?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Repair of volumetric muscle loss (VML) injuries is a complicated endeavour which necessitates the collaborative use of different regenerative approaches and technologies. Herein is proposed the development of fibrin-based microbeads (FMs) alone or as a bone marrow mesenchymal stem cell (MSC) encapsulation matrix for modular muscle engineering. FMs were generated through the ionotropic gelation of alginate and fibrinogen obtained from the platelet-rich plasma of whole blood, and then removing the alginate by citrate treatment. FMs were first characterized by FT-IR, SEM and water uptake tests. Then, the stability of FMs and the mitochondrial dehydrogenase activity of the MSCs encapsulated in FMs were evaluated under in vitro culture conditions. Eventually, the regenerative capacity of the cell-devoid and MSCs-encapsulated FMs was evaluated in a rat VML injury model involving 8 × 4×4 mm3-size bilateral defects in the biceps femoris muscles. The histochemical, immunohistochemical and semi-quantitative histomorphological scoring results retrieved at 30, 60 and 180 days demonstrated that the cell-devoid FMs supported muscle regeneration to a great extent. Moreover, MSCs-encapsulated FMs were more effective in shortening the regeneration period of the injured tissue of the rat VML, resulting in good myofibre orientation, while the Sham group resulted in incomplete repair with fibrotic scar tissue formations.
Introduction
Major traumatic injuries of the skeletal muscle affect hundreds of millions of people throughout the world causing long-term pains and organ dysfunctions [Citation1,Citation2]. Regional tissue loss in the skeletal muscle, caused by accidents, gun battles, natural disasters, etc. may lead to traumatic skeletal muscle injuries [Citation1–3]. To date, several therapeutic approaches among which are drug therapies and surgical procedures with varying and usually limited efficiency have been used to treat skeletal muscle injuries [Citation3–5].
Despite the fact that skeletal muscle has a good self-regeneration capacity, spontaneous healing of acute volumetric muscle loss (VML) is known to be restricted [Citation1]. Activation of the myogenic process and the consequent inflammation are both involved in the modulation of regeneration [Citation2]. Certain biomaterials could serve as structural scaffolds for the regenerating new tissue [Citation5,Citation6]. Hence, a number of natural or synthetic biomaterials have been identified for muscle repair applications with varying levels of success [Citation5–8]. Nevertheless, the regeneration of a VML remains to be an unsolved issue. Previous studies indicate that co-regulation of inflammation, regeneration and fibrosis play key roles in functional muscle recovery [Citation2,Citation9]. Thus, the most appropriate biomaterial to be used in skeletal muscle tissue engineering is to contain components that regulate these three phases [Citation8,Citation10]. The use of stem cells alone or in combination with regenerative biomaterials for the engineering of the skeletal muscle tissue could have potential as a biological alternative for aiding the modulation of regeneration [Citation11–14]. This approach could support the VML region in the aftermath of severe damage and provide faster healing by allowing for an exogenous regenerative response to complement an endogenous one [Citation10]. In particular, the incorporation of mesenchymal stem cells (MSCs) to the appropriate biomaterial scaffold may have the potential to improve the overall regenerative milieu [Citation15]. The optimal biomaterial should not only support the viability of MSCs, accelerate tissue ingrowth and prevent fibrotic scar tissue formation, but also have a biodegradation rate compatible with de novo myogenesis, and promote neovascularization and innervation at the regeneration site.
Platelet rich plasma (PRP) is an autologous source of a wide variety of growth factors including platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), insulin growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF), which all can promote tissue regeneration [Citation16]. PRP is used in a broad range of wound-healing processes due to the fact that it supports the regeneration/remodelling of damaged tissues [Citation17,Citation18]. PRP is obtained through the centrifugation of a patient's own peripheral blood. Therefore, no immune response against PRP occurs in the patient’s body. As there is interest for platelets as a wound-healing accelerator and immunomodulator [Citation19], the usage of PRP is wide-spread in many fields of surgery [Citation20–22]. It is also observed that there is an extensive use of PRP in skeletal muscle treatments. Some studies indicate that PRP induces the repair of the damaged skeletal muscle tissue via specific processes, such as the modulation of pro-inflammatory cytokines and myogenic regulatory factors [Citation23,Citation24].
Fibrin is a critical blood component responsible for haemostasis, and it is formed by thrombin and calcium-mediated activation of fibrinogen. Owing to its intrinsic bioactivity, it has been extensively used as a temporary matrix for the cells in the wound-healing applications [Citation1,Citation25,Citation26]. Apart from that, platelet-poor plasma (PPP)-derived fibrin glue is used in a variety of surgical applications as well [Citation27,Citation28]. Both PRP and PPP can safely be used as a delivery vehicle and a scaffolding matrix for tissue engineering applications. It is known that fibrin in combination with an appropriate cell source can be utilized as a structural scaffold for the engineering of bone [Citation29,Citation30], cartilage [Citation31], heart [Citation32] and nerve tissues [Citation33]. There are some studies available in literature which show that fibrin plays a major role in the regeneration of the connective tissue and skeletal muscle [Citation34]. However, the number of studies using the fibrin matrix for muscle tissue engineering applications is still limited. It is anticipated that PRP-derived fibrin structure, due to its higher platelet content can accelerate the wound-healing process more efficiently than PPP. By providing a temporary extracellular matrix (ECM) in the wound area, fibrin supports the formation of the new tissue. Consequently, the use of PRP-derived fibrin biomaterials in muscle tissue engineering can be considered to be promising. In most of the studies, PRP has been used alone in fibrin gel form or in combination with a grafting material. While, there are no studies on the use of PRP in microbead form for the healing of VML injuries, cell encapsulation may be utilized within this context since this technology provides a three-dimensional tissue-like semi-permeable hydrogel environment for the regenerative cells during transplantation [Citation35–37].
Given the regeneration capacity of stem cells, MSC-based therapies have emerged as an attractive treatment option for tissue repair and regeneration. On the other hand, PRP is widely used in tissue engineering and regenerative medicine due to its rich content in growth factors that take part in the wound healing process. Notably, several studies have shown the mitogenic effects of PRP on MSCs [Citation38]. In the light of this information, we thus aimed to combine the healing and mitogenic properties of PRP with the regenerative effects of MSCs to obtain a more efficient way of muscle tissue repair.
In this study, fibrin-based microbeads (FMs) were produced by using alginate and PRP (and PPP) obtained from the whole blood, and then the alginate-free FMs were generated by using sodium citrate and characterized as an encapsulation matrix for rat bone marrow-derived MSCs (BM-MSCs). The efficiency of cell-devoid and MSCs-encapsulated FMs was evaluated for the regeneration of a VML injury in rats, as a potential approach for modular muscle tissue engineering.
