?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Co-encapsulation of drugs provides a convenient means for treating different symptoms of a disease. Celastrol (Cel) shows potent anti-arthritic activity and Indomethacin (Indo) is effective in relieving inflammatory pain. Nanostructured lipid carriers loaded with Celastrol and Indomethacin (Cel-Indo-NLCs) were prepared by emulsification evaporation–solidification method, optimized by the Box–Behnken design and characterized by transmission electron microscopy (TEM), Fourier transform infrared (FTIR) and powder X-ray diffraction analysis (PXRD). Visualization of transdermal translocation of Cel-Indo-NLCs was achieved by confocal laser scanning microscope (CLSM). Further, Cel-Indo-NLCs were incorporated into Carbopol 940 for transdermal delivery. The in vitro studies were evaluated by using the Franz diffusion cells. Cel-Indo-NLCs depicted small particle size (26.92 ± 0.62 nm) and PDI (0.201 ± 0.01), high entrapment efficiency (96.56 ± 1.41%) and drug load (3.65 ± 0.05%). Moreover, Cel-Indo-NLCs showed prominent effect of decreasing paw oedema, inhibiting inflammation and pain by regulating the levels of IL-1β, TNF-α, β-endorphin and Substance P. After the administration of Cel-Indo-NLCs-gel, no skin irritation was observed in rats. There was no difference of gastrointestinal tract between different groups of rats when they were sacrificed. The histological analysis showed no renal and reproductive toxicity. Therefore, it can be concluded that co-encapsulation strategy based NLCs have the potential to provide safe transdermal delivery and are promising in treatment of pain and inflammation associated with rheumatoid arthritis.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease, characterized by infiltration of inflammatory cells and proliferation of synovial fibroblasts, which causes destruction of the synovial membrane, progressive bone destruction and joint deformity [Citation1]. The treatment principle of RA is to slow down the development of the disease, reduce the rate of disability, and improve the quality of life of RA patients.
Tripterygium wilfordii Hook f. (TWHF), a traditional Chinese medicine, has potent anti-inflammatory and immunosuppressive properties, which has been applied to treat RA with promising clinical results. Celastrol (Cel) is a bioactive component of TWHF, and gains great interest because of its potential anti-inflammatory, anti-arthritic and anti-cancer activities. Many literatures have reported that Cel can regulate levels of chemokines and cytokines and mediate cellular infiltration into joints to achieve its anti-arthritic activity [Citation2,Citation3]. Indomethacin (Indo) is a nonsteroidal anti-inflammatory drug that is effective to inflammatory pain and commonly used to relieve RA. Therefore, this paper focuses on the combination of anti-inflammatory and anti-arthritic activities of Cel and powerful analgesic effect of Indo in treatment of RA. However, long-term oral administration can cause toxicity, especially on renal and reproductive systems for Cel and gastrointestinal system for Indo. Hence, it is urgent to explore an alternative route for effective delivery of Cel and Indo without any toxicity to internal organs. Apparently, transdermal delivery is an attractive way to achieve the above objectives and has the advantages of easy application and improved patient compliance.
Stratum corneum (SC) is the main barrier to overcome for delivering drugs into skin. Based on lipid nanoparticles, nanostructured lipid carriers (NLCs) are the most attractive strategy for improving penetration of drugs into skin, especially for drug co-encapsulation [Citation4]. Composed of solid and liquid lipids, NLCs with small size can form a monolayer on skin, which can prevent transepidermal water loss. The hydration of skin may open intergaps between corneocytes, thus facilitating penetration of drugs into skin [Citation5]. NLCs are frequently applied for topical delivery due to their remarkable properties such as high drug load, low leakage, sustained release property and excellent biocompatibility. However, the efficacy and safety of transdermal delivery of nanostructured lipid carriers loaded with Celastrol and Indomethacin (Cel-Indo-NLCs) to relieve RA has not fully researched.
At present, several studies have focused on the way in which nano- or micro-emulsion enters the skin. Some studies reported that nanoemulsions could enhance permeation into skin via intercellular and intracellular routes [Citation6]. However, other researchers believed that the encapsulated drugs are released from nanoemulsions firstly and diffuse through the SC [Citation7,Citation8]. Su et al. [Citation9] demonstrated that trans-follicular route was the primary way that nanoemulsions permeate into deep sites of skin. Several topical NLCs have been approved for preclinical study and achieved good therapeutic effects, e.g. methotrexate [Citation10], tripterine [Citation11], lappaconitine and ranaconitine [Citation12]. However, in vivo fate of NLCs after transdermal administration has not been fully understood.
This study was to develop and optimize a topical delivery system based on NLCs loaded with Cel and Indo. The NLCs were prepared by emulsification evaporation–solidification method and optimized by the Box–Behnken design (BBD). The in vitro transdermal characteristics of Cel-Indo-NLCs were investigated. To elucidate the transdermal delivery mechanism of Cel-Indo-NLCs, visualization of the transport of NLCs was performed using confocal laser scanning microscope (CLSM). Moreover, the pharmacodynamics, gastrointestinal and reproductive system toxicity were investigated after percutaneous administration of Cel-NLCs, Indo-NLCs and Cel-Indo-NLCs in a RA model, aiming to investigate the priority of combination of Cel and Indo by comparing with their separated application.
Materials and methods
Materials
Celastrol was purchased from Nanjing Zelang Medical Technology Co., Ltd. (Nanjing, China). Indomethacin, Polyoxy 35 castor oil and isopropyl palmitate were purchased from Shanghai YuanYe Biological Technology Co., Ltd. (Shanghai, China). Capric triglyceride, Oleic acid, Labrasol ALF, Compritol 888 ATO, Precirol ATO-5, Isopropyl Myristate were purchased from GATTEFOSSE (Paramus, NJ). Tween 80, Span 80, PEG400, PEG4000, Poloxamer 188 were purchased from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). Glyceryl monostearate (GMS), stearic acid (SA), Miglyol 812 N, Cremophor RH40 and Carbopol 940 were purchased from Beijing Fengli Jingqiu Pharmaceutical Co., Ltd. (Beijing, China). HPLC grade methanol and acetonitrile were purchased from Sigma Aldrich (St. Louis, MO). All other chemicals used were of analytical grade.
