?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Artemisinin is used as an antimalarial and anticancer agent with minimal toxic effects on the host body. Biotin-PEG-PCL polymers have been used for targeted drug delivery to cancer, as well as to improve the pharmacokinetics of the drug and reduce its effects. In this study, biotin-conjugated copolymers were fabricated with polymerization of the ring opening method and the properties of copolymer and nanoparticles were investigated using various techniques. The toxicity of artemisinin and its nanoparticles have been investigated on MCF-7 and normal HFF2 cells. The results showed that the encapsulation efficacy of artemisinin in nanoparticles was 45.5 ± 0.41%. The release profile of the drug indicates that the release is slow and controlled and is approximately pH dependent. The results of artemisinin cell culture on human breast cancer cells showed that biotin-PEG-PCL nanoparticles had an inhibitory effect on MCF-7 cells and had no toxic effects on HFF2 cells. Anticancer activity in vivo in the 4T1 breast cancer model showed that tumour volumes were decreased up 40 mm3 by ART-loaded micelles and 76 mm3 by free ART, compared to the control group (2150 mm). In vivo results showed that this formulation significantly increases the accumulation of substances in the tumours. Therefore, the molecular formulation of ART-based copolymers can be a desirable process for cancer treatment purposes.
Introduction
Copolymers have been developed as delivery systems for medicinal products as well as various carriers for therapeutic agents and different contrast agents in diagnostic imaging applications [Citation1–4]. With the structure of amphiphilic polymeric micelles, hydrophobic drug compounds are soluble within the hydrophobic nuclei, while the shell retains a hydration barrier that protects the integrity of each microcell [Citation5,Citation6]. As a result, polymeric micelles can be used as efficient containers for undesired solvents with low stability in physiological environments [Citation7,Citation8]. Additionally, the ability to categorize multiple groups provides a significant scope for accurately adjusting drug loading and drug-targeted propagation properties. Selective drugs for cancer cells are an interesting task [Citation9–11]. It is specially treated for the treatment of chemical cancer, since most anticancer drugs cannot detect cancerous and healthy cells. A well-known method for active tumour targeting is the chemical bond of specific ligands to the micelles outer layer that can detect the exact molecular signatures of cancer cells [Citation12,Citation13]. Special attention has been paid to the development of drug carriers with active targeting capabilities to increase the delivery efficiency of drug and special cancer treatment. Polymer micelles can be targeted to specific sites delivered by targeting passive, size dependent or target active activity using the assembly of the target molecule [Citation14,Citation15]. Nanoparticles that fuse target molecules can reach a high degree of selectivity from specific organs and increase the drug's intensity in target cells [Citation16–18]. Significantly, cells that are rapidly being divided, such as those in solid tumours, require a large number of certain vitamins, including folate, vitamin B12, and vitamin H; as a result, excessive amounts of receptors in the absorption of the vitamin are expressed on the levels of cancer cells [Citation19–21].
Folic acid (folate) is one of the most important and most effective targeting molecules. Folate receptors are expressed in a large variety of cancers, including breast, uterine, lung, and brain and colon cancer. Folate specifically attaches to the folate receptor [Citation22]. Although cancerous tumours exhibit higher biotin content than the normal soft tissue, many studies have analyzed the delivery systems using vitamin H (biotin) as a ligand for cancer targeting [Citation23,Citation24]. In this study, the physicochemical properties of biotin-PEG-PCL copolymer and conjugated nanoparticles with biotin-containing artemisinin and its cytotoxicity on cancerous and normal cells will be investigated. We prepared a biodegradable amphiphilic block composed of poly(ethylene glycol) (PEG) and poly(ε-caprolactone) (PCL) which can be used to form a nanopolymer with shell structure and loading capacity of a hydrophilic drug [Citation25]. Polyethylene glycol is a neutral polymer with end hydroxyl groups that have poor hydrogen bonds and can be added to drug carriers through a number of different ways, including covalent binding. It is also used as a protective layer in polymeric micelles to increase the circulation time of nanoparticles in the blood and to reduce detection by macrophages of the reticuloendothelial system (RES). Polyethylene glycols with a high molecular weight have a longer cycle time. Artemisinin (ART) is extracted from a single-year-old plant of the Asteraceae family. A native of Asia and most likely of China, which has been used for centuries in traditional Chinese medicine against fever and malaria. is a natural antimalarial sesquiterpene lactone with anticancer properties. ART and its derivatives are commonly used in malaria therapy [Citation26–30]. Despite ART efficacy, ART, have pharmacokinetic limitations [Citation31]. Artemisinin is insoluble and should be taken orally. It is absorbed rapidly and within 1 to 2 h, its concentration in the plasma reaches the maximum. After taking artemisinin within 1 to 3 h, its amount in the blood is about half than that of the plasma [Citation32]. It is suggested that ART in the treatment of invasive cancers, without resistance, can be a therapeutic alternative [Citation33–35]. They also show cooperation with other antitumour drugs that do not increase the toxicity of normal cells [Citation36]. This showed that ART semisynthetic derivatives exhibit much antitumour activity than their monomeric supplements through apoptosis, cell cycle arrest and oxidative stress [Citation36]. The core impartial of this study is to prepare effective PEG/PCL copolymers functionalized by biotin and to investigate their potential as ligand mediators for anticancer treatment. The biotin-conjugated PEG/PCL block copolymers were determined with FT-IR, 1H-NMR, DSC and GPC measurements. Particle size, surface properties and the amount of anticancer drug loading were also investigated. In vitro, an anticancer drug was determined from biotin-derived PEG/PCL mice. In addition, a cytotoxic study was conducted in vitro using natural and cancerous cells. Antitumor in vivo behavior of methyl biotin-PEG-PCL loaded with ART was investigated for the possibility of methyl biotin-PEG-PCL as a drug targeting.