Materials and methods
Animals
The in vivo study was reviewed and approved by the Ethics Committee for Animal Experimentation of Ankara University (Approval Number: 2012-7-053) and carried out in accordance with the international guidelines. A total of 38 healthy Wistar rats (10–12 weeks-old, weighing 250 ± 25 g) were used for the in vivo experiments: three rats were used for isolation of the MSCs; eight rats were used for obtaining PRP, and the remaining 27 rats were used for the surgeries.
Materials
All chemicals used for the generation of fibrin microbeads were purchased from Sigma-Aldrich (St. Louis, MO) and were used without further purification. MSCs isolated from the rat bone marrows were seeded on tissue culture polystyrene (TCPS) flasks (Corning, NY) and cultured in the Minimum Essential Medium Alpha (α-MEM; Lonza, Basel, Switzerland), l-glutamine (Lonza, Basel, Switzerland), penicillin/streptomycin solution (Pen/Strep, Lonza, Basel, Switzerland) and foetal bovine serum (FBS; Lonza, Basel, Switzerland). Trypsin (Sigma-Aldrich, St. Louis, MO) and ethylenediaminetetraacetic acid (EDTA; Sigma-Aldrich, St. Louis, MO) were used to harvest the expanded MSCs. The antibodies used were as follows: anti-MyoD1 antibody (ab16148 AbCam, Cambridge, MA), anti-Desmin antibody (ab8470 AbCam), anti-myogenin (ab1835 AbCam). Whole blood, used as the source of PRP and PPP, was obtained from adult Wistar rats.
Isolation and culture of MSCs
MSCs were isolated from the femurs of donor Wistar rats (n = 3; 250 ± 25 g) according to standard protocols [Citation39]. Briefly, after anaesthesia with ketamine hydrochloride (60 mg/kg) and xylazine (10 mg/kg), the femurs were dissected out aseptically. Muscle and fat tissue around the bones were removed as clean as possible with 70% ethanol and the lower and upper parts of the femurs were cut afterwards. Bone marrow was collected by flushing the diaphysis by aid of a syringe with 25 G tip containing 5 mL of α-MEM. Then, the marrow suspension was centrifuged at 1100 rpm for 10 min. The upper part of the suspension was discarded and cells were centrifuged three times for 5 min at 1100 rpm and were washed with FBS-free culture medium. After the washing step, the cells were pooled and then transferred into T25 cell culture plates (Corning, NY) containing the expansion medium (α-MEM containing 20% FBS, 2 mM l-glutamine, 100 µL/mL Pen/Strep) and cultured inside an incubator at 37 °C, 5% CO2, 95% air and 90% humidity. At the third day of culture, the non-adherent cells were carefully removed by changing the culture medium. The adherent cell culture was maintained by changing the medium three times a week. The cells were passaged by 0.05% trypsin and 1.0 mM EDTA, when they reached a confluence of ∼70%. Rat BM-MSCs obtained from the passages 2 and 3 were used in all experiments after confirming their MSC character [Citation40]. The cells demonstrating CD29+, CD44+, CD90+, CD34– and CD45– immunophenotype and trilineage potential were used in the encapsulation studies.
Preparation of PRP and PPP
Whole blood was drawn from healthy rats via cardiac puncture by aspiration. PRP was obtained from anti-coagulated whole blood samples through centrifugation steps. Briefly, ∼9–10 mL whole blood from each donor was collected and transferred to centrifuge tubes containing a solution of citrate as an anticoagulant, and then centrifuged at 1800 rpm. A two-phase mixture was obtained after centrifugation. The upper phase containing the PPP was transferred to another centrifuge tube and centrifuged at 3600 rpm. After the second centrifugation, the upper part (PPP) was removed to obtain the PRP. Both PRP and PPP were kept at room temperature, agitated on an axial rotator and were used as fresh.
Encapsulation of MSCs in FAMs and FMs
summarizes the experimental procedures involving encapsulation of cells in FAMs and FMs. To put it briefly, sodium alginate powder (0.2 g) was added into 10 mL of either PRP or PPP and then stirred on a magnetic stirrer until they were completely solubilized (yielding 2 wt% solutions). MSCs were harvested by using trypsin/EDTA and suspended in a 100 µL solution containing 140 mM NaCl, 5 mM KCl, 10 mM HEPES, 10 mM glucose and 0.02% EDTA [Citation41]. The cell suspension was added into 3900 µL PRP (or PPP)-alginate solution, resulting in 4 mL of PRP (or PPP)-alginate with cells. A homogenous mixture was obtained by carefully pipetting and then transferred to a syringe.
Figure 1. Schematic presentation of the experimental procedures involving isolation and encapsulation of cells in FAMs and FMs.
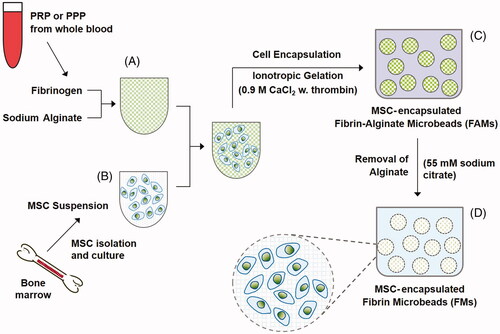
MSCs-suspended in PRP (or PPP)-alginate gel mixture was added dropwise into a solution of 0.9 M calcium chloride (in 0.85% NaCl) containing 10 U/mL thrombin, by using a syringe (with 21 G needle tip) while stirring on a magnetic stirrer. The resulting microbeads were stirred in the CaCl2 solution containing thrombin for the completion of the ionotropic gelation process for 15 min. The obtained FAMs were washed twice with a solution containing 140 mM NaCl, 5 mM KCl, 10 mM HEPES, 10 mM glucose and 0.02% EDTA.
FAMs were incubated with a solution of 55 mM sodium citrate, 150 mM NaCl and 30 mM EDTA (pH 6.8) for 15 min to remove the alginate [Citation37]. Obtained FMs were washed with the culture medium and then cultured under cell culture conditions. Alginate microbeads were also fabricated and used as a control for FTIR and water uptake experiments.
MTT assay
The mitochondrial dehydrogenase activity of the cells encapsulated inside FMs was investigated at specific time points by using the MTT assay based on the reduction of the yellow MTT salt [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide] to blue-purple coloured formazan crystals by mitochondrial enzymes. For this purpose, MSCs-encapsulated in FMs and in FAMs (from three separate encapsulation batches) were cultured in 48-well plates for 0 (3 h), 7 and 14 days. Then, the culture medium was removed from the wells at time points. After washing of the microbeads with serum-free medium, 270 µL medium and 30 µL MTT solution were added to each well, incubated at 37 °C for 4 h. The formation of insoluble blue-purple formazan crystals were monitored by an inverted microscope. Then, the resulting formazan crystals were dissolved in 300 µL MTT solvent. The optical densities were determined at 570 nm wavelength against blank using a microplate reader (Spectromax M5, Molecular Devices, Sunnyvale, CA).