Animals
Male Sprague-Dawley rats, weighing 200 ± 20 g, were used in this study. The animal experiments were conducted with the approval of the Animal Ethical Committee of Beijing University of Chinese Medicine.
Quantification of Cel and Indo
HPLC system (Thermo Scientific, Waltham, MA) with UV spectrophotometric detector was used to determine the encapsulation efficiency. The separation was carried out on Thermo Scientific AcclaimTM 120 C18 column (250 mm × 4.6 mm, 5 μm). The mobile phase for Cel was 95:5 of methanol:2% phosphoric acid. The mobile phase for Indo was 70:30 of acetonitrile:2% phosphoric acid. The flow rate was 1.0 ml/min and the detection wavelengths used were 431 nm and 329 nm for Cel and Indo, respectively.
Concentrations of Cel in receiving fluid were analysed using a Waters ACQUITY UPLC system with Xevo TQ-S triple quadrupole mass spectrometer (Waters, Milford, MA). The experiment was performed with a Waters UPLC BEH C18 column (100 × 2.1 mm, 5 μm). The mobile phase consisted of methanol:0.5% formic acid (80:20), and the flow rate was 0.4 ml/min. An electrospray ionization source interface set in positive ion mode was employed. The precursor-to-production transitions were monitored at m/z 451.3–201.1 for Cel using a selected reaction monitoring mode.
Preparation of Cel-Indo-NLCs
Selection of lipids
Selection of liquid lipid
Excess Cel and Indo were dispersed in tubes containing 5 g liquid lipids and the mixtures were stirred for 1 h. [Citation13] Thereafter, the tubes were kept in an isothermal orbital shaker at 37 °C for 48 h to reach equilibrium. The samples were centrifuged at 5000 rpm for 30 min, and the supernatants were diluted with methanol and analysed by HPLC.
Selection of solid lipid
The solid lipids for selecting included GMS, Compritol 888 ATO, Precirol ATO-5, SA and PEG 4000 [Citation14]. The method of visual observation under light was applied to estimate the solubility of drugs in melted solid lipid. Two grams solid lipid was melted at 85 °C in different beakers in ultrasonic cleaning machine (Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China). Cel or Indo was added in the increment of 2 mg to the beakers and dissolved at the ultrasonic frequency of 40 kHz until saturation was achieved. The amount of drug solubilized in melted solid lipids was recorded.
The miscibility of solid and liquid lipids
The miscibility of lipids was confirmed by visual observation. The solid and liquid lipids with better solubility of drugs were mixed in different ratios (i.e. 90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80 and 10:90). The mixtures were melted at 85 °C, vortexed for 5 min and maintained at 85 °C for 2 h before cooled to room temperature. After 12 h, mixtures were placed on filter paper. The combination of having a melting point of more than 40 °C and no oil drops on the paper was selected as the lipid phase for the design of NLCs.
Screening of surfactant
Surfactants are essential and important for fabrication of NLCs. Different surfactant aqueous solutions (Cremophor RH40, Kolliphor HS 15, PEG 400 and Poloxamer 188) kept in 85 °C thermostatic water bath were added to the melted lipids to examine which surfactant could emulsify the lipids sufficiently.
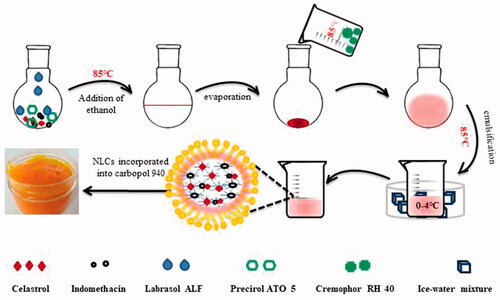
Preparation process of Cel-Indo-NLCs
Cel-Indo-NLCs were prepared by emulsification evaporation–solidification method (). Briefly, Cel (10 mg), Indo (10 mg), lipids were dissolved in 15 ml of ethanol at 85 °C, the lipid phase was formed after ethanol was totally rotary evaporated. The aqueous phase was prepared by dissolving surfactant into 10 ml distilled water at 85 °C. After that, the aqueous phase was added into the lipid phase rapidly. The mixture was homogenized in a rotator (85 °C) for 10 min to obtain the pre-emulsion. Cel-Indo-NLCs were obtained after cooled quickly in ice-water mixture. The blank NLCs were prepared by the above method without adding Cel and Indo.
Optimization design
In this study, three factors and three levels () in the BBD were applied for optimization to assess the relationship between the independent variables like concentration of total lipids (A), concentration of surfactant (B), amount of added Cel and Indo (mg/g) (C), and dependent variables, i.e. particle size (Y1), mean entrapment efficiency (EE) (Y2) and total drug load (DL) (Y3). To execute this design, total 17 formulations were prepared and evaluated ().
Table 1. Factors and levels for the experimental design.
Table 2. Randomized design and results for Box–Behnken design of total 17 experiments.
Characterization of Cel-Indo-NLCs
Particle size and polydispersity
Particle size and polydispersity (PDI) were determined by dynamic light scattering using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) at room temperature.
Entrapment efficiency and drug load
The EE and DL of Cel and Indo in NLCs were determined by ultrafiltration method. Briefly, Cel-Indo-NLCs suspension was placed in tubes and centrifuged at a rate of 10,000 r/min for 30 min. The supernatant was transferred to the upper chamber of a centrifuge tube matched with a 30 kDa ultrafilter and centrifuged at a rate of 6000 r/min for 30 min. The encapsulated Cel and Indo were still remained in the upper chamber, whereas the aqueous dispersion medium in the lower chamber containing free Cel and Indo. The free drug (Wfree) and the centrifuged supernatant (Wtotal) were diluted with methanol and determined by HPLC. The EE and DL were calculated using the following equations: EE = (Wtotal – Wfree)/Wtotal × 100%; DL = (Wtotal – Wfree)/Wlipids × 100%, where Wlipids is the weight of total lipids.