Materials and methods
Materials
Dimethylthiazol-2-yl-3,5-diphenylformazan, thiazolyl blue formazan (Aldrich, St. Louis, USA, CAS. 57360–69-7), polyethylene glycol (Mn =6000 Da) (Aldrich, St. Louis, USA, CAS.81323), biotin (Aldrich, St. Louis, USA), Sn(Oct)2 (Aldrich, St. Louis, USA, CAS. 301100), ε-caprolactone (98% purity) (Acros, New Jersey, USA, CAS.502443), ART (Merck, Darmstadt, Germany, Art No. 820354).
Synthesis of Biotin-PEG–PCL copolymers
In the first step, 0.4 g of PEG with a molecular weight of 6000 g/mol and 0.09 g biotin was dissolved in 20 mL DMF in a round-bottomed balloon that was fed with a magnetic stirrer. We placed them the container under a nitrogen atmosphere to prevent oxidation of the substances in the reaction. Then, NHS 1/14 mg and DCC 25.5 mg were added to the balloon at 25 °C. The resulting DMF mixture was placed at ambient pressure and temperature to dry. After evaporation, a milky colored material was obtained. The material was dissolved in chloroform of 7 ml and transferred to crystallizer and diethyl ether was added in mL (so that the non-reacted material is removed from the environment; in other words, our precipitate only contains biotin-PEG) and for 20–15 min at 20 °C to make the deposition more stable. The supernatant is removed using the sampler, and the sediment is removed using plain paper. For the synthesis of biotin-conjugated PEG/PCL block copolymer, the resulting biotin-conjugated PEG reacted with ε-caprolactone in the presence of stannous octoate. We weighed 0.5 g of biotin-PEG and 1 g of caprolactone and placed in a balloon of two openings and under vacuum and at 80 °C to melt (4 g monocaprolactone was poured into a balloon and taken up in calcium hydride for 1 week, after which the desired time was completely dissolved by the Buchner funnel and the vacuum pump); then, the nitrogen gas was added to the system containing biotin-PEG and caprolactone. We immersed them in a magnetic stirring and brought the temperature to 100 °C in an oil bath. After melting, 100 μL of tin octane as a catalyst was added to the balloon and the reaction proceeded at 100 °C for 12 h. The system was then cooled, and the copolymer was dissolved in chloroform and deposited in a cool diethyl ether. The precipitate was collected by filtration paper and dried.
Characterization of Biotin-PEG-PCL copolymers
The HNMR device was used to determine the biotin-PEG-PCL binary copolymer structure. NMR spectra were taken with a Bruker 400 MHz device at a temperature of 25 °C. Chloroform diet (CDCl3) was used as solvent and tetramethylsilane (TMS) as the internal standard. The FT-IR spectrum was used to confirm the formation of biotin-PEG-PCL copolymers as well as biotin-PEG-PCL nanoparticles (through interaction between drugs and copolymers in the nanoparticle, as well as observing vibrations of each of them in the nanoparticle). The spectra of the samples were recorded using the FT-IR spectroscopy (Bruker, Tensor). DSC analysis was used to study the thermal behavior of biotin-PEG-PCL copolymer and their nanoparticles. The samples were heated at a velocity of10 °C /min and measured in a range of 250–200 °C using the 9.3 Mettler Toledo, Star SW manufacturing facility in England. The average molecular mass and molecular mass distribution of biotin-PEG-PCL copolymers were analyzed by gel permeation chromatography analysis. The sample was dissolved in completely pure tetrahydrofuran, and after injecting into the column and comparing with the calibration diagram drawn by using standard polyesters with a mass of molecules between 4500 g/mol and 29000 g/mol, the molecular mass, and polydispersity copolymer were calculated. For GPC analysis, a German Knauer HPLC with a refractive index detector was used with a column with a specification of 4.30 mm × 4.6 and a mobile phase containing tetrahydrofuran.
Preparation of ART loaded micelles
Nanoparticles loaded with artemisinin were prepared by nanoprecipitation. In this way, we added 5 mg of artemisinin and 20 mg of biotin conjugated copolymer and dissolved them in 2 mL of chloroform and then added to 20 mL solution with 1% PVA, which was stirred by a magnetic stirrer. After adding the organic solvent, the solution was stirred for 15 min and then stirred for 2–3 h at room temperature until evaporation of the organic solvent was continued. The resulting solution was then transferred to a filtered falcon with a cathode of 10,000 and was subjected to centrifugation at about 5000 for 15 min for removal. To ensure the removal of surfactant, the precipitate was washed two times with deionized water and centrifuged. The supernatant was used after the dilution for stability and load testing.
Characterization of the micelles
Particle morphology
The AFM device (JPK Nanowizard, Berlin, Germany) was used to capture images. Then, some of the nanoparticles are diluted using distilled water, and then, one drop is placed on a plate of micaceous material for one microlitre of the device; then, the mica is dried and placed inside the device and a picture of it is taken.
Determination of particle size
The distribution method and the mean particle size were determined using Zeta Sizer (Malvern Zetasizer Nano ZS90). The range can be measured by this device from 6 to 6 μm. After defining a standard operating instruction for them (including sample absorption at the wavelength, the refractive index of the nanoparticles and type of solvent), the average particle size distribution was obtained. It should be noted that this was done in three replicates for each type of reaction.
Stability of micelles
To evaluate the stability of nanoparticles, their particle size of the suspension was measured over a period of 6 months and their related size was used to analyze the physical stability.