SEM imaging
The surface morphology and pore structure of FMs were investigated by using a Jeol JSM 5600 model scanning electron microscope (Tokyo, Japan). Samples were fixed overnight with 2.5% glutaraldehyde solution (in 0.1 M PBS; pH 7.4) and then washed in distilled water to remove any remaining fixative. After that, microbeads were dehydrated by treating with ethanol series (50%, 70%, 80%, 90% and 95%) and allowed to air-dry. The specimens were sputter-coated with a thin layer of gold–palladium (∼15 nm) and then examined with SEM operating at 20 kV.
Fourier-transform infrared spectroscopy
Fourier-transform infrared (FTIR) spectroscopy was used to reveal whether or not alginate was removed from the fibrin microbeads. Samples were lyophilized overnight to remove the water using a freeze dryer at –55 °C (Christ Alpha 1-4 LD-plus, Osterode am Harz, Germany). FTIR spectral analysis of FM-R/FM-P, FAM-R/FAM-P and sodium alginate were performed with a Perkin Elmer-Spectrum 100 model device (Waltham, MA). Spectral scanning was done in the range of 650–4000 cm−1 and in the resolution of 0.5 cm−1 for four times. Data were evaluated by using Essential FTIR software.
Water uptake
Water uptake experiments were carried out to determine the structural characteristics of the FMs and compare the water uptake capacities of FMs and FAMs. Initially, all microbeads were lyophilized and the dry weights of FMs and FAMs were measured (M0). Then, the microbeads were immersed in distilled water at 37 °C to reach swelling equilibrium. After 48 h, microbeads were collected carefully, removing excessive water on the surface of samples and then the final weights of microbeads were weighed (Mt).
The percentage of water uptake was calculated according to the following equation:
(1)
(1)
VML and surgical studies
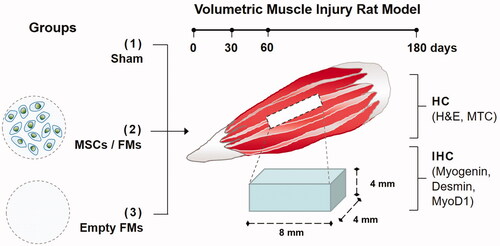
Twenty-seven adult Wistar rats were used for surgical studies. Anaesthesia was realized by an intraperitoneal injection of ketamine hydrochloride (60 mg/kg) and xylazine (10 mg/kg) for recipient rats. To create the VML, the muscle tissue (of 8 × 4×4 mm3) from the central part of biceps femoris of each rat leg was resected using a scalpel. Bleeding was controlled by mild compression of the resection area. The study design, and the experimental procedures related to VML are presented in . Animals were grouped as follows: (1) Sham group without implantation; (2) transplantation group I: 15–16 FM-R microbeads containing ∼1.3 × 105 MSCs (i.e. 8.5 × 103 MSCs per microbead) and (3) transplantation group II: 15–16 FM-R microbeads devoid of MSCs. Each wound was closed in multiple layers. 30, 60 and 180 days after transplantation, the defect area was carefully explanted under anaesthesia and then fixed in 10% formalin solution for histochemical and IHC analyses.
Histochemistry and immunohistochemistry
FM-R with/without MSCs-transplanted muscle tissues were retrieved at specific time points, fixed in 2.5% GA in phosphate buffer (pH 7.4). Fixed tissue samples were embedded in paraffin and cut into 5-µm sections. After standard preparations, the tissue sections were stained with either haematoxylin and eosin (H&E), or Masson’s Trichrome (MTC), then examined under a light microscope (Nikon, Tokyo, Japan). Morphological scoring system of the muscles associated with the stage of repair was implemented on the experimental samples according to . The regenerative efficiencies of MSC-free FMs, MSCs-containing FMs and the Sham group were compared. The histomorphometric analyses for each experimental group and each time point were performed on nine slides from three different animals for H&E and MTC stainings.
Table 1. Morphological scoring system of the muscles associated with stage of repair.
For immunohistochemistry, samples were incubated overnight at 4 °C with primary mouse monoclonal antibodies at the respective concentrations: anti-myogenin (1:100; Abcam cat. no. ab1835), anti-Desmin (1:50; Abcam cat. no. ab8470) and anti-MyoD1 (1:100; Abcam cat. no. ab16148). Sections were washed three times with PBS and the secondary antibody was applied for 1 h at room temperature. Sections were washed three times with PBS, and examined under the light microscope (Nikon, Tokyo, Japan).
Statistical analysis
Quantitative data analyses were done in triplicate and expressed as the mean ± standard deviation. The data were analysed by Student’s t test, and statistical significance was defined as *p< .05, ** p< .01 or ***p< .001. Cell-containing groups were compared with the cell-free groups using one-way ANOVA followed by Bonferroni's post hoc test using the GraphPad Prism software (La Jolla, CA).
Results and discussion
Blood source, processing and use of alginate for encapsulation
In this study, first of all, the method of producing fibrin microbeads with/without MSCs was established. In vivo studies were designed to evaluate the regeneration capacity of FMs on the rat VML model. Whole blood, the source of PRP and PPP was obtained allogeneically, as this is a more favourable option, compared to the xenogeneic sources [Citation42,Citation43]. Larger donors can be used in order to increase the amount of whole blood for transplantations to rodents; however, it is essential that immunological reaction is kept to minimum during transplantations. Centrifugation procedures used in the literature tested primarily with the object of obtaining PRP and PPP from whole blood [Citation44]. High-speed centrifugation operations may cause haemolysis of the blood cells, while low-speed centrifugation prevents the separation of blood components. For this reason, a suitable centrifuge method in which PRP and PPP components are separated from whole blood and keeping platelets alive was selected for the experiments.
Generally, fibrin is used in gel form either together with cells or alone as tissue adhesive in surgeries [Citation28,Citation45]. Using fibrin in the form of microbead allows to control or sustain the release of the growth factors contained in the platelets. Besides, transplantation of stem cells in free form may have several disadvantages, such as the difficulty of localizing the cells at the injury site, rapid loss of cell viability, and the possibility of tissue malformations [Citation46]. It is thought that migration of stem cells can be prevented by the encapsulation approach. But, due to its sticky property, there are difficulties in producing microbeads only by using fibrin. Some groups have produced FMs by using the heated oil emulsion method [Citation47]. However, cells cannot be encapsulated inside the FMs, they can only be attached to the surface of the microbeads by this method.
In this study, alginate was used as the supporting material for the generation of FAM microbeads and encapsulation of MSCs at the initial stage. Biodegradation time span of cell-encapsulating microbeads is a critical parameter, because it is essential for the microbeads to release the growth factors from the MSCs to the injury site, while keeping the encapsulated live MSCs for a certain period of time. Then follows their timely biodegradation towards the end of the tissue regeneration process. Moreover, alginate is inherently non-degradable in mammals, as they lack the enzyme which can cleave the polymer chains [Citation48]. Thus, alginate microbeads alone do not degrade in vivo and are not suitable for this type of application. For this reason, in order to obtain the alginate-free FMs, alginate was removed from the structure of the FAMs by using citrate buffer.