Surface morphology
The surface morphology of Cel-Indo-NLCs was observed by transmission electron microscopy (TEM) (JEM-1230(HC), Tokyo, Japan)). The Cel-Indo-NLCs were dropped onto a carbon-coated copper grid and stained with 2% (w/v) phosphotungstic acid. The grid was applied for observation after dried.
Fourier transform infrared (FTIR) spectroscopy
FTIR spectra were recorded by FTIR spectrometer (Thermo Scientific Nicolet iS10, Waltham, MA) to analyse the interaction between drugs and excipients. Cel, Indo, Mixture, freeze-dried Blank-NLCs and Cel-Indo-NLCs were mixed with KBr in a ratio of 1:100 and pressed into pellets. Afterwards, the pellets were observed by FTIR spectrometer and the spectrums were recorded.
Powder X-ray diffraction analysis (PXRD)
PXRD was used to investigate the crystalline structures of particles. The PXRD for Cel, Indo, Mixture, Blank-NLCs and Cel-Indo-NLCs were recorded on X-ray diffractometer (Xpert Pro MPD, Panalytical, Almelo, Netherlands). The X-ray source was Cu Kα radiation and operated at 40 kV and 40 mA. The samples were analysed at 2θ from 5° to 60.0° at a scanning speed of 4°/s.
Transdermal translocation of NLCs penetration into skin
Skin has the ability to prevent body from losing water. SC and epidermis contain about 15% and 75% water, respectively [Citation15]. P4, one of NIR fluorescent probes developed by Wei Wu group, has a sensitive aggregation-caused quenching effect when contacting with water through π–π stacking [Citation16–18]. Due to the highly hydrophobic feature, P4 can be embedded into lipids, where they can emit fluorescence signals. However, once P4 released from nanoparticles due to degradation of lipids and contacted with water in skin, the fluorescence quenches immediately. So, it can be used to track the in vivo fate of NLCs.
Preparation of P4-C6-NLCs
P4 was loaded into lipids to track the transdermal translocation of NLCs, while coumarin-6 (C6) was embedded to represent the drugs. The preparation of P4-C6-NLCs was the same with Cel-Indo-NLCs, except Cel and Indo were replaced by P4 and C6.
In vivo skin permeation studies
The rats were anaesthetized by intraperitoneal injection of 10% chloral hydrate aqueous solution and fixed by a rat fixator. The abdominal hair of the rats was removed and the donor cell of the Franz diffusion cells was fixed on the abdominal surface. Two millilitres of P4-C6-NLCs were applied into the donor cell. The rats were sacrificed at 1, 2, 4, 8, 12, 24, 36 and 48 h after administration. The skins were excised and frozen at –80 °C.
Frozen section and confocal laser scanning microscope
The skins were embedded in the OCT compound. Cryosections of vertical skins of different times and horizontal skins of every 10 μm (starting from SC, the first slice out of every five sections) of 24 h were collected. These slices were further subjected to DAPI and immunofluorescence staining. CLSM (Carl Zeiss Inc., Oberkochen, Germany) was performed to visualize the slides. P4 signal was excited by Alexa 633 channel. Fluorescence from DAPI and C6 was excited by corresponding default channels.
Preparation and optimization of Cel-Indo-NLCs-gel
Preparation of Cel-Indo-NLCs-gel
A hydrogel was introduced to obtain viscosity levels adequate for transdermal application. Hence, 0.5% (w/v) of Carbopol 940 was added to Cel-Indo-NLCs and left to hydrate overnight. The dispersion was subsequently neutralized by using triethanolamine to promote gelation. The preparation of Cel-Indo-NLCs-gel is illustrated in .
Optimization of Cel-Indo-NLCs-gel
Rheological behaviour
A Rheometer (Anton Paar, MCR-102; Austria) was used to determine the rheological behaviour of NLCs-gel. NLCs-gel was put on the plate and the parameters were adjusted according to the equipment recommendations. The viscosity and the corresponding shear stress were measured isothermally at 32 °C. The apparent viscosity at each speed was recorded by software Rheoplus and the flow behaviour of sample was determined by flowcurve test. The oscillation frequency sweep test was carried-out by measuring G′ (storage modulus), G″ (loss modulus) and complex viscosity (η*) as the angular frequency (ω) ranging. This test was used to monitor sample behaviour at constant strain and on changing frequency.
In vitro skin permeation studies
The skin permeation of Cel and Indo from Cel-Indo-NLCs and Cel-Indo-NLCs-gel was measured by using the Franz diffusion cells. The full-thickness abdominal skins of male Sprague-Dawley rats were detached after they were sacrificed and the subcutaneous fats and connective tissues were carefully removed before washed with saline. The prepared skins were mounted between the donor and receptor compartments, with the SC side facing up. The Franz diffusion cells with an effective diffusion area of 3.14 cm2, and a receptor volume of 9 ml were used to assess in vitro skin permeation. The donor medium consisted of Cel-Indo-NLCs or Cel-Indo-NLCs-gel, and the receptor medium was comprised of ethanol and phosphate-buffered saline at a ratio of 3:7 [Citation11,Citation19]. The stirring rate was 200 rpm/min and receptor compartments were maintained at 37 ± 0.1 °C to ensure the temperature of skin surface was 32 °C to mimic human skin conditions. 1.0 ml of the receptor medium at various intervals (2, 4, 6, 8, 10, 12, 24, 36 and 48 h) was removed and immediately replaced with 1.0 ml fresh medium. The release studies were performed in triplicate. The cumulative amount of Cel and Indo diffused per unit area of the excised skin (Qn) is: Qn = Cn × V/A, where Cn was the drug concentration of receptor medium at each sampling time, V represents the volumes of receptor compartment and A represents the effective diffusion area.