Determination of loading efficiency
In EquationEquation (1)(1)
(1) , the percentage of drug loaded is equal to the weight of the loaded drug to the total weight of the nanoparticle:
(1)
(1)
In EquationEquation (2)(2)
(2) , the percentage of encapsulation efficiency is equal to the amount of drug loaded in the nanoparticles to the weight of the primary drug:
(2)
(2)
Drug loading from ART was defined by high-performance liquid chromatography (HPLC). Moving phase consists of acetonitrile and water at a volume ratio of 60:40. It was delivered using a double-valve pump at a flow rate of 1.0 mL/min. The wavelength of the analysis was 210 nm (Waters, MA, USA, Model Breeze). The sample was injected through a 20-μL sample loop. An analytical column C8 (150 mm ×4.6 mm, particle size 5 μm; still incomplete, MZ-Analysentechnik, Germany) was applied by the support column of the same package.
Drug release study
To study the release of artemisinin, 5 mg of nanoparticle powder was mixed with 2 mL of buffer and transferred into a 12000 cut-off bag and placed it inside a container containing 13 mL phosphate buffer (pH =4.40) and tween (2% V/V). The dish of the dialysis bag was placed at 37 °C then 1 cc, of the the sample was removed from the external environment of the dialysis bag and replaced with fresh buffer and the amount of drug released by HPLC was measured at a wavelength of 210 nm. Each of the measurements was repeated three times, and the average of these three data was used to calculate the concentration of the released drug. Measurement results were drawn for a period of 168 h. The same was done with phosphate buffer pH =5/5.
Cell cultures and in vitro experiments
Experiments performed on adenocarcinoma cellular cells (MCF-7), mouse carcinoma cell lines (4T1) and human Human foreskin fibroblasts (HFF2), from the National Bank of Iran (Pasteur Institute of Iran, Tehran, Iran). Cells are cultured in RPMI-1640 (GIBCO, USA) that do not contain protein, fat or growth factor. Therefore, RPMI 1640 medium needs supplementation, usually with 10% foetal bovine serum (FBS), 2 mM L-glutamine, penicillin (50 IU/ml), streptomycin (50 μg/ml) and sodium bicarbonate system Carbonate (2.0 g/L) and therefore, to maintain a physiological pH, an environment of 5 to 10% of CO2 is required and incubated at 37 °C. When MCF-7 and HFF-2 cells were reached into 80%, they were separated by trypsin/EDTA (0.05%). The cells were then added again to a calture plate at a concentration of 104 cells/cm2 (e.g. 200 mL per layer). To absorb the background, some of the wells are left without cells, which means empty control.
Cell viability by MTT assay
Around 65%–65% distillation (24 h after seed), cultured cells with fresh concentrations (0.0005 to 400 μg/mL) of ART and equivalent dose of PE/PCL ART/biotin-functionalized and diabetic biotin-PEG-PCL alone are in solution for 48 and 72 h, and a group without treatment for control is used. Six wells were not treated as controls. Then, the MTT solution at concentrations (25 mL of solution 4 mg/mL MTT per 100 μL environment) was added to each well, and then, the plates were incubated at 3.5 °C and 37 °C with 5% CO2. Remaining MTT solution was removed and 100 μL DMSO was added to each loop to eliminate flask crystals. The plates were shaken for 20 min in a plate shaker to ensure proper solubility. The optical density (OD) of the wells was determined using a plate reader at a wavelength of 570 nm and a reference wavelength of 690 nm.
Gene expression profile in 4T1 cells
Total RNA from 4T1 cells was extracted using an RNA isolation kit (iNtRON easy-BLUE1500 District Avenue Suite 2097Burlington, MA 01803, USA). The cDNAs are manufactured using the Qiagen Company kit according to the manufacturer's instructions. The cDNAs are stored at –20 °C. Real-time PCR was performed in a 15 μL reaction solution using a sequence of specific primers on the 96-cell optical plates (.
Table 1. Characteristics of the primers used in the real-time polymerase chain reaction.
Relative gene expression was analyzed by ΔΔCt 2 method. The change in target gene cDNA relative to GAPDH (internal control) was by Fold change =2–ΔΔCt where ΔΔCt = sample (CtTarget gene–Ct GAPDH) – Reference sample (CtTarget gene–Ct GAPDH).
In vitro hemolytic test
The experimental method described here is the standard modification of the F-756–00, based on the color recognition of the red hemoglobin cyanate in the solution. In this test, the micelles are placed in the incubator hemoglobin released by damaged cells is oxidized to methemoglobin by ferricyanide in the presence of bicarbonate, and then cyanide converts the methemoglobin to cyanmethemoglobin 0.7 mL of micells (biotin-PEG-PCL and ART/biotin-PEG-PCL) at various concentrations in 0.1 mL of rat red blood cells were incubated at 37 °C and for 3.5 h. After incubation, the solution was centrifuged at 4500 rpm for 12 min. To determine supernatant hemoglobin, 0.7 mL of Drabkin’s solution was added to 0.3 mL of the microwave, and the specimen was allowed to stand for 20 min. The amount of hemoglobin in supernatant was measured by the spectrophotometer with a wavelength of 540 nm and then compared with a control. The amount of cyanmethemoglobin in the supernatant was measured by spectrophotometer (Spectro UV–vis Auto UV-1800) at a wavelength of 540 nm and then compared to a standard curve (hemoglobin concentrations ranging from 0.004 to 1.1 mg/mL)., distilled water and saline solution were used as positive and negative, respectively [Citation37].
In vivo LD50 measurement for biotin-PEG-PCL micelles by up-and-down method
To test the LD50, at first, different doses of Biotin-PEG-PCL (control 160, 240 and 360 mg/kg; the highest dose of fluids in solution) were administrated on 10 BALB/c mice (male: 22–25 g) and every 5 micce was placed in an ideal laboratory condition in chamber for seven days. The University's Research and Ethics Committee confirmed the experimental and ethical protocol, and all animals were sacrificed at the end of the experiment. The mortality rate was measured 48 h after treatment. The number of animals that died during this period was expressed as the percentage of LD50. LD50 was determined by measuring the number of deaths/total number. After surviving all animals within 48 h, two additional animals were treated at the highest dose, if these animals survived, LD50 was more than the limit and the test stopped.