Characterization of FMs: FTIR, water uptake, and SEM
FTIR analysis was performed to confirm the removal of alginate from the FAMs. As the result, the alginate-specific peaks were observed in the FTIR spectra of FAM-R and FAM-P (citrate-untreated), and sodium-alginate (). On the other hand, the intensity of the –OH (3428 cm−1), >C=O– (1618 cm−1), –CC (1418 cm−1) and –COC peaks (1091 cm−1 and 1031 cm−1) characteristic for alginate [Citation49] had decreased in the FM-R and FM-P groups (indicated in black circles). Fibrin-specific amide II peak (1500 cm−1) (indicated in black triangles) was observed in the FTIR spectra of the FMs and FAMs groups (). Both the decrease in the intensity of alginate-specific peak in FMs and the observation of fibrin-specific amide II peak (1500 cm−1) in FTIR spectra of the FMs and FAMs confirmed that, the incorporated alginate was removed from the FAMs with the aid of citrate buffer. Besides, there was no marked difference between the FM-R and FM-P, due to the fact that fibrin is available both in PRP and PPP.
Figure 3. (A) FTIR spectra of FMs obtained from PPP and PRP, FAMs obtained from PRP and PPP, and sodium alginate. (B) Water uptake capacity of FMs and FAMs after 48 h. The data were statistically analysed by one-way ANOVA followed by Bonferroni's post hoc comparison tests (**p<.01). (C) SEM micrographs showing the surface, and cross-section structure of the fibrin microbeads.
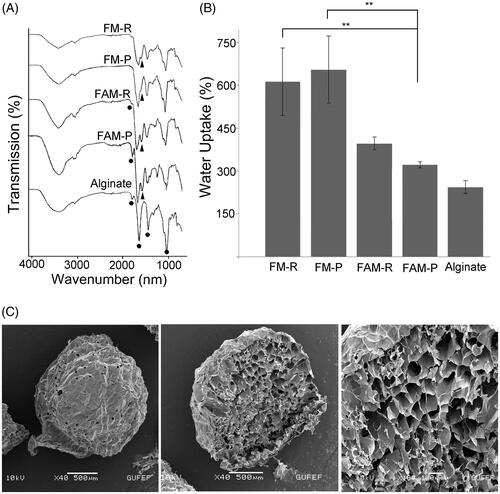
The water uptake capacity of a regenerative biomaterial may be a significant feature. Thus, the water uptake capacities of FMs and FAMs were determined prior to the in vivo studies. Percent water uptake of the microbeads increased over time. FMs provided higher levels of water uptake (>600%) compared to the alginate microbeads (∼230%) and FAMs (∼350%), after 48 h at 37 °C () (both p < .01). These results show that FMs possessed higher water uptake capacity than that of sodium alginate and FAMs, which reveals that fibrin is more permeable and swellable than the alginate network. The highly porous structure of fibrin may provide a large surface area for attachment and proliferation of cells encapsulated inside the FMs. In addition, the water uptake capacity of the FMs is greater than the FAMs. This indicates that alginate has been largely removed from the FAM structure following citrate treatment. SEM analyses revealed that FMs had a highly porous, homogenous structure, with a pore size around 70–120 µm (). This feature of FMs provides a large surface area suitable for cell migration and growth. Besides, SEM also indicates the spherical structure of the FMs.
In vitro properties of FMs: mechanical stability and encapsulated cell viability
compares the microbead yield, encapsulated cell content and the sizes of different types of microbeads (FM-R and FM-P) produced together with or without MSCs. Differences in the microbead yield of FM-R + MSCs vs. cell-devoid FM-R, as well as FM-p + MSCs and cell-devoid FM-P were found to be statistically significant (p < .001 and p < .01, respectively). While not significantly different, the FM-R had a smaller mean diameter compared to that of the FM-P microbeads (1600 ± 275 µm vs. 1700 ± 330 µm). Besides, MSC-containing microbeads had a smaller size than the cell-devoid microbeads (1200 ± 210 µm vs. 1600 ± 275 µm for FM-R, and 1400 ± 280 µm vs. 1700 ± 330 µm for FM-P). The cell encapsulation yields of FM-R and FM-P were found to be statistically insignificant (p>.05).
Table 2. Comparison of microbead yield, encapsulated cell content and size of different microbeads.
Phase-contrast images of the FMs are given in . From the inverted micrographs at day 0, the homogenous smooth spherical shape of both FMs is clearly visible. FM-P was slightly larger than FM-R at day 0. From day 0 to day 7, the size change of the FM-R was not significant demonstrating volume stability of FM-R, however at day 14, the size slightly increased but still preserved its spherical shape. Size of the FM-P increased from day 0 to day 7; swelling of FM-P was visible, and deformations were detected at day 14, indicating that FM-P is less stable than FM-R in cell culture conditions for the duration of the experiment.
Figure 4. (A) Phase contrast micrographs of the MSC-encapsulated FM-R and FM-P at specific culture time points. (B) Viability of rat MSCs encapsulated in FM-R and FM-P. MTT data obtained were analysed by two-way ANOVA followed by Bonferroni's post hoc comparison tests (*p<.05).
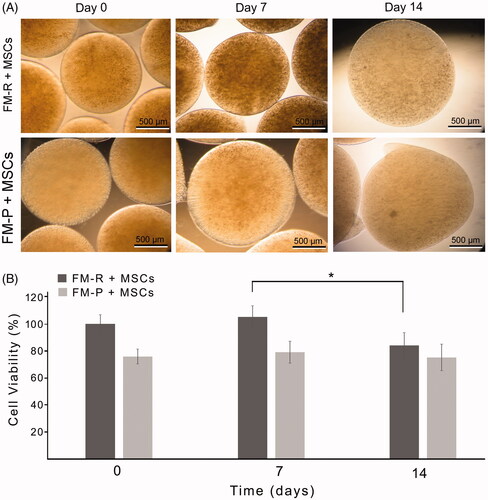
In order to evaluate whether the microbeads provided a suitable environment for the MSCs or not, the metabolic activity of the cells was determined by using the MTT assay at 0, 7 and 14 days of culture. It is clearly seen that the mitochondrial dehydrogenase activity of the MSCs in FMs was maintained for the duration of cell culture experiments and the cells stayed alive during the 14-day experimental period (). The difference in cell metabolic activity between the FM-R and FM-P-encapsulated groups can be attributed to the difference in platelet content of the microbeads; the cell density was greater in FM-R due to the fact that PRP has higher number of platelets. Consequently, both types of microbeads preserved mitochondrial activity of the cells. However, FM-P microbeads were not preferred for use during the in vivo studies, because FM-Ps which had regular spherical shape at the beginning of culture were eventually deformed in the following days.