In vivo anti-rheumatic activity assessment
RA model and monitoring
Complete Freund’s adjuvant (CFA)-induced RA model was employed to investigate anti-rheumatic activity of Cel-Indo-NLCs-gel [Citation20–22]. Two hundred microlitres of CFA was injected subcutaneously into right hind paw of rats after they were anesthetized. Another booster dose of 100 μl of CFA was injected in the same paw on day 6 of the initial immunization. On day 7, rats were randomly divided into six groups, including (A) Normal group (normal rats), (B) Model group (RA rats without treatment), (C) Safety control group (normal rats with Cel-Indo-NLCs-gel), (D) Cel-NLCs-gel group (RA rats with Cel-NLCs-gel), (E) Indo-NLCs-gel group (RA rats with Indo-NLCs-gel) and (F) Cel-Indo-NLCs-gel group (RA rats with Cel-Indo-NLCs-gel). The formulations were topically applied three times a day on paws and joints of rats at a dose equivalent to 5 mg/kg. A plethysmometer was used to measure the paw volume of each rat on day 7, 10, 13, 16, 19, 22, 25, 28, 31 and 34.
Estimation of pro-inflammatory cytokines
Blood was collected by abdominal aortic method and allowed to clot for 1 h, and the serum was collected by centrifugation at 3000 rpm for 15 min. Concentrations of IL-1β, TNF-α, β-endorphin and Substance P (SP) were quantified in the serum by ELISA according to the manufacturer’s protocols.
Histological analysis
After the completion of experiment, ankle joints, ankle skins, kidneys and testes of rats were collected and fixed with 10% formalin. The tissues were sectioned, embedded in paraffin and stained with haematoxylin and eosin. Slides were viewed under an optical microscope (BH-2; Olympus, Tokyo, Japan) for histopathological changes. The severity of arthritis was assessed according to infiltration of leukocytes and the extent of hyperplasia of synovium.
Statistical analysis
Results are presented as the mean ± standard deviation. Differences were analysed using Student’s t-test or one-way ANOVA using SPSS software (SPSS Inc., Chicago, IL). p < .05 was considered statistically significant.
Results and discussion
Lipids and surfactant screening
Selection of lipids
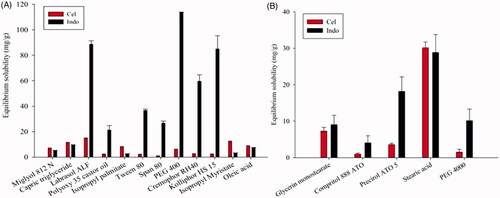
Solubility of drugs in melted lipid is known to be a major factor to obtain sufficient EE and DL. The desirable liquid lipid should not only have a good ability to dissolve the drugs, but also possess the quality to form a homogenous mixture with solid lipid. So, different lipids were selected to dissolve Cel and Indo. The solubility of the drugs in different lipids is presented in . According to the result, Labrasol ALF was chosen as the liquid lipid because of its maximal solubility of two drugs. It was worth noting that although the solubility of drugs in SA and GMS was higher than Precirol ATO-5, the melted lipids containing SA or GMS with Labrasol ALF showed phase separation. So, Precirol ATO-5 was chosen as the solid lipid.
The miscibility of lipids
Precirol ATO-5 and Labrasol ALF mixed in different ratios were carried out to exam the miscibility of lipids. The melting point about melted lipids mixture of all ratios was greater than 40 °C. However, the combination of solid and liquid lipids with ratios of 90:10, 80:20 and 70:30 had no oil drops on the filter paper. Considering the larger solubility of liquid lipids to drugs, 70:30 was chosen as the ratio in order to achieve a higher DL.
Surfactant screening
The species and percentages of surfactants are important for the fabrication of NLCs. It was interesting that only moderate aqueous solution of Cremophor RH40 could emulsify the melted lipids sufficiently, resulting in NLCs with clear and transparent appearance. However, other groups produced turbid and opaque suspensions or flocculated precipitate in the process. So, Cremophor RH40 was chosen as the surfactant.
Optimization of Cel-Indo-NLCs preparation
The optimal conditions for preparation of Cel-Indo-NLCs were selected using a BBD. According to the Design Expert software, 17 runs of Cel-Indo-NLCs were prepared and evaluated by response parameters (). The summary of results about regression analysis for the three responses is given in . The results depicted that the independent variables showed a significant effect on dependent variables (p < .05). The observed responses were fitted to 2FI, quadratic and quadratic models. The R2 values for particle size, mean EE and total DL were found to be 0.8514, 0.9134, 0.9866, which implied that 85.14%, 91.34% and 98.66% of variations in responses in test domain were attributed to independent variables.
Table 3. Summary of the results of regression analysis for responses, and analysis of variance for particle size, mean EE and total DL.
The polynomial equations were generated by the statistical analysis of the results:
A positive value in equations favours relationship between the factors and responses, while a negative value corresponds to an inverse to the relationship.
From the equation for particle size, it is observed that an increase in concentration of total lipids and the added drugs led to an increase in particle size of NLCs, as the concentration of surfactant increased, the particle size decreased. The higher lipid concentration might thicken the “shell” of the NLCs, while the greater the amount of added drugs, the thicker the “nucleus” of the NLCs, resulting in increased particle size. While the higher concentration water solution of surfactant might cover the surface of tiny lipid droplets by enough emulsifier, which in turn impeded their coalescence by increasing the repulsion between them [Citation23].
For mean EE, an increase in concentration of total lipids and surfactant resulted in increased EE and the added drugs showed inverse relationship. The higher total lipid concentration, the stronger loading capacity could achieve, so it is much easier to entrap the drugs in lipids completely. Moreover, an increase in the surfactant concentration led to an increase in EE, which might be due to retention of drug molecules within the lipids by producing a large number of NLCs in a certain amount of lipid [Citation24,Citation25].