In vivo anticancer activity in the 4T1 breast cancer model
Thirty five BALB/c mice (6 to 8 weeks) were obtained using the Animal Breeding Stock Facility, Razi Institute of Karaj, Iran. Mice were kept under standard housing conditions with a 12-h regular dark cycle at 25 ± 1 °C. All experiments were carried out using animals under the conditions established by the Zanjan Medical Ethics Committee for the care of laboratory animals. The 4T1 cells presented in the logarithmic growth stage and the female BALB/c tumour model was developed with a 0.2-mL injection of 4T1 cell suspension (∼106 cells/mL) to the upper right of the female BALB/c mouse. In order to create a tumour, the injected mice were kept at Zanjan University of Medical science. After 14 days, when the tumours increased to 220 mm, animals were randomly assigned to different treatment groups (day 0). 1: Control (PBS), 2: biotin micelle PEG-PCL, 3: ART, 4: ART/PEG-PCL mice and 5: ART/biotin-PEG-PCL micelles. In each group, seven animals (a total of 35 animals) were selected for treatment. The mice were given 12 intravenous injections, one every other day of 100 μL ART (60 mg/kg) or ART/Biotin-PEG-PCL (ART 60 mg/kg) in sterile saline. The control group received sterile PBS without treatment. At different times, the mice were weighed and the size of the tumour was measured by Vernier Calliper. The tumour volume was determined as TV (cm3)=L × W2/2, where L is the length of the tumour and W is the tumour width.
Statistical analysis
Analysis of RT-PCR and MTT data was performed using one-way variance analysis using Graph Pad Prism 7 with a p values <.05. For RT-PCR data, Ct and the mean ratio of gene expression and correlation coefficient (R2) were determined before statistical calculation.
Results and discussion
Synthesis and characterization of biotin-PEG-PCL copolymer
Synthesis of PCL-PEG diblock copolymer conjugated to biotin was performed by ring polymerization of anhydrous caprolactone monomer in the presence of biotin-PEG as the reaction initiator and Sn (Oct)2 catalyst (). The structure and composition of biotin, biotin-PEG and biotin-PEG-PCL-doped copolymer were determined by HNMR spectroscopy and shown in .
Figure 1 Schematic synthesis path of the conjugated biotin-PEG-PCL copolymer. And HNMR spectrum of biotin-PEG-PCL copolymer.
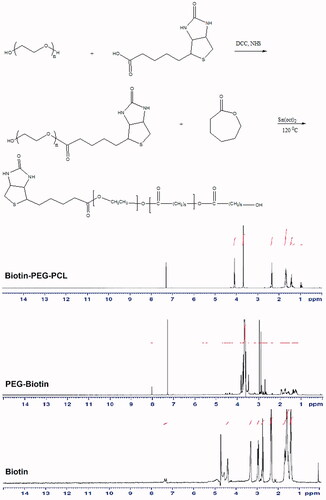
According to , in the biotin spectrum, the peak of the N-atoms in the imidazole ring in the range between 7–8 ppm and its alkenyl chain hydrogens in the region of 0.5 ppm, were also found in the region between 4–5 ppm and peak hydrogen atoms adjacent to the carbon atoms in the thiophene ring in the region of 3–4 ppm. Regarding the biotin-PEG spectrum, PEG alkyl chain hydrogen in the region between 3 and 4 ppm and peak biotin were also observed in this spectrum. Regarding the biotin-PEG-PCL spectrum, the biotin and PEG hydrogens in the region between 3–4 ppm and peak of polyacroploton and biotins hydrogen in the region of 1–2.5 ppm and 4–5 ppm were observed.
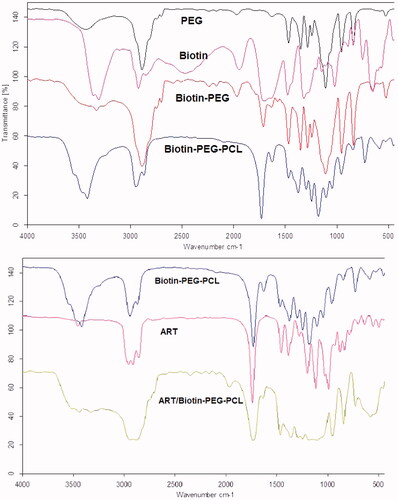
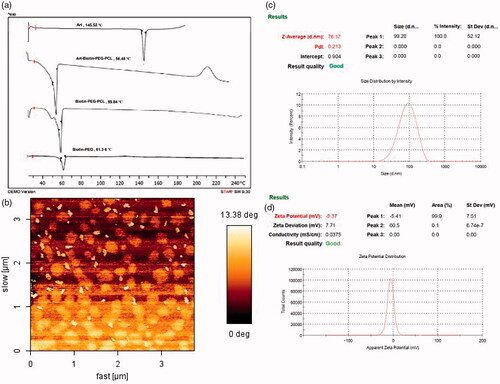
In , the FT-IRs of PEG, biotin, biotin-PEG and biotin-PEG-PCL are shown. In the FTIR spectrum of PEG, the absorbance band at 3500 cm–1 is related to the OH end and the 3000 cm–1 is related to the stretching C-H and 1110 cm–1 corresponding to the ether-C-O. In the biotin spectrum of the absorption band at an acidic OH of 3400 cm–1, the stretching bond C-H is about 3000 cm–1 and the carbonyl group is 1700 cm–1.In the spectrum of biotin-PEG, the absorption band at a frequency of 3000 cm–1 is related to the stretching bond C-H and 1700 cm–1 of the carbonyl group. In the biotin-PEG-PCL spectrum, the absorbance band at 1750 cm–1 is related to the carbonyl group, and as compared to other spectra, it was concluded that copolymer synthesis was successful. DSC can also provide supportive evidence for the synthesis of copolymer PEG/PCL conjugated with biotin. shows pure biotin, biotin-PEG and biotin-PEG-PCL thermography. In the DSC thermography (), the melting point of biotin-PEG and biotin-PEG-PCL appeared at 61.36 and 59.84 °C, respectively. The DSC thermogram of biotin-PEG and the biotin-PEG-PCL bond show an peak at 61.36 and 59.84 °C, indicating a melting point of the copolymer. As it is clear, there is no visible point near the melting point of biotin , which confirms the formation of the bond and the absence of unconjugated biotin in the product. In addition, GPC results showed that the average molecular weight and polydispersity index of conjugated polymer were 24.23 KD and 1.05, respectively.
