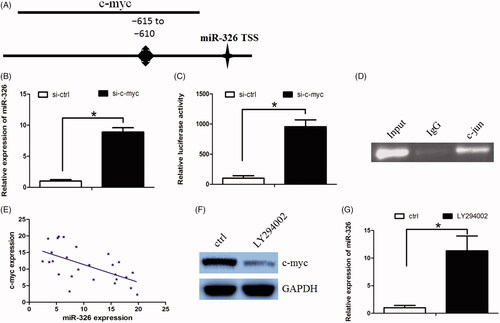
Furthermore, we asked whether the PI3K/AKT signalling pathway could serve as a negative feedback by suppressing miR-326 expression. We used UCSC and PROMO bioinformatics software to analyze a 1-kb region upstream of the transcription start site of miR-326. The c-myc-binding motif at −615 to −610 was identified inside the putative promoter region upstream of the miR-326 transcriptional start site (TSS) (). Subsequently, we used si-RNAs to knock down c-myc expression in Huh7 cells and found that miR-326 expression was significantly increased in these cells when c-myc was down-regulated (). In addition, c-jun down-regulation increased miR-326 promoter luciferase activity (). Finally, the Chromatin immunoprecipitation (ChIP) assay confirmed that c-myc protein was recruited to the binding site in the putative miR-326 promoter in Huh7 cells (). We further revealed that miR-326 expression was negatively correlated with c-myc expression (, Spearman's correlation coefficient, R = −0.6635). In addition, PI3K inhibitor LY294002 decreased c-myc expression, while increased miR-326 expression in Huh7 cells (). Taken together, our data suggest that the PI3K/AKT/c-myc axis negative regulated miR-326 expression.
Figure 4. c-myc decreased miR-326 expression through biding at its promoter. (A)The putative transcription factor-binding site of c-myc at miR-326 promoter region. (B) c-myc down-regulation increased miR-326 expression. (C) c-myc down-regulation increased miR-326 promoter luciferase activity. (D) The CHIP assay confirmed that c-myc protein was recruited to the binding site in the putative miR-326 promoter. (E) miR-326 expression was negatively correlated with c-myc expression in HCC tissues. (F) and (G) PI3K inhibitor LY294002 decreased c-myc expression, while increased miR-326 expression in Huh7 cells.
AuNPs delivered miR-326 decreased HCC cell growth in vitro and in vivo
We fabricated a nano-carrier (gold-PEI) to deliver miR-326 into HCC cells. The size of Gold-PEI nano-carrier was about 20–30 nm as determined by dynamic lighting scatter (DLS) (Supplementary Figure 1(H), left panel). The nano-miR-326 displayed an approximately spherical shape with good dispersion and its size was similar to that of gold-PEI observed by TEM (Supplementary Figure 1(H), right panel). To validate the transfection efficiency at the cellular level, we determined the cellular uptake of FITC-labelled AuNP-miR-326 by fluorescence microscopy. After 1 h incubation, Huh7 cells treated with AuNP-miR-326 demonstrated significant green fluorescence in almost all cells, which indicated high efficient and rapid uptake of AuNP-miR-326 by the cells (Supplementary Figure 1(I)). When treated by nano-miR-326, HCC cells growth was accordingly reduced (). Similarly, nano-miR-326 treatment decreased Huh7 cells colony formation ability () and led to cell cycle arrest at G1 phase (). In addition, the relevant signals (PDK1, p-Akt, c-Myc, cyclinD1 and CDK4) were also decreased (). nano-miR-326 treatment also decreased Huh7 cells migration (). Interestingly, the E-cadherin expression was elevated, while N-cadherin and Vimentin expression were decreased in nano-miR-326-treated cells ().
Figure 5. AuNPs delivered miR-326 decreased HCC cell growth in vitro and in vivo. (A) When treated by nano-miR-326, HCC cells growth was accordingly reduced. (B) nano-miR-326 treatment decreased Huh7 cells colony formation ability. (C) nano-miR-326 treatment led to cell cycle arrest at G1 phase. (D) PDK1, p-Akt, c-Myc, cyclinD1, and CDK4 expression levels were decreased in nano-miR-326-treated cells. (E) nano-miR-326 treatment decreased Huh7 cells migration ability. (F) The E-cadherin expression was elevated, while N-cadherin and Vimentin expression were decreased in nano-miR-326-treated cells. (G) nano-miR-326-treated Huh7 cells grew more slowly. (H) The mean weight of xenograft tumours was less in the nano-miR-326 group when compared with that in the nano-miR-ctrl group. (I) The KI-67 stain assay revealed that nano-miR-326 decreased Huh7 cell proliferation ability.