For total DL, the increase of surfactant concentration and the amount of drugs caused an increase in DL whereas the amount of lipids showed inverse relationship. This is obvious because the higher surfactant concentration could emulsify sufficiently the melted mixture of lipids and drugs, thus avoiding drugs leaking to the external water phase. As quantitative lipids could load a certain amount of drug, while in this range as the drug increased, the drug load increased. If the amount of drug was decided, the more the lipids added, the lower the DL.
Average particle size, PDI, mean EE and total DL of the optimized Cel-Indo-NLCs were 26.92 ± 0.62 nm, 0.201 ± 0.01, 96.56 ± 1.41% and 3.65 ± 0.05%, respectively. The low RSD values between predicted and experimental values obtained based on optimal formula generated by BBD () proved that the optimization process was stable and feasible.
Table 4. Predicted and experimental values obtained based on optimal formula generated by BBD (n = 3).
Characterization of Cel-Indo-NLCs
Surface morphology
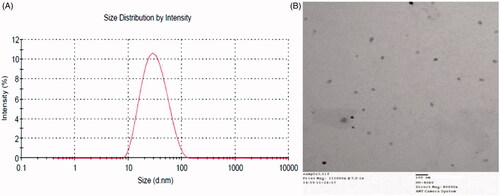
The TEM study indicated that Cel-Indo-NLCs were in spherical shape and smooth surface (). In addition, the size observed with TEM was in good agreement with particle size obtained by Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). The uniformity in particle size distribution correlated with the small PDI.
FTIR analysis
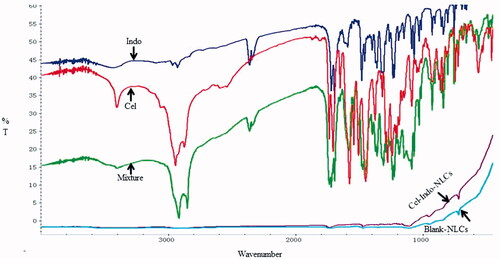
As shown in , the principle peaks of Indo were observed at 1717.94 cm−1(C=O stretching of carboxyl), 1691.71 cm−1 (C=O stretching of amide bond) and 1234.35 cm−1 (=C–O–CH3). 1704.04 cm−1 (C=O stretching of carboxyl), 1734.85 cm−1 (=C=O), 2943.38 cm−1 (–CH in –CH3) and 3402.87 cm−1 (–OH of carboxyl) were peaks of Cel, whereas the peak for Blank-NLCs was observed at 750 cm−1. The characteristic peaks of Cel and Indo were appeared in Mixture and disappeared in Cel-Indo-NLCs. The peaks of Cel-Indo-NLCs were similar to Blank-NLCs, which revealed that drugs may exist in amorphous form in NLCs.
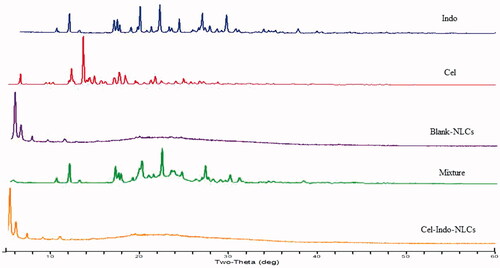
Powder X-ray diffraction analysis
The diffraction pattern of drugs in free form and NLCs was studied by PXRD. As shown in , the characteristic peak of 100% relative intensity at 2θ position of 21.914° and 13.497° was for Indo and Cel, respectively. Other important peaks were observed at 2θ between 10° and 30°, indicating their highly crystalline nature. However, the characteristic peaks were appeared in Mixture and Cel-Indo-NLCs, which confirmed the absence of crystallinity of drugs.
In vivo transdermal translocation of NLCs
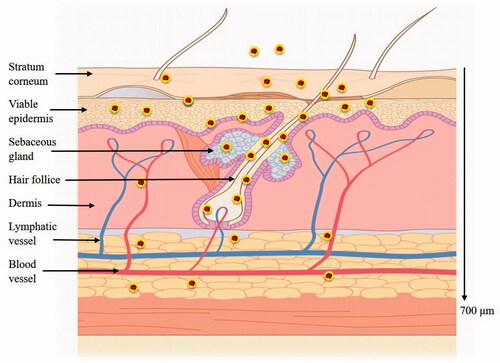
DAPI was used to stain nucleus of living cells to visualize the overall transdermal translocation of NLCs. As shown in , it is obvious that strong fluorescence signals of P4 and C6 were observed on the surface of viable epidermis and dermis at 1 h, which attributed to the small particle size. The strong fluorescence signals all over the skin could last to 24 h and after that the intensity decreased because P4 was released after lipids degradation and quenched when contacted with water. The fluorescence in the hair follicles, sebaceous glands and sweat ducts remained strong at 36 h. The results confirmed that NLCs could penetrate into skin in a complete nanoparticle form while some NLCs could enter the skin through the hair follicle. The results could also support the sustained release of NLCs.
Figure 6. CLSM images of vertical section of the skin treated with Cel-Indo-NLCs at different time. The slides were stained with DAPI. White arrows indicate the hair follicles.