VML model and in vivo studies
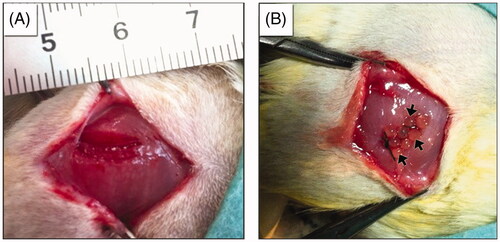
The in vivo studies were designed to determine the effectiveness of FM-R with and without MSCs in a VML injury rat model. For this purpose, bilateral defects, each in the sizes of 8 × 4×4 mm3 were created in the biceps femoris muscle of rats (). Aarimaa et al. [Citation50] had previously reported that an initial muscle loss of >20% of the total tissue volume, would result in fibrosis, and failure of regeneration. The VML model applied in this study had a removed muscle volume slightly >20% of the total. Three groups including (1) the Sham group, (2) 15–16 FM-R microbeads containing ∼1.3 × 105 MSCs and (3) 15–16 FM-R microbeads devoid of MSCs were set up to transplant into the injury sites. In the Sham group, the same volumetric injury was created and then sutured without performing any transplantation. The healing process of each group was periodically pursued.
Figure 5. Macrographs showing (A) the created volumetric muscle loss of 8 × 4×4 mm3 at the rat biceps femoris muscle and (B) transplantation of fibrin microbeads into the VML site.
Several studies evaluating the synergistic effects of PRP and MSCs for the treatment of various types of tissue injuries have shown that use of PRP alone was not sufficiently effective for tissue regeneration. Pieri et al. [Citation51] pointed out that the combinational use of PRP and MSCs supported the development of the new bone tissue formation, due to the osteogenic differentiation potential of the MSCs. MSCs-free groups resulted in a limited degree of bone formation with poor biomechanical stability, whereas the MSCs/PRP combination with a carrier biomaterial significantly increased the amount of new bone formation [Citation51]. Similarly, Teng et al. [Citation52] reported that the combination of PRP and MSCs promoted the healing of the tendon–bone defects. The contribution of MSCs to new bone formation is explained by the differentiation of the MSCs from immature cells to osteoblast-like cells in the presence of many growth factors PRP possesses [Citation51,Citation53]. Furthermore, there are studies describing the mitogenic effects of PRP on MSCs [Citation38]. The present study also indicated that the combinational use of PRP/MSCs promoted the regeneration of the damaged muscle tissue.
H&E histochemical findings: fibrosis or regeneration
Muscle healing has a series of processes including degeneration, inflammation, regeneration and fibrosis [Citation54]. Active muscle degeneration and inflammation occur in a month after injury. Scar tissue formation, i.e. fibrosis, starts around the second and third weeks of injury and the size of the scar tissue increases over time. The formation of scar tissue is the final stage of the muscle repair process. As long as the scar tissue is forming, it is understood that the muscle regeneration is not completed. Tissue regeneration at the injury site was evaluated by histochemical ( and ) and immunohistochemical (; SCitation2) analyses, as well as by semi-quantitative histomorphological scoring ().
Figure 6. Representative H&E-stained sections of the muscle explants retrieved from subjects with VML injury at 30, 60 and 180 days post-transplantation. Asterisks indicate the microbeads, black arrows indicate the capillaries. Scale bars: 300 µm.
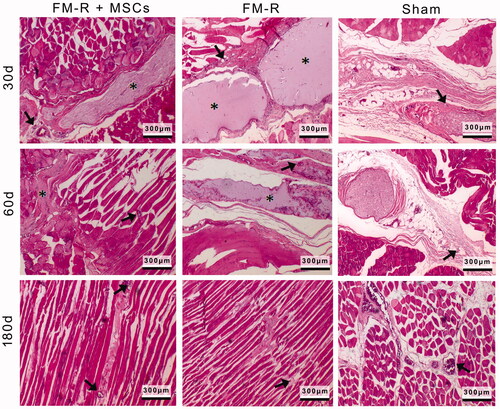
Figure 7. Representative Masson’s trichrome-stained sections of the muscle explants retrieved from subjects with VML injury at 30, 60 and 180 days post-transplantation. Collagen deposition (indicated with arrow heads) was evident between muscle fibres and microbeads (asterisks) in the FM-R and FM-R + MSCs groups at 30 and 60 days, whereas collagen deposition had decreased and muscle regeneration was noted after 180 days in these groups. However, in the Sham group, collagen deposition was strongly evident after 60 days, which later demonstrated an incomplete repair process with fibrotic scar tissue at 180 days. Scale bars: 300 µm.
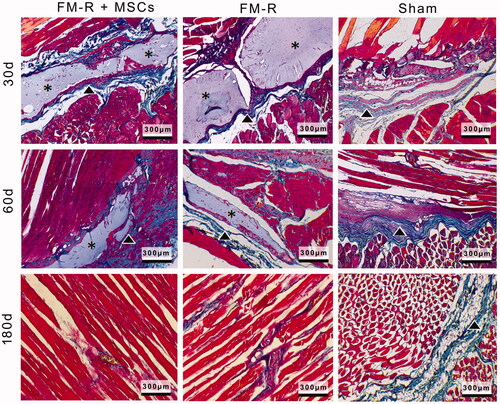
Figure 8. Representative anti-Desmin-stained sections of the muscle explants retrieved from subjects with VML injury at 30, 60 and 180 days post-transplantation. Scale bars: 300 µm.
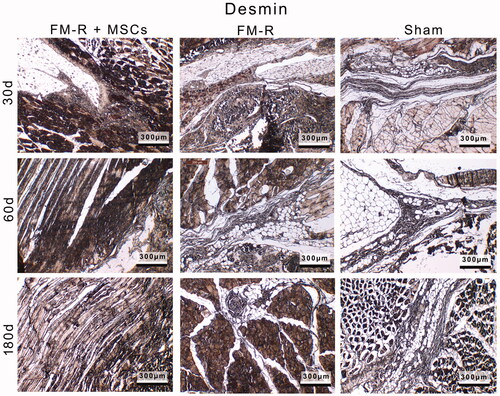
Table 3. Morphological scoring results of the muscles associated with stage of repair.