Preparation and characterization of copolymeric micelles
Biotin-PEG-PCL copolymer has a self-assemble structure due to the presence of hydrophobic and hydrophilic parts in the presence of a suitable solvent. Nanoprecipitation method was used for preparation of Biotin-PEG-PCL micelles loaded with artemisinin [Citation38,Citation39]. The morphology of the nanoparticles was investigated using the AFM technique, and the average size of nanoparticles and its image are shown in . According to the image, nanoparticles are almost uniform and have an average size of 70.33 ± 8.61 nm (mean ± SD (n = 30)). The size of the micelles was also measured by dynamic light scatering. As shown in , the z-average of ART/biotin-PEG-PCL are 76.17 nm and their respective PDI is 0.21 (). The diameter of the micelles determined by the DLS shows the hydrodynamic diameter, while the AFM results from the decayed rods after evaporation of water. The ratio of loading and encapsulation efficiencies of ART/L to PEG-PCL micelles was 12.0 ± 09.09 and 45.1 ± 0.41%, respectively.
Stability assay of micelles by determination of size
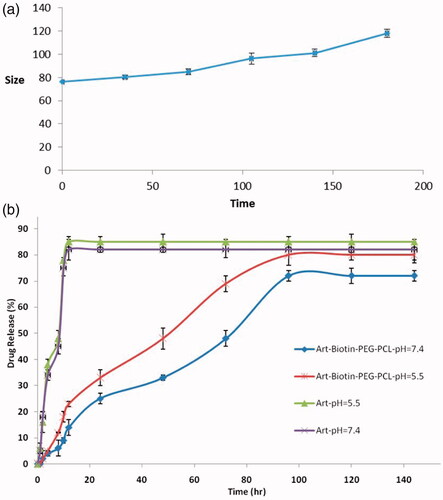
In the clinical aspect of the nanoparticles, the size and stability of the nanostructures are very important. Micelles should be stable enough to be used as a scheduled drug delivery system in order to provide the time to deliver medication and accumulate in the target area. In this study, the stability of particle size over a 6-month period was investigated. The size of the micelle change as the role of incubation time is shown in . The size of all micelles increased somewhat during the measurement period. These observations cannot be a symbol of accumulation, which usually leads to an increase in multiple loads. Some inflammation and/or hydration copolymers (due to the presence of PEG segments at the micelles) can do this.
FTIR analysis
The FTIR spectrums of biotin-PEG-PCL, ART, and ART/biotin-PEG-PCL are presented in . The FTIR spectrum of ART shows feature bands indicated at 1722.47 cm-1 for C=O bands, 2987.45 cm-1 (C-H stretching) for ART. By comparing these data with the spectrum of drug load micelles, the presence of ART attributes in the micelles range can express the successful loading of ART in the micelles. Most of the specific features of the FTIR spectrum were the blue variation of vibration C = O, ranging from 1728 to 1737 cm–1 for ART loaded over the biotin-PEG-PCL spectrum. Changes in the micelles spectrum show that there is some form(s) of association between the ART and the C = O functional group of biotin-PEG-PCL.
DSC analysis
As seen in in the DSC spectrum of the biotin-PEG-PCL copolymer, a peak appeared in the region of 59.84 °C corresponding to the melting point of the copolymer and the single-peak of the spectrum was shown to be the synthesis of copolymer purity. In the DSC spectrum, artemisinin peak observed in the region of 145.52 °C is related to the melting point of the drug. In the range of drug-loaded nanoparticles, a peak was observed in the region of 56.48 °C which was related to the melting point of the copolymer. The reason for not observing the artemisinin melting point in the nanoparticles is that the amount of drug used is much less than that of copolymer and artemisinin is amorphous and its peak is not observed
In vitro release of ART
In order to investigate the effect of chemical and biochemical properties on the release of ART from micelles, an experimental study was performed on ART-loaded micelles in PBS (pH =7.4) and acidic solution of PBS (pH 5.5). As a control, the free ART release was examined to verify that dispersion of drug molecules through the dialysis membrane is not at the liberating step of the rate-limiting step. It was observed that ART is released freely and is 85% and 82%, respectively, at the first 12 h at pH 7.4 and 5.5, respectively. shows the release of ART of drug-loading micelles at pH 7.4 and 5.5. Certainly, no significant burst release of ART from micelles has been identified. As shown in , the ART released from the micelles increases, with a pH of 7.4 to 5.5. For example, after 72 h of incubation, the amount of ART released in a medium at pH 7.4 and 5.5 was 48% and 69%, respectively. The reason for this is the pH of the release of ART from micelles because the copolymer decomposes in acidic conditions by hydrolysis. Also, release is faster in acidic pH than in neutral. This behavior is a very good feature in many applications, particularly in the delivery of anticancer drugs, in which micro environments are extracellular spaces of tumours, intracellular lysosomes and acidic endosomes that can release the drug from micelles to activate the achieved results. The results showed that the maximum release of the drug was 72% and 80%, respectively, for PBS pH =7.4 and pH 5.5 after 96 h. The sustained release of ART can be attributed to the trap of ART in the core of the micelles. Therefore, the combined polymer mixtures can be considered as a very attractive carriers for the delivery of controlled micelles for hydrophobic medications for the success of different therapeutic goals.