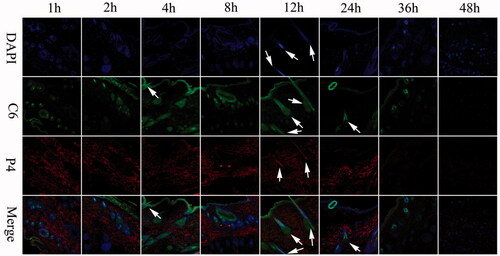
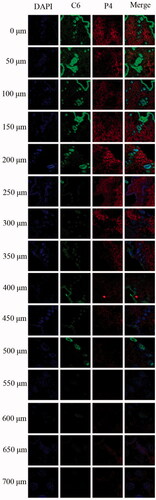
In order to further characterize the penetration depth of NLCs, continuous horizontal sections of skin samples of 24 h after administration were performed. As observed from the vertical sections (), NLCs could efficiently penetrate across the intact skin. Fluorescence in the form of a circle indicated that NLCs entered the skin through the hair follicle, while other red fluorescence indicated that NLCs entered the skin in the form of complete NLCs. As the depth increased (700 μm), the fluorescence decreased. The transdermal mechanism and depth of NLCs into skin are shown in .
Rheological behaviour
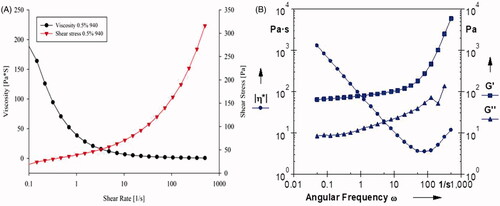
The rheological properties are important to gel formulation since they influence the ease of application and both adhesion and retention at the site of application. The viscosity of Cel-Indo-NLCs-gel was 38.5 Pa·s when the shear rate was 1/s and shearing stress was 39.3 Pa (). The value of flow behaviour index was 0.3072, which revealed a pseudo-plastic behaviour as indicated by a decrease in viscosity with increasing shear speed, which is a desirable feature for efficient topical formulations since it ensures maximum area coverage upon application. As shown in , no cross-over of G′ and G″ was seen, and G′ was quite higher than G″, which further assured the high elasticity of Cel-Indo-NLCs-gel that retained virtue of less dissipation of energy. So, Cel-Indo-NLCs-gel could be easily transported and stored at ambient temperature assuming it is not subjected to any shear changes which might alter their viscosity.
In vitro skin permeation studies
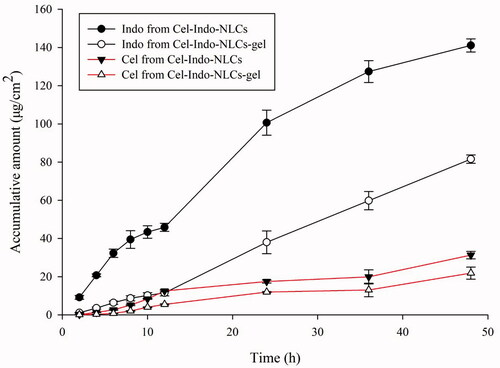
illustrates the in vitro skin permeation of Cel and Indo from Cel-Indo-NLCs and Cel-Indo-NLCs-gel. The Q48 of Cel in them was 31.26 ± 2.00, 21.87 ± 3.14 μg/cm2, and 141.10 ± 3.42 and 81.58 ± 2.21 μg/cm2, for Indo. The figure depicted that the transdermal release of Cel was sustained. Indo has been sustained released for the first 12 h, and the amount of percutaneous penetration increased subsequently. This may attribute to the moderate log P of Indo, so it could penetrate into the receptor through skin, while Cel continued to accumulate in skin because of a large log P and low permeability.
Effects on paw swelling
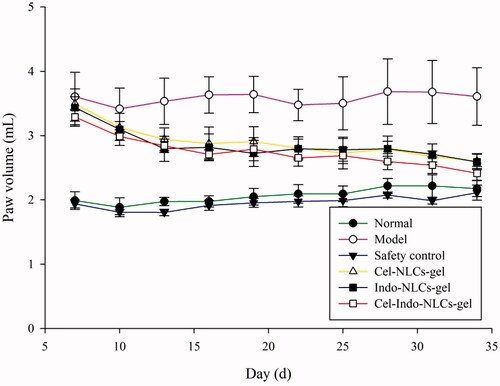
Paw volume changes during the experiment is a crucial parameter to evaluate the inflammatory effect. The rats exhibited a significant increase in volume of the injected paw after injected by FCA. shows the paw changes of different groups, the treated groups reversed paw-swelling trend. The Cel-Indo-NLCs-gel group significantly reduced the paw swelling and showed the best inhibition effect.
Anti-inflammatory and analgesic effects
TNF-α [Citation26] and IL-1β [Citation27] are pro-inflammatory cytokines that play a significant role in pathogenesis. As shown in , there was a significant increase in the levels of TNF-α and IL-1β in RA rats compared to normal and safety control group. RA rats treated with Cel-NLCs-gel or Indo-NLCs-gel significantly reduce the levels of TNF-α and IL-1β, while the serum of rats in Cel-Indo-NLCs-gel group showed the lowest level. The results showed that the transdermal delivery of Cel-Indo-NLCs-gel provided the most prominent inhibitory effect on inflammatory cytokines.
Figure 12. Effects of different treatments on serum levels of inflammatory and pain factors in rats. **p < .01, *p < .05 compared with the model group (n = 6). A, B, C, D, E and F respectively represent Normal (normal rats), Model (RA rats without treatment), Safety control (normal rats with Cel-Indo-NLCs-gel), Cel-NLCs-gel (RA rats with Cel-NLCs-gel), Indo-NLCs-gel (RA rats with Indo-NLCs-gel) and Cel-Indo-NLCs-gel (RA rats with Cel-Indo-NLCs-gel) group.
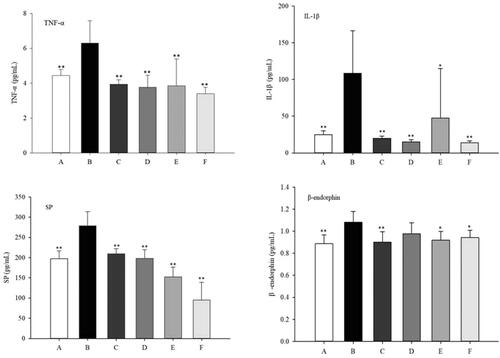
The neurotransmitters β-endorphin and SP [Citation28,Citation29] are vital in the pathophysiology of pain development. The concentrations of SP in serum of treatment groups were significantly lower than that of model group (). While concentrations of β-endorphin in the serum of Cel-NLCs-gel group had no significant difference with model group, indicating that the analgesic effect of Cel was not caused by the regulation of β-endorphin or its analgesic effect was inferior to Indo in this experiment.