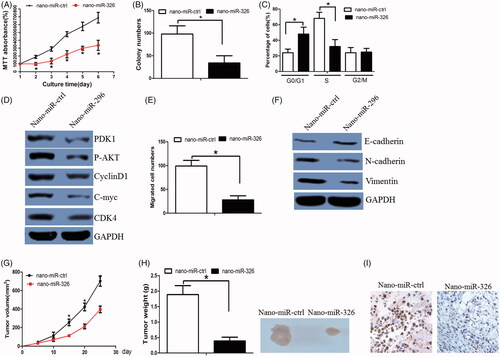
Subsequently, we asked whether nano-miR-326 inhibited tumour growth in vivo. When treated with nano-miR-326, Huh7 cells grew more slowly (). The mean weight of xenograft tumours was less in the nano-miR-326 group when compared with that in the nano-miR-ctrl group (). Tumour sections were stained for Ki-67 expression to quantitatively assess the proliferation index in xenograft tumours which was lower in the nano-miR-326 group (). Taken together, these data support the growth-suppressive effect of nano-miR-326 in vivo.
Discussion
MicroRNA play significant role in regulating cancer cell progression and invasion [Citation14–16]. Literatures have documented that miR-326 played a significant role in regulating cancer development. MiR-326 was identified as a tumour-suppressive microRNA in a serious of cancer [Citation17–19]. MiR-326 expression was usually down-regulated in cancer tissues and its down-regulation was negatively associated with tumour patient poor prognosis [Citation20,Citation21]. Previous report documented that miR-326 was down-regulated in HCC and miR-326 could be applied for HCC diagnostic biomarkers [Citation5]. Recently, it was indicated that miR-326 inhibited HCC cell growth and invasion [Citation22]. In parallel, our data revealed that miR-326 was downed in HCC tissues and cell lines. Low-level miR-326 expression was correlated with a shorter overall survival time and disease-free time in HCC patients. In addition, we found that attenuated miR-326 expression was negatively associated with T classification, N classification and clinical stages of HCC patients. The functional experiment revealed that miR-326 decreased HCC cell growth. We then present evidence that miR-326 induced cell cycle transition obstacle by repressing the expression of cell cycle factors and promoted cell apoptosis, which result in the inhibition of cell growth. In addition, miR-326 decreased cell migration via inhibiting the expression of matrix metalloproteinase (i.e. MMP-2 and MMP-9). Interestingly, miR-326 also reversed the EMT phenotype, which was parallel to previous study [Citation23,Citation24]. These results suggested miR-326 serve as a tumour suppressor in HCC.
The Akt constituted an important pathway modulating multiple biological processes, especially in cancer development [Citation25,Citation26]. AKT signalling pathway can promote cancer cell growth, invasion and induce EMT phenotype [Citation27]. Phosphorylation of some kinases (such as PDK1) can activate Akt [Citation28,Citation29]. Our study revealed that PDK1 was a down-stream target of miR-326. MiR-326 modulated PDK1 expression and thereby regulated AKT signalling pathway. Interestingly, we revealed that c-myc, a down-stream target of PDK1/AKT signalling, could negatively regulated miR-326 expression by directly binding at miR-326’s promoter. We demonstrated there was a negative feedback loop between miR-326 and PDK1/AKT/c-myc pathway.
Subsequently, we developed a gold-PEI nanocarrier with good transfection efficiency to deliver miR-326 and elicited its potential therapeutic effect. We found that nano-miR-326 suppressed cell proliferation and tumour growth effectively both in vitro and in vivo. These results had a clinical implication for HCC treatment involving miRNA intervention by nano-delivery.
In summary, we revealed that nano-miR-326 was tumour suppressor, which was down-regulated in HCC cell lines and tissues. MiR-326 inhibited the PDK1/Akt pathway and the down-stream signalling. In addition, we developed a gold nanocarrier to deliver miR326 and elicited its potential therapeutic effect in animal models. Although miRNA-based therapeutics is still in their infancy, our findings are encouraging and miR-326 may be potential therapeutic targets for the treatment of HCC patients.
supplementary_Figure_1.tif
Download ()Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Marquardt JU, Thorgeirsson SS. SnapShot: hepatocellular carcinoma. Cancer Cell. 2014;25:550.
- Xu Y, Zhang C, Liang H, et al. Dishevelled 1, a pivotal positive regulator of the Wnt signalling pathway, mediates 5-fluorouracil resistance in HepG2 cells. Artif Cells Nanomed Biotechnol. 2018;27:1–9.
- Yuan JH, Yang F, Wang F, et al. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–681.
- Zhang J, Du J, Liu Q, et al. Down-regulation of STAT3 expression using vector-based RNA interference promotes apoptosis in hepatocarcinoma cells. Artif Cells Nanomed Biotechnol. 2015; 44:1–5.
- Lu M, Kong X, Wang H, et al. A novel microRNAs expression signature for hepatocellular carcinoma diagnosis and prognosis. Oncotarget. 2017;8:8775–8784.