Histochemical findings based on H&E stainings are presented in . At 30 days, as a sign of chronic inflammatory response to VML, cellular infiltration of macrophages and lymphocytes at the interface of the FM-Rs can be observed. Generally speaking, microbeads (both MSCs-devoid and MSCs-encapsulated) remarkably increased the regeneration in the defect area compared to the Sham group (). In both of the FM-R transplantation groups, the gradual degradation of the microbeads (asterisks) and integration of the muscle fibres with the injured muscle are evident from the micrographs of 30 and 60 days. However, MSC-encapsulated FM-Rs showed a faster degradation and remodelling as compared the FM-Rs without MSCs. After 180 days, complete degradation and remodelling of the FM-Rs were observed along with de novo muscle regeneration demonstrated by muscle fibre formations in the FM-R and FM-R + MSCs groups, which also shows that the microbeads did not interfere but facilitated cell alignment (). In the Sham group, the muscle fibres appeared to collapse around the injury after 30 and 60 days. Inflammatory cells were observed along with fibroblasts-like cells, demonstrating scar formations. An incomplete repair was achieved after 180 days in the Sham group.
PRP contains high amounts of platelets, and owing to the fact that platelets release some of the growth factors involved in modulating the regeneration process, it is not surprising that the MSC-devoid FMs still have a regenerative effect on the VML site. The inflammation phase in the FM-R transplantation groups (with/without MSCs) passed faster than the Sham group [Citation55]. Growth factors released from FM-Rs may also have an influence in decreasing scar tissue formation. On the other hand, it is well-known that MSCs secrete a number of growth factors and cytokines which induce the regeneration process [Citation56]. The paracrine effect, along with immunomodulatory properties of the MSCs, is thought to shorten the inflammation process thus preventing the formation of scar tissue.
MTC histochemical findings
MTC staining results demonstrated typical muscle changes during remodelling/regeneration processes in all groups (). At 30 and 60 days post-surgery, extensive cellular infiltration of macrophages and lymphocytes was noted both at the interface of the microbeads and in the vicinity of the VML of the Sham group. However, there were no significant signs of necrosis in the groups after 30 days. In the FM-R + MSC and FM-R groups, collagen deposition (indicated with arrow heads) was clearly visible after 30 and 60 days post-surgery, but was largely deficient after 180 days, suggesting that myogenesis had occurred after this period of time. Muscle repair in the MSCs-encapsulated FM-R group was atop of MSC-devoid FM-R group. We observed new capillary formations at the vicinity of the VML site indicating that neovascularization was taking place. Another observation was that, MSCs-encapsulated FM-Rs had a faster biodegradation rate compared to that of the cell-free FM-Rs, demonstrating the fact that MSCs create a suitable microenvironment for tissue remodelling. This finding also suggests that the MSCs-encapsulated FMs are biologically active and show paracrine effect [Citation57]. In the Sham group, muscle remodelling was accompanied by collagen deposition, leading to apparent fibrotic tissue formation after 180 days at the VML site.
Histomorphological assessment
The state of muscle regeneration at the injury site was also assessed by semi-quantitative histomorphological scoring. The morphological scoring system of the muscles associated with the stage of repair is presented in . The table shows the repair stages of F1 (inflammatory stage), F2 (granulation tissue and matrix formation) and F3 (quality of the myofibres). In , the semi-quantitative morphological scoring results of the muscles associated with stage of repair, based on the histological (H&E and MTC) evaluations are presented. The semi-quantitative scoring results were in line with the histological evaluations.
In the Sham group, a high level of inflammation was noted after 30 days, which slowed down after 60 days. Granulation tissue and matrix formation, which was demonstrated by collagen fibre deposition together with abnormal or irregular myofibre formations continued. In the transplantation groups, a milder inflammatory stage was observed after 30 days, which turned into the regenerative phase (F2 and F3 phases). MSC-encapsulated FM-Rs showed a faster regeneration of the muscle tissue when compared with the MSC-devoid FM-Rs.
In the injury-repair-regeneration cascade, proteases and hydrolases that promote tissue damage are activated, then the mitogens of the related immune and muscle cells are produced by the activated enzymes, which eventually lead to the progression of membrane repair [Citation54]. It is probable that the immunomodulatory MSCs intervene with this process by modulating the transition of the proinflammatory phase (early-invading M1 macrophages) into the repair phase (anti-inflammatory M2 macrophages), leading to a faster regeneration of the VML.
After 180 days in the MSC-encapsulated FM group, mostly normal myofibres were evident, which showed the successful regeneration of the VML site. MSC-devoid FM-Rs showed slightly lower but a close level of regeneration following the same period of time. These findings suggest that MSC-devoid FM-Rs have a regenerative effect close to that of the MSC-encapsulated FM-Rs in the rat VML model. However, it is also clear that the MSCs, encapsulated in the FM-Rs have an accelerating effect on muscle regeneration.
Immunohistochemistry
Immunohistochemical analyses supported the findings obtained from the histochemical and semi-quantitative histomorphological assessments. Representative anti-Desmin-stained sections of the muscle explants retrieved from subjects with VML injury at 30, 60 and 180 days post-transplantation are given in . At 30, 60 and 180 days post-surgery, the myogenic marker Desmin was detected at different levels in the tissue sections demonstrating the regeneration of the muscle. Desmin immunoreactivity was weak in the Sham group demonstrating that the myogenic response is limited. However, in the FM-R transplantation groups, Desmin immunoreactivity was high in regions in close proximity to the injured muscle showing that the regeneration levels of both the MSC-devoid and MSC-encapsulated FM-R groups were high.
Among the transplantation groups, the MSC-encapsulated FM-R group showed a faster regeneration at the injury site; however, the MSC-devoid FM-R group almost reached the regeneration level of the MSC-containing group after 180 days. Thus, it is established that PRP promotes muscle regeneration and reduces scar tissue formation due to the abundance of growth factors, which are important for regulating the healing processes [Citation58,Citation59]. In addition, the anti-myogenin and anti-MyoD1 stainings of the tissue specimens are given in the Supplemental section (SCitation2). These myogenic markers were also observed at the vicinity of the VML site at different levels. In all of the analysed tissue specimens, a clear correlation between anti-Desmin, anti-myogenin and anti-MyoD1 stainings was detected.
The observations regarding the functionality of the animals are as follows: The rats receiving the transplants (MSC-encapsulated and MSC-devoid FM-Rs) were quite active starting from the third week, and there was almost no difference in their activities at the termination of the study (after 180 days), when compared with the untreated healthy animals. On the other hand, the subjects of the Sham group could only drag their feet after 60 days, and demonstrated some disabilities while walking after 180 days post-treatment.