Histological analysis
Histological analysis in ankle joints of normal rats showed no infiltration of inflammatory cells and the articular cartilage was intact (). Sections of the model group displayed severe inflammatory infiltration, synovium destruction and articular cartilage erosion. The degree of severity for treated groups was scored from 0 to +++ (0 = normal and +++ = severe). The rats treated with Cel or Indo-NLCs-gel also showed synovium destruction and infiltration of inflammatory cells. In contrast, the inflammatory cells and synovial hyperplasia in Cel-Indo-NLCs-gel group were substantially decreased compared to model group.
Figure 13. Effects of different administrations on paw appearances, histopathological images of ankle joints and skins. The degree of severity of inflammation is presented as 0 = normal; + =mild; ++=moderate; +++ =severe. The images presented are dedicated for (A) Normal (normal rats), (B) Model (RA rats without treatment), (C) Safety control (normal rats with Cel-Indo-NLCs-gel), (D) Cel-NLCs-gel (RA rats with Cel-NLCs-gel), (E) Indo-NLCs-gel (RA rats with Indo-NLCs-gel) and (F) Cel-Indo-NLCs-gel (RA rats with Cel-Indo-NLCs-gel) group.
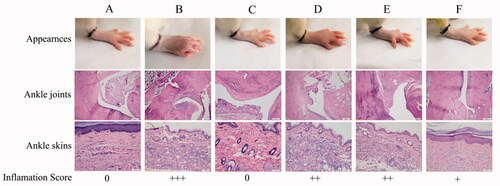
The histopathology in ankle skins of RA rats showed severe inflammatory infiltration. The rat skins of safety control group were same with normal group, indicating that no skin irritation was caused after long-term administration of Cel-Indo-NLCs-gel. It could be attributed to biocompatible nature of NLCs. Compared with model group, inflammatory infiltration degree of treated groups was decreased, which was particularly obvious in Cel-Indo-NLCs-gel group.
The severity of inflammation of ankle joints and skins is seen in the following order: model group > Indo-NLCs-gel > Cel-NLCs-gel > Cel-Indo-NLCs-gel > normal and safety test group. The above results suggested that co-load of Cel and Indo into NLCs could inhibit infiltration of inflammatory cells effectively and alleviate arthritis in CFA induced rats.
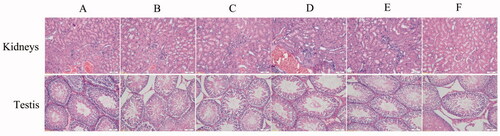
Safety evaluation
In the process of dissection, the gastrointestinal tract, testis and kidneys of all groups appeared smooth and normal in colour and size. The common gastrointestinal adverse reactions of Indo included nausea, abdominal pain and diarrhoea. In order to avoid the side effects, there are many transdermal delivery formulations of Indo on the market, for example, Indomethacin Cataplasm, Indomethacin Liniment, Indomethacin Ointment, Indomethacin Cream, Compound Indometacin Cream, and so on. In the process of experiment, rats of all groups did not suffer from anorexia, diarrhoea and other abnormal symptoms through daily behaviour observation. Hence, the reproductive toxicity of Cel-Indo-NLCs-gel should be attached with great importance compared with gastrointestinal side effect. As shown in , sections of kidneys showed normal physiological structure and cell morphology. Sections of testis revealed that there were mature sperm cells and sperms in the testicular seminiferous tubules and the structure of seminiferous tubules, Sertoli cells, and the count of spermatogonia, spermatocytes and sperm were normal. The results revealed that no toxicity was caused after the topical administration of Cel-Indo-NLCs-gel and the preparation had better bioavailability.
Figure 14. Histopathological images of kidneys and testis of different groups. The images presented are dedicated for (A) Normal (normal rats), (B) Model (RA rats without treatment), (C) Safety control (normal rats with Cel-Indo-NLCs-gel), (D) Cel-NLCs-gel (RA rats with Cel-NLCs-gel), (E) Indo-NLCs-gel (RA rats with Indo-NLCs-gel) and (F) Cel-Indo-NLCs-gel (RA rats with Cel-Indo-NLCs-gel) group.
Conclusions
In this study, an NIR fluorescent probe was employed to track the transdermal translocation of NLCs, which demonstrated NLCs can penetrate into skin by its small particle size and hair follicle pathway. The potential of Cel combined with Indo co-encapsulated in NLCs was investigated. The optimized Cel-Indo-NLCs were incorporated into gel for convenient skin application. The pharmacodynamics test indicated the enhanced anti-inflammatory effect, analgesic effect and anti-rheumatic effect of Cel-Indo-NLCs-gel in FCA-induced RA model. Cel-Indo-NLCs-gel could relieve arthritis by inhibiting cytokines expression and inflammatory infiltration into the inflamed joints. These results suggested that Cel-Indo-NLCs-gel could be a promising sustained transdermal delivery to relieve RA and avoid side effects on the gastrointestinal and reproductive systems.
Acknowledgements
We thank Professor Wei Wu from Fudan University for gifting the P4 dye.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Doss HM, Ganesan R, Rasool M. Trikatu, an herbal compound ameliorates rheumatoid arthritis by the suppression of inflammatory immune responses in rats with adjuvant-induced arthritis and on cultured fibroblast like synoviocytes via the inhibition of the NFκB signaling pathway. Chem-Biol Interact. 2016;258:175–186.
- Venkatesha SH, Astry B, Nanjundaiah SM, et al. Suppression of autoimmune arthritis by Celastrus-derived Celastrol through modulation of pro-inflammatory chemokines. Bioorg Med Chem. 2012;20:5229–5234.