In vitro cell toxicity
To use medicine, toxicity is an important aspect to consider when evaluating your applications. For medical and pharmaceutical applications, polymer nanoparticles are intentionally designed to communicate with cells, and it is essential to ensure that these advances do not have a negative effect. Cytotoxic study of ART, copolymer and biotin-PEG-PCL was performed using HFF2 cell with different concentrations. The cells were incubated with samples for 48 and 72 h at concentrations of 0.0005, 0.31, 0.948, 14.22, 106.66 and 400 μM. compares cell survival as ART, copolymer and ART/biotin-PEG-PCL efficacy. With increasing concentrations, the toxicity of ART and ART/biotin-PEG-PCL increased. However, biotin-PEG-PCL copolymers with similar concentrations do not show toxic effects on cells. In all cases, MTT experiments did not show the effects of polymer and biotin-PEG-PCL inhibitors on cell growth. The results of biocompatibility tests indicate that these biotin-conjugated copolymers are highly compatible with each other and do not have a toxic effect. These as-prepared micelles are likely to be used for more in vivo applications.
Figure 5 MTT assay of ART, polymer, and ART/biotin-PEG-PCL micelles (a) HFF-2 cell line after 48 h; (b) HFF-2 after 72 h; (c) MCF-7 after 48 h and (d) MCF-7 after 72-h incubation time period.
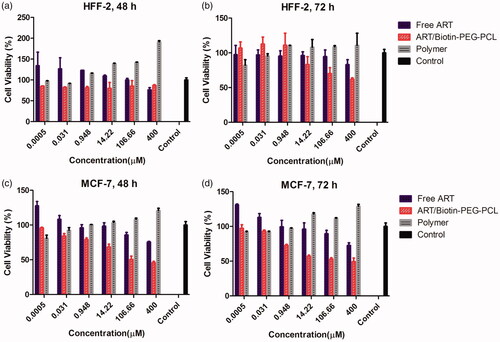
Using different concentrations of ART/biotin-PEG-PCL micelles for different times indicated the effect of cytotoxicity in cancer cells after 48 and 72 h (). MTT results showed a significant reduction in the number of live MCF-7, IC50 cells with 106.66 μg/mL (48 h) and 49.87 μg/mL (72 h). Treatment of biotin-PEG-PCL from MCF-7 cells after 48 and 72 h did not significantly reduce cell proliferation. The MTT test showed that treatment with ART at a concentration of 400 μg/mL had a toxic effect on MCF-7 cells. The results showed that the treatment of MCF-7 cells with ART/biotin-PEG-PCL micelles significantly reduced living cells compared with ART. Toxicity in cancer cells and no inhibitory effect on healthy cells showed specific cell cytotoxicity.
Real-time quantitative PCR analysis
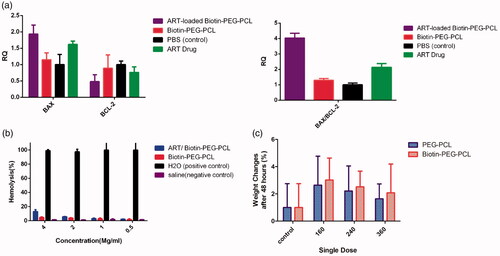
To investigate the effect of ART/biotin-PEG-PCL-micelles on 4T1 cells, the expression of selected genes was analyzed by real-time PCR method. The mitochondria are vital regulators of the pathway of apoptosis, the members of the Bcl-2 family, including pro- and anti-apoptotic proteins, are the main mitochondrial-dependent regulators of apoptosis, ART blocks, the release of cytotoxic C by induction of the Bcl-2 family, controls this process, including inhibiting Bcl-2 (anti-apoptotic) and activating Bax (pro-apoptotic) [Citation40]. Therefore, expression of the target gene in 4T1 cells after treatment with ART/biotin-PEG-PCL mixtures was investigated in comparison with ART and biotin-PEG-PCL micelles. Real-time PCR analysis showed that after 48 h of treatment, ART/biotin-PEG-PCL micelles significantly reduced Bcl-2 expression by 0.48, p<.05. Compared to control group, in addition, ART/biotin-PEG-PCL micelles significantly (p<.05) can adjust the expression of BAX to 1.935 and also significantly (BA) increases the level of BAX mRNA relative to ART. The results showed that biotin-PEG-PCL micelles can significantly improve the effects of ART in increasing the expression of BAX expression. Biotin-PEG-PCL alone does not change the expression of Bcl-2 and BAX (). These results are consistent with our expectations of ART behavior and prove that biotin-PEG-PCL micelles improve ART's efficacy in controlling apoptosis and the expression of metastatic gene expression. Bio-PEG-PCL treatment using ART has been shown to increase Bax expression and reduce Bcl 2 expression with high Bax/Bcl 2 ratio ().
In vitro hemolysis test
The biocompatibilities of biotin-PEG-PCL micelles and ART/biotin-PEG-PCL micelles in the presence of red blood cells (RBCs) were examined by a hemolysis test. As shown in , the percentage of hemolysis increased accordingly with the concentration of micelles. ART/biotin-PEG-PCL at a concentration of 4 mg/mL caused a slight increase in hemolysis when compared with that of the negative control (saline solution). The results also demonstrated that the hemolytic activity of micellar ART depended on the ART concentration ().