Indeed, it is not clear whether the main factor that induces muscle healing is the bioactive molecules originating from the PRP or MSCs, or both. The influential positive regenerative effect may be due to the molecular mechanisms elicited by the PRP on MSCs. Despite the wide use of PRP in therapeutic applications, in vivo studies investigating this issue are not available. On the other hand, there are several in vitro studies which have evaluated the combinational use of PRP and MSCs. For example, Mishra et al. have found that PRP enhances the expression of Sox9 and Aggrecan, and increases the extracellular cartilage matrix synthesis of differentiating MSCs [Citation60]. Sassoli et al. have reported that PRP supports cell viability, increases cell proliferation, and also activates the AKT and Notch-1 pathways in the BM-MSCs [Citation61]. D'Esposito et al. have shown that PRP accelerates MSC migration, proliferation and G1-S cell cycle progression, and reduces the Caspase 3 cleavage [Citation62]. However, such in vitro studies may not truly reflect the in vivo situation. Additionally, PRP may also have an influence on different cell types present in the host tissues [Citation63]. For those reasons, comprehensive molecular studies could be helpful for elucidating the possible pathways affected by the combinational use of PRP and MSCs in the future. In addition, it should be pointed out that the possible effects of PRP on MSCs may vary according to the composition of the PRP due to differences in preparation methodology or variations in the plasma contents between different donors.
Conclusions
Repair of VML injuries is a complicated endeavour which necessitates the collaborative use of different regenerative approaches and technologies. The combinational use of autologous MSCs, and autologous blood in prospective studies, for developing an efficient cell-encapsulation biomatrix may have potential from the viewpoint of personalized medicine. In this study, we have demonstrated that cell-devoid fibrin microbeads support muscle regeneration to a great extent. Our findings support the notion that, MSCs-encapsulated fibrin microbeads obtained from PRP are effective in shortening the regeneration period of the injured tissue of the rat VML, resulting in good myofibre orientation. Evidently, detailed muscle functionality assessments can be planned in studies with a larger animal model in the future. Furthermore, it appears that the described MSC-encapsulated fibrin microbead system may also have potential for other regenerative applications.
SUPPLEMENTARY_FIGURES.pdf
Download ()Disclosure statement
Y.M.E. is the founder and shareholder of Biovalda Health Technologies, Inc. (Ankara, Turkey). The authors have patent applications in relation to regenerative biomaterials.
References
- Pollot BE, Corona BT. Volumetric muscle loss. Methods Mol Biol. 2016;1460:19–31.
- Corona BT, Wenke JC, Ward CL. Pathophysiology of volumetric muscle loss injury. Cells Tissues Org. 2016;202:180–188.
- Lipman A, Strauss E. Treatment of pectoralis major muscle ruptures. Bull Hosp Jt Dis. 2016;74:63–72.
- Greising SM, Dearth CL, Corona BT. Regenerative and rehabilitative medicine: a necessary synergy for functional recovery from volumetric muscle loss injury. Cells Tissues Org. 2016;202:237–249.
- Qazi TH, Mooney DJ, Pumberger M, et al. Biomaterials based strategies for skeletal muscle tissue engineering: existing technologies and future trends. Biomaterials. 2015;53:502–521.
- Quarta MC, Chacon R, Blonigan J, et al. Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nat Commun. 2017;8:15613.
- Bian W, Bursac N. Tissue engineering of functional skeletal muscle: challenges and recent advances. IEEE Eng Med Biol Mag. 2008;27:109.
- Longo UG, Loppini M, Berton A, et al. Tissue engineered strategies for skeletal muscle injury. Stem Cells Int. 2012;2012:1.
- Danna NR, Beutel BG, Campbell KA, et al. Therapeutic approaches to skeletal muscle repair and healing. Sports Health. 2014;6:348–355.
- Grasman JM, Zayas MJ, Page RL, et al. Biomimetic scaffolds for regeneration of volumetric muscle loss in skeletal muscle injuries. Acta Biomater. 2015;25:2–15.
- Brazelton TR, Nystrom M, Blau HM. Significant differences among skeletal muscles in the incorporation of bone marrow-derived cells. Dev Biol. 2003;262:64–74.
- Peçanha R, Bagno LL, Ribeiro MB, et al. Adipose-derived stem-cell treatment of skeletal muscle injury. J Bone Joint Surg Am. 2012;94:609–617.
- Caseiro AR, Pereira T, Bártolo PJ, et al. Mesenchymal stem cells and biomaterials systems—perspectives for skeletal muscle tissue repair and regeneration. Proc Eng. 2015;110:90–97.
- Goldman SM, Henderson B.E.P, et al. Evaluation of bone marrow mononuclear cells as an adjunct therapy to minced muscle graft for the treatment of volumetric muscle loss injuries. Stem Cell Res Ther. 2017;8:142.
- Parmaksiz M, Elçin AE, Elçin YM. Decellularization of bovine small intestinal submucosa and its use for the healing of a critical‐sized full‐thickness skin defect, alone and in combination with stem cells, in a small rodent model. J Tissue Eng Regen Med. 2017;11:1754–1765.
- Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114:1502–1508.
- Alsousou J, Thompson M, Hulley P, et al. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery. J Bone Jt Surg Br. 2009;91-B:987–996.
- Vu TD, Pal SN, Ti LK, et al. An autologous platelet-rich plasma hydrogel compound restores left ventricular structure, function and ameliorates adverse remodeling in a minimally invasive large animal myocardial restoration model: a translational approach: Vu and Pal “Myocardial Repair: PRP, Hydrogel and Supplements”. Biomaterials. 2015;45:27–35.
- Fernandez-Moure JS, Van Eps JL, Cabrera FJ, et al. Platelet-rich plasma: a biomimetic approach to enhancement of surgical wound healing. J Surg Res. 2017;207:33–44.
- Kasten P, Vogel J, Geiger F, et al. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008;29:3983–3992.
- Zhao T, Yan W, Xu K, et al. Combined treatment with platelet-rich plasma and brain-derived neurotrophic factor-overexpressing bone marrow stromal cells supports axonal remyelination in a rat spinal cord hemi-section model. Cytotherapy. 2013;15:792–804.
- Chen WH, Lo WC, Hsu WC, et al. Synergistic anabolic actions of hyaluronic acid and platelet-rich plasma on cartilage regeneration in osteoarthritis therapy. Biomaterials. 2014;35:9599–9607.
- Dimauro I, Grasso L, Fittipaldi S, et al. Platelet-rich plasma and skeletal muscle healing: a molecular analysis of the early phases of the regeneration process in an experimental animal model. PLoS One. 2014;9:e102993.
- McClure MJ, Garg K, Simpson DG, et al. The influence of platelet‐rich plasma on myogenic differentiation. J Tissue Eng Regen Med. 2016;10:E239–E249.
- Bacakova M, Musilkova J, Riedel T, et al. The potential applications of fibrin-coated electrospun polylactide nanofibers in skin tissue engineering. Int J Nanomed. 2016;11:771–789. DOI:10.2147/IJN.S99317
- Ahmed TAE, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng B. 2008;14:199–215.
- Dahlstrøm KK, Weis-Fogh US, Medgyesi S, et al. The use of autologous fibrin adhesive in skin transplantation. Plast Reconstr Surg. 1992;89:968–972.
- Ahmed TAE, Giulivi A, Griffith M, et al. Fibrin glues in combination with mesenchymal stem cells to develop a tissue-engineered cartilage substitute. Tissue Eng A. 2011;17:323–335.