- Cascão R, Vidal B, Raquel H, et al. Effective treatment of rat adjuvant-induced arthritis by Celastrol. Autoimmun Rev. 2012;11:856–862.
- Vitorino C, Almeida J, Gonçalves LM, et al. Co-encapsulating nanostructured lipid carriers for transdermal application: from experimental design to the molecular detail. J Control Release. 2013;167:301–314.
- Wissing SA, Müller RH. Cosmetic applications for solid lipid nanoparticles (SLN). Int J Pharm. 2003;254:65.
- Khurana S, Jain NK, Bedi PMS. Nanoemulsion based gel for transdermal delivery of meloxicam: physico-chemical, mechanistic investigation. Life Sci. 2013;92:383–392.
- Kogan A, Garti N. Microemulsions as transdermal drug delivery vehicles. Adv Colloid Interface Sci. 2006;123–126:369–385.
- Shakeel F, Baboota S, Ahuja A, et al. Skin permeation mechanism of aceclofenac using novel nanoemulsion formulation. Pharmazie. 2008;63:580–584.
- Su R, Fan W, Yu Q, et al. Size-dependent penetration of nanoemulsions into epidermis and hair follicles: implications for transdermal delivery and immunization. Oncotarget. 2017;8: 38214–38226.
- Garg NK, Singh B, Tyagi RK, et al. Effective transdermal delivery of methotrexate through nanostructured lipid carriers in an experimentally induced arthritis model. Colloids Surf B: Biointerfaces. 2016;147:17–24.
- Chen Y, Zhou L, Yuan L, et al. Formulation, characterization, and evaluation of in vitro skin permeation and in vivo pharmacodynamics of surface-charged tripterine-loaded nanostructured lipid carriers. Int J Nanomedicine. 2012;7:3023–3033.
- Guo T, Zhang Y, Li Z, et al. Microneedle-mediated transdermal delivery of nanostructured lipid carriers for alkaloids from Aconitum sinomontanum. Artif Cells Nanomedicine Biotechnol. 2017;1–11.DOI:10.1080/21691401.2017.1376676
- Ghate VM, Lewis SA, Prabhu P, et al. Nanostructured lipid carriers for the topical delivery of tretinoin. Eur J Pharm Biopharm. 2016;108:253–261.
- Moghddam SM, Ahad A, Aqil M, et al. Optimization of nanostructured lipid carriers for topical delivery of nimesulide using Box–Behnken design approach. Artif Cells Nanomed Biotechnol. 2017;45(3):617–624.
- Kumar A, Pathak K, Bali V. Ultra-adaptable nanovesicular systems: a carrier for systemic delivery of therapeutic agents. Drug Discov Today. 2012;17:1233–1241.
- Hu X, Fan W, Yu Z, et al. Evidence does not support absorption of intact solid lipid nanoparticles via oral delivery. Nanoscale. 2016;8:7024–7035.
- Xie Y, Hu X, He H, et al. Tracking translocation of glucan microparticles targeting M cells: implications for oral drug delivery. J Mater Chem B. 2016;4:2864–2873.
- Hu X, Zhang J, Yu Z, et al. Environment-responsive aza-BODIPY dyes quenching in water as potential probes to visualize the in vivo fate of lipid-based nanocarriers. Nanomedicine. 2015;11:1939–1948.
- Wang X, Xue M, Gu J, et al. Transdermal microemulsion drug delivery system for impairing male reproductive toxicity and enhancing efficacy of Tripterygium Wilfordii Hook f. Fitoterapia. 2012;83:690–698.
- Jia M, Deng C, Luo J, et al. A novel dexamethasone-loaded liposome alleviates rheumatoid arthritis in rats. Int J Pharm. 2018;540:57–64.
- Kaur A, Bhoop BS, Chhibber S, et al. Supramolecular nano-engineered lipidic carriers based on diflunisal-phospholipid complex for transdermal delivery: QbD based optimization, characterization and preclinical investigations for management of rheumatoid arthritis. Int J Pharm. 2017;533:206–224.
- Zeb A, Qureshi OS, Yu C-H, et al. Enhanced anti-rheumatic activity of methotrexate-entrapped ultradeformable liposomal gel in adjuvant-induced arthritis rat model. Int J Pharm. 2017;525:92–100.
- Joshi SA, Jalalpure SS, Kempwade AA, et al. Fabrication and in-vivo evaluation of lipid nanocarriers based transdermal patch of colchicine. J Drug Deliv Sci Technol. 2017;41:444–453.
- Liu J, Hu W, Chen H, et al. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int J Pharm. 2007;328:191–195.
- Castro GA, Coelho ALLR, Oliveira CA, et al. Formation of ion pairing as an alternative to improve encapsulation and stability and to reduce skin irritation of retinoic acid loaded in solid lipid nanoparticles. Int J Pharm. 2009;381:77–83.
- Pu Y, Cao D, Xie C, et al. Anti-arthritis effect of a novel quinazoline derivative through inhibiting production of TNF-α mediated by TNF-α converting enzyme in murine collagen-induced arthritis model. Biochem Biophys Res Commun. 2015;462:288–293.
- Wu Q, Wang Y, Wang Q, et al. The bispecific antibody aimed at the vicious circle of IL-1β and IL-17A, is beneficial for the collagen-induced rheumatoid arthritis of mice through NF-κB signaling pathway. Immunol Lett. 2016;179:68–79.
- Riley JL, Cruz-Almeida Y, Dasilva Ribeiro MC, et al. Age differences in the time course and magnitude of changes in circulating neuropeptides after pain evocation in humans. J Pain. 2017;18:1078–1086.
- Zheng B, Hu L, Song X, et al. Analgesic effect of different moxibustion durations in rheumatoid arthritis rats. J Trad Chin Med. 2014;34:90–95.