- Bhola NE, Freilino ML, Joyce SC, et al. Antitumor mechanisms of targeting the PDK1 pathway in head and neck cancer. Mol Cancer Ther. 2012;11:1236–1246.
- Mo Y, Lu Y, Wang P, et al. Long non-coding RNA XIST promotes cell growth by regulating miR-139-5p/PDK1/AKT axis in hepatocellular carcinoma. Tumour Biol. 2017;39:101042831769099.
- Raimondi C, Falasca M. Targeting PDK1 in cancer. Curr Med Chem. 2011;18:2763–2769.
- Deng X, Ma L, Wu M, et al. miR-124 radiosensitizes human glioma cells by targeting CDK4. J Neurooncol. 2013;114:263–274.
- Lesch HP, Laitinen A, Peixoto C, et al. Production and purification of lentiviral vectors generated in 293T suspension cells with baculoviral vectors. Gene Ther. 2011;18:531–538.
- Elbakry A, Zaky A, Liebl R, et al. Layer-by-layer assembled gold nanoparticles for siRNA delivery. Nano Lett. 2009;9:2059–2064.
- Hurst SJ, Lytton-Jean AK, Mirkin CA. Maximizing DNA loading on a range of gold nanoparticle sizes. Anal Chem. 2006;78:8313–8318.
- Gagliardi PA, Puliafito A, Primo L. PDK1: at the crossroad of cancer signaling pathways. Seminars in Cancer Biology 2018 Feb;48:27–35. doi: 10.1016/j.semcancer.2017.04.014.
- Shang A, Lu WY, Yang M, et al. miR-9 induces cell arrest and apoptosis of oral squamous cell carcinoma via CDK 4/6 pathway. Artif Cells Nanomed Biotechnol. 2018 Dec;46(8):1754–1762. doi: 10.1080/21691401.2017.1391825
- Lu Y, Tang L, Zhang Q, et al. MicroRNA-613 inhibits the progression of gastric cancer by targeting CDK9. Artif Cells Nanomed Biotechnol. 2018;46:980–984.
- Xue K, Yang J, Hu J, et al. MicroRNA-133b expression associates with clinicopathological features and prognosis in glioma. Artif Cells Nanomed Biotechnol. 2018;46:815–818.
- Wu H, Wang Y, Wu C, et al. Resveratrol induces cancer cell apoptosis through MiR-326/PKM2-mediated ER stress and mitochondrial fission. J Agric Food Chem. 2016;64:9356–9367.
- Ji S, Zhang B, Kong Y, et al. miR-326 inhibits gastric cancer cell growth through downregulating NOB1. Oncol Res. 2017;25:853–861.
- Liang Z, Wu H, Xia J, et al. Involvement of miR-326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance-associated protein 1. Biochem Pharmacol. 2010;79:817–824.
- Li Y, Gao Y, Xu Y, et al. Down-regulation of miR-326 is associated with poor prognosis and promotes growth and metastasis by targeting FSCN1 in gastric cancer. Growth Factors. 2015;33:267–274.
- Zhang ZL, Bai ZH, Wang XB, et al. miR-186 and 326 predict the prognosis of pancreatic ductal adenocarcinoma and affect the proliferation and migration of cancer cells. PloS One. 2015;10:e0118814.
- Hu S, Ran Y, Chen W, et al. MicroRNA-326 inhibits cell proliferation and invasion, activating apoptosis in hepatocellular carcinoma by directly targeting LIM and SH3 protein 1. Oncol Rep. 2017;38:1569–1578.
- Cai M, Wang Z, Zhang J, et al. Adam17, a target of Mir-326, promotes Emt-induced cells invasion in lung adenocarcinoma. Cell Physiol Biochem. 2015;36:1175–1185
- Liu W, Zhang B, Xu N, et al. miR-326 regulates EMT and metastasis of endometrial cancer through targeting TWIST1. Eur Rev Med Pharmacol Sci. 2017;21:3787–3793.
- Li L, Zhu X, Shou T, et al. MicroRNA-28 promotes cell proliferation and invasion in gastric cancer via the PTEN/PI3K/AKT signalling pathway. Mol Med Rep. 2018;17:4003–4010.
- Xiao N, Qi XY, Tang LN, et al. VEGF promotes cardiac stem cells differentiation into vascular endothelial cells via the PI3K/Akt signaling pathway. Artif Cells Nanomed Biotechnol. 2014;42:400–405.
- Rafael D, Doktorovova S, Florindo HF, et al. EMT blockage strategies: targeting Akt dependent mechanisms for breast cancer metastatic behaviour modulation. Cgt. 2015;15:300–312.
- Bader AG, Kang S, Zhao L, et al. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–929.
- Carnero A, Blanco-Aparicio C, Renner O, et al. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Ccdt. 2008; 8:187–198.