LD50 and acute toxicity
To test LD50, we treated mice with a wide range of doses. Mice have been treated for 360 mg/kg after 48 h, and none of our mice have died. In addition, body weight change is an important indicator for the potential toxicity of formulation, shows weight change in mice after 48 h because of any weight gain of normal rats.
Effect of ART/biotin-PEG-PCL micelles in tumour development
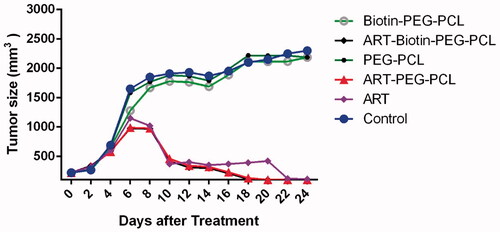
The BALB/c tumour model was made by infusion of 0.2 mL of 4T1 cell suspension (∼ 106 cells/mL) to the right of female BALB/c mice. Injection mice were kept for 14 days to create a tumour (. Antitumour efficacy of ART and/or biotin-PEG-PCL micelles were calculated using BALT/c mice. The tumour growth rate of mice treated with ART, ART/biotin-PEG-PCL and PBS is presented in . The use of ART micelles and ART/biotin-PEG-PCL is effective in preventing tumour growth. However, ART load inhibited tumour volume inhibition versus free ART. Tumor volumes were decreased up 40 mm3 by ART -loaded micelle and 76 mm3 by free ART, compared to that of control group (2150 mm3), respectively. Based on the above results, the ART/biotin-PEG-PCL micelles indicates the effectiveness of tumour targeting and more therapeutic effects than ART. Later, hydrophobic drugs that are encapsulated in biotin-PEG-PCL micelles have the benefits of long-term circulation, preventing the use of RES, and targeting polymeric micelles to the tumour by biotin receptor.
Conclusions
Briefly, biotin-PEG-PCL micelle-encapsulated ART was constructed and designed to improve insolubility of ART. This ART/biotin-PEG-PCL micelles can slowly release ART, promote systemic circulation in vivo and inhibit the growth of cancer cells in vitro and in vivo by demonstrating promising therapies for cancer treatment. To be in vitro cytotoxicity study showed that micelles is safe and low toxicity. Our study showed that these micelles provide a suitable system for the delivery of ART to breast cancer cells. This nanodrug delivery system can also be used as a promising base for designing more micelles systems to improve the effectiveness of ART therapy in the future.
Ethical considerations
This study was approved by the Ethics Committee of the Zanjan University of Medical Sciences, and the study participants signed an informed consent.
Acknowledgement
This work was supported by the deputy of research of Zanjan University of Medical Sciences (Grant No: A-12-430-19).
Disclosure statement
No potential conflict of interest was reported by the authors.
Reference
- Romio M, Morgese G, Trachsel L, et al. Poly (2-oxazoline)-pterostilbene block-copolymer nanoparticles for dual-anticancer drug delivery. Biomacromolecules. 2017;19:103–111.
- Zhang W-J, Hong C-Y, Pan C-Y. Efficient fabrication of photosensitive polymeric nano-objects via an ingenious formulation of RAFT dispersion polymerization and their application for drug delivery. Biomacromolecules. 2017;18:1210–1217.
- Green JJ, Shi J, Chiu E, et al. Biodegradable polymeric vectors for gene delivery to human endothelial cells. Bioconjugate Chem. 2006;17:1162–1169.
- Li Y, Ding J, Zhu J, et al. Photothermal effect-triggered drug release from hydrogen bonding-enhanced polymeric micelles. Biomacromolecules. 2018;19(6):1950–1958.
- Jiang Y, Wong S, Chen F, et al. Influencing selectivity to cancer cells with mixed nanoparticles prepared from albumin–polymer conjugates and block copolymers. Bioconjugate Chem. 2017;28:979–985.
- Du B, Jia S, Wang Q, et al. A self-targeting, dual ros/ph-responsive apoferritin nanocage for spatiotemporally controlled drug delivery to breast cancer. Biomacromolecules. 2018;19:1026–1036.
- Ting JM, Porter WW, III, Mecca JM, et al. Advances in polymer design for enhancing oral drug solubility and delivery. Bioconjugate Chem. 2018;29:939–952.
- Xu W, Ding J, Chen X. Reduction-responsive polypeptide micelles for intracellular delivery of antineoplastic Agent. Biomacromolecules. 2017;18:3291–3301.
- Liu Q, Xu N, Liu L, et al. Dacarbazine-loaded hollow mesoporous silica nanoparticles grafted with folic acid for enhancing antimetastatic melanoma response. ACS Appl Mater Interfaces. 2017;9:21673–21687.
- Shiao Y-S, Chiu H-H, Wu P-H, et al. Aptamer-functionalized gold nanoparticles as photoresponsive nanoplatform for co-drug delivery. ACS Appl Mater Interfaces. 2014;6:21832–21841.
- Karimi M, Sahandi Zangabad P, Ghasemi A, et al. Temperature-responsive smart nanocarriers for delivery of therapeutic agents: applications and recent advances. ACS Appl Mater Interfaces. 2016;8:21107–21133.
- Sinha A, Chakraborty A, Jana NR. Dextran-gated, multifunctional mesoporous nanoparticle for glucose-responsive and targeted drug delivery. ACS Appl Mater Interfaces. 2014;6:22183–22191.
- Martinez AP, Qamar B, Fuerst TR, et al. Biodegradable “smart” polyphosphazenes with intrinsic multifunctionality as intracellular protein delivery vehicles. Biomacromolecules. 2017;18:2000–2011.