- Pripatnanont P, Nuntanaranont T, Vongvatcharanon S, et al. The primacy of platelet-rich fibrin on bone regeneration of various grafts in rabbit’s calvarial defects. J Craniomaxillofac Surg. 2013;41:191–200.
- Zhou H, Xu HHK. The fast release of stem cells from alginate-fibrin microbeads in injectable scaffolds for bone tissue engineering. Biomaterials. 2011;32:7503–7513.
- Wang W, Li B, Yang J, et al. The restoration of full-thickness cartilage defects with BMSCs and TGF-beta 1 loaded PLGA/fibrin gel constructs. Biomaterials. 2010;31:8964–8973.
- Thomson KS, Korte FS, Giachelli CM, et al. Prevascularized microtemplated fibrin scaffolds for cardiac tissue engineering applications. Tissue Eng A. 2013;19:967–977.
- Scott JB, Afshari M, Kotek R, et al. The promotion of axon extension in vitro using polymer-templated fibrin scaffolds. Biomaterials. 2011;32:4830–4839.
- Page RL, Malcuit C, Vilner L, et al. Restoration of skeletal muscle defects with adult human cells delivered on fibrin microthreads. Tissue Eng A. 2011;17:2629–2640.
- Koc A, Emin N, Elcin AE, et al. In vitro osteogenic differentiation of rat mesenchymal stem cells in a microgravity bioreactor. J Bioact Comp Polym. 2008;23:244–261.
- Emin N, Koc A, Durkut S, et al. Engineering of rat articular cartilage on porous sponges: effects of TGF-beta 1 and microgravity bioreactor culture. Artif Cell Blood Sub. 2008;36:123–137.
- Durkut S, Elcin AE, Elcin YM. In vitro evaluation of encapsulated primary rat hepatocytes pre- and post-cryopreservation at-80 degrees C and in liquid nitrogen. Artif Cell Nanomed Biotechnol. 2015;43:50–61.
- Fernandes G, Yang S. Application of platelet-rich plasma with stem cells in bone and periodontal tissue engineering. Bone Res. 2016;4:16036.
- Celebi B, Elcin YM. Proteome analysis of rat bone marrow mesenchymal stem cell subcultures. J Proteome Res. 2009;8:2164–2172.
- Celebi B, Elcin AE, Elcin YM. Proteome analysis of rat bone marrow mesenchymal stem cell differentiation. J Proteome Res. 2010;9:5217–5227.
- Perka C, Arnold U, Spitzer RS, et al. The use of fibrin beads for tissue engineering and subsequential transplantation. Tissue Eng. 2001;7:359–361.
- Murphy MB, Blashki D, Buchanan RM, et al. Adult and umbilical cord blood-derived platelet-rich plasma for mesenchymal stem cell proliferation, chemotaxis, and cryo-preservation. Biomaterials. 2012;33:5308–5316.
- Man Y, Wang P, Guo Y, et al. Angiogenic and osteogenic potential of platelet-rich plasma and adipose-derived stem cell laden alginate microspheres. Biomaterials. 2012;33:8802–8811.
- Simman R, Hoffmann A, Bohinc RJ, et al. Role of platelet-rich plasma in acceleration of bone fracture healing. Ann Plast Surg. 2008;61:337–344.
- Suzuki Y, Kuroda Y, Morita A, et al. Fibrin glue sealing for the prevention of pancreatic fistulas following distal pancreatectomy. Arch Surg. 1995;130:952–955.
- Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111.
- Gurevich O, Vexler A, Marx G, et al. Fibrin microbeads for isolating and growing bone marrow-derived progenitor cells capable of forming bone tissue. Tissue Eng. 2002;8:661–672.
- Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–126.
- Huang H, Zeng X, Li W, et al. Reinforced conducting hydrogels prepared from in-situ polymerization of aniline in an aqueous solution of sodium alginate. J Mater Chem A. 2014;2:16516–16522.
- Aarimaa V, Kaariainen M, Vaittinen S, et al. Restoration of myofiber continuity after transection injury in the rat soleus. Neuromuscul Disord. 2004;14:421–428.
- Pieri F, Lucarelli E, Corinaldesi G, et al. Effect of mesenchymal stem cells and platelet-rich plasma on the healing of standardized bone defects in the alveolar ridge: a comparative histomorphometric study in minipigs. J Oral Maxillofac Surg. 2009;67:265–272.
- Teng C, Zhou C, Xu D, et al. Combination of platelet-rich plasma and bone marrow mesenchymal stem cells enhances tendon–bone healing in a rabbit model of anterior cruciate ligament reconstruction. J Orthop Surg Res. 2016;11:96.
- Ogino Y, Ayukawa Y, Kukita T, et al. The contribution of platelet-derived growth factor, transforming growth factor-β1, and insulin-like growth factor-I in platelet-rich plasma to the proliferation of osteoblast-like cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2006;101:724–729.
- Tidball JG. Mechanisms of muscle injury, repair, and regeneration. Compr Physiol. 2011;1:2029–2062.
- Jeong W, Yang CE, Roh TS, et al. Scar prevention and enhanced wound healing induced by polydeoxyribonucleotide in a rat incisional wound-healing model. IJMS. 2017;18:1698.
- Ruehle MA, Stevens HY, Beedle AM, et al. Aggregate mesenchymal stem cell delivery ameliorates the regenerative niche for muscle repair. J Tissue Eng Regen Med. 2018;12(8):1867–1876. DOI:10.1002/term.2707 [Epub ahead of print]
- Su N, Gao P-L, Wang K, et al. Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: a new dimension in cell-material interaction. Biomaterials. 2017;141:74–85.
- Mosca MJ, Rodeo SA. Platelet-rich plasma for muscle injuries: game over or time out? Curr Rev Musculoskelet Med. 2015;8:145–153.
- Lyras DN, Kazakos K, Agrogiannis G, et al. Experimental study of tendon healing early phase: is IGF-1 expression influenced by platelet rich plasma gel? Orthop Traumatol Surg Res. 2010;96:381–387.
- Mishra A, Tummala P, King A, et al. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods. 2009;15:431–435.
- Sassoli C, Vallone L, Tani A, et al. Combined use of bone marrow-derived mesenchymal stromal cells (BM-MSCs) and platelet rich plasma (PRP) stimulates proliferation and differentiation of myoblasts in vitro: new therapeutic perspectives for skeletal muscle repair/regeneration. Cell Tissue Res. 2018;372:549–570.
- D'Esposito V, Passaretti F, Perruolo G, et al. Platelet‐rich plasma increases growth and motility of adipose tissue‐derived mesenchymal stem cells and controls adipocyte secretory function. J Cell Biochem. 2015;116:2408–2418.
- Rubio-Azpeitia E, Andia I. Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles Ligaments Tendons J. 2014;4:52–62.