- Tian J, Xu L, Xue Y, et al. Enhancing photochemical internalization of dox through a porphyrin-based amphiphilic block copolymer. Biomacromolecules. 2017;18:3992–4001.
- De Luca S, Chen F, Seal P, et al. Binding and release between polymeric carrier and protein drug: ph-mediated interplay of coulomb forces, hydrogen bonding, van der Waals interactions, and entropy. Biomacromolecules. 2017;18:3665–3677.
- Mathews PD, Patta ACMF, Gonçalves JV, et al. Targeted drug delivery and treatment of endoparasites with biocompatible particles of pH responsive structure. Biomacromolecules. 2017;19:499–510.
- Cheng H, Fan X, Wang X, et al. Hierarchically self-assembled supramolecular host-guest delivery system for delivery of chemotherapeutics to drug resistant cancer tumours. Biomacromolecules. 2018;19:1926–1938.
- Miyazaki M, Yuba E, Hayashi H, et al. Hyaluronic acid-based pH-sensitive polymer-modified liposomes for cell-specific intracellular drug delivery systems. Bioconjugate Chem. 2017;29:44–55.
- Peng X-H, Qian X, Mao H, et al. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy. Int J Nanomed. 2008;3:311.
- Sanna V, Sechi M. Nanoparticle therapeutics for prostate cancer treatment. Nanomed: Nanotechnol, Biol Med. 2012;8:S31–S36.
- Jochum FD, Roth PJ, Kessler D, et al. Double thermoresponsive block copolymers featuring a biotin end group. Biomacromolecules. 2010;11:2432–2439.
- Říhová B, Jelinkova M, Strohalm J, et al. Antiproliferative effect of a lectin-and anti-Thy-1.2 antibody-targeted HPMA copolymer-bound doxorubicin on primary and metastatic human colorectal carcinoma and on human colorectal carcinoma transfected with the mouse Thy-1.2 gene. Bioconjugate Chem. 2000;11:664–673.
- Mandracchia D, Rosato A, Trapani A, et al. Design, synthesis and evaluation of biotin decorated inulin-based polymeric micelles as long-circulating nanocarriers for targeted drug delivery. Nanomed Nanotechnol, Biol Med. 2017;13:1245–1254.
- Lesniak W, Kariapper M, Nair B, et al. Synthesis and characterization of nanodevices for targeted tumor therapy. Nanomed Nanotechnol, Biol Med. 2006;2:318.
- Chen S, Yang K, Tuguntaev RG, et al. Targeting tumor microenvironment with PEG-based amphiphilic nanoparticles to overcome chemoresistance. Nanomed Nanotechnol, Biol Med. 2016;12:269–286.
- Nakase I, Gallis B, Takatani-Nakase T, et al. Transferrin receptor-dependent cytotoxicity of artemisinin–transferrin conjugates on prostate cancer cells and induction of apoptosis. Cancer Lett. 2009;274:290–298.
- Chen Y, Lin X, Park H, et al. Study of artemisinin nanocapsules as anticancer drug delivery systems. Nanomed Nanotechnol, Biol Med. 2009;5:316–322.
- Van Nijlen T, Brennan K, Van den Mooter G, et al. Improvement of the dissolution rate of artemisinin by means of supercritical fluid technology and solid dispersions. Int J Pharm. 2003;254:173–181.
- Sharafi A, Hashemi Sohi H, Sharafi A, et al. Tissue culture and regeneration of an antimalarial plant, Artemisia sieberi Besser. Res J Pharmacogn. 2014;1:15–20.
- Mirzaee H, Sharafi A, Sohi HH. In vitro regeneration and transient expression of recombinant sesquiterpene cyclase (SQC) in Artemisia annua L. S Afr J Bot. 2016;104:225–231.
- Woodrow C, Haynes R, Krishna S. Artemisinins. Postgraduate Med J. 2005;81:71–78.
- Li QG, Peggins JO, Fleckenstein LL, et al. The pharmacokinetics and bioavailability of dihydroartemisinin, arteether, artemether, artesunic acid and artelinic acid in rats. J Pharm Pharmacol. 1998;50:173–182.
- Shutava TG, Balkundi SS, Vangala P, et al. Layer-by-layer-coated gelatin nanoparticles as a vehicle for delivery of natural polyphenols. ACS Nano. 2009;3:1877–1885.
- Wang T, He N. Preparation, characterization and applications of low-molecular-weight alginate–oligochitosan nanocapsules. Nanoscale. 2010;2:230–239.
- Safari J, Zarnegar Z. Advanced drug delivery systems: nanotechnology of health design A review. J Saudi Chem Soc. 2014;18:85–99.
- Crespo-Ortiz MP, Wei MQ. Antitumor activity of artemisinin and its derivatives: from a well-known antimalarial agent to a potential anticancer drug. BioMed Res Int. 2012;(2011):247597.
- Cuong N-V, Jiang J-L, Li Y-L, et al. Doxorubicin-loaded PEG-PCL-PEG micelle using xenograft model of nude mice: effect of multiple administration of micelle on the suppression of human breast cancer. Cancers. 2010;3:61–78.
- Khoee S, Rahmatolahzadeh R. Synthesis and characterization of pH-responsive and folated nanoparticles based on self-assembled brush-like PLGA/PEG/AEMA copolymer with targeted cancer therapy properties: a comprehensive kinetic study. Eur J Med Chem. 2012;50:416–427.
- Kim SY, Cho SH, Lee YM, et al. Biotin-conjugated block copolymeric nanoparticles as tumor-targeted drug delivery systems. Macromol Res. 2007;15:646–655.
- Desagher S, Martinou J-C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377.