Abstract
Stem cells can be obtained from a variety of sources. To compare the effect of cell source on the osteogenic differentiation potential, buccal fat pad-derived mesenchymal stem cells (BFP-MSCs), bone marrow-derived MSCs (BM-MSCs) and unrestricted somatic stem cells (USSCs) with different accessibility in time and region, were cultured on bioceramic (Bio-Oss®) coated electrospun polycaprolactone (PCL) scaffold (PCL-Bio). After scaffold characterization, stem cells proliferation and osteogenic differentiation were investigated by MTT and Alizarin red staining, alkaline phosphatase activity, calcium content and gene expression assays. Proliferation rate of the stem cells was not significantly different with each other, only USSCs showed significantly lower proliferation rate while cultured on PCL-Bio; although, PCL-Bio showed better proliferation support in comparison with tissue culture plate and PCL. Mineralization of the BM-MSCs was significantly higher than others, while BFP-MSCs were close to it. Highest ALP activity was detected in BFP-MSCs cultured on PCL-Bio. USSCs demonstrated higher gene expression level in three genes, although differences were not huge compared to others. According to the results and due to the availability, facilitated preparation procedure and less patients suffering, BFP-MSCs have a better choice than BM-MSCs and USSCs for use in bone tissue engineering.
Introduction
Today, several factors, including the aging of the world’s population and their lifestyle in advanced societies, have caused develop bone diseases to be one of the serious problems facing humanity. Diseases such as osteoporosis, osteogenesis imperfecta, osteodystrophy and osteosarcoma, along with a variety of bone fractures resulting from accidents, have caused suffering to many people around the world [Citation1,Citation2]. The current therapeutic strategy is bone grafting that divided to the grafting from one person to another (allograft) or from another part of the patient’s body (autograft), which has many limitations [Citation3,Citation4]. For this reason, in recent years, cell therapy with tissue engineering has been able to provide new methods for treating this patient. The two main parts of these methods are based on the type of stem cells used and the type of scaffolds suitable for accelerating bone repair and reconstruction [Citation5,Citation6]. The safest and most widely used stem cell for clinical treatment is Adult stem cells that are multipotent undifferentiated cells with mesenchymal stem cells (MSCs) characteristics that can be extracted from several sources such as dental pulp stem cells (DPSCs), buccal fat pad-derived MSCs (BFP-MSCs), bone marrow-derived MSCs (BM-MSCs), unrestricted somatic stem cells (USSCs) from human umbilical cord blood and adipose tissues derived stem cells (ADSCs) [Citation7,Citation8].
As another main part of the tissue engineering, scaffolds help stem cells to grow as well as tissue formation because they are pseudo three-dimensional organization. In the area of damaged tissues, the mechanical structure, the ability to transport efficient food and other growth factors has also been destroyed [Citation9,Citation10]. Using these scaffolds, in addition to the platform needed to control the growth of stem cells, the problems mentioned above are also overcome. When the stem cells to form a three-dimensional tissue structure, the scaffold is gradually degraded and only differentiated cells remain. In such a system using growth factors, stem cell differentiation into a variety of different tissues is possible without changing the basic matrix materials [Citation11,Citation12]. Bioactive ceramics such as hydroxyapatite (HAP), tri-calcium phosphate (TCP) and bioactive glasses are known as ceramics that are applied to induce specific biological activity in damaged bone via reaction with physiological fluids and through cellular interactions form tenacious bonds to achieve tight fixation [Citation13,Citation14]. Bio-ceramics usually have higher Young’s modulus than human cortical bone; therefore, they should combine with synthetic biomaterials to modify their mechanical properties. Examples of these composite nanofibrous scaffolds such as bioactive hybrids are polycaprolactone (PCL)/silica hybrids, PCL/bioactive glass hybrid, PCL/HAP composite and PCL/calcium phosphate [Citation15]. Currently, it has been reported that PCL nanocomposite with forsterite (Mg2SiO4) as a new bioceramic shows adequate mechanical properties and good bioactivity [Citation15]. It was also demonstrated that addition of bio-ceramics could modulate PCL degradation [Citation16–18]. Moreover, PCL/bioceramic nanocomposite scaffolds resulted in greater protein adsorption and enhanced mineralization. Improving both mechanical strength and bioactivity could be beneficial in the processing of bone cements [Citation15].
In this study, we investigated the osteoinductive potential of Bio-Oss® coated PCL when cultured with three different stem cells such as BFP-MSCs, BM-MSCs and USSCs. The aim of this study was survey osteogenic differentiation potential of three different adult stem cells while cultured on the surface of the same bioceramic coated nanofibrous scaffold.
Methods and materials
Scaffold preparation
PCL nanofibers were fabricated using electrospinning method as described previously [Citation19]. Briefly, 5% (w/w) solution of poly(ε-caprolactone) (Mw=80,000 g/mol) (Sigma-Aldrich, St. Louis, MO) was prepared in chloroform and N-dimethylformamide (DMF, Merck, Darmstadt, Germany) and then was fed to a 5 mL syringe that was connected to a blunted needle with a distance of 15 cm from the collector with collecting rate of 250 rpm. Voltage was 20 kV and flow rate was 1 mL/h. Fabricated nanofibers were placed in vacuum for evaporation of residual solvent for 24 h.
Scaffold surface modifications
After scaffold fabrication, surface modification was carried out in the two steps: at first scaffold treated by oxygen plasma using a cylindrical quartz reactor (Diener Electronics, Ebhausen, Germany) with frequency of 44 GHz and pressure of 0.4 mbar for 5 min. At the next step, Bio-Oss® particles (GeistlichPharma AG, Wolhusen, Switzerland) were dispersed in deionized water (1%, w/v) by an ultrasonic bath for 20 min. Then solution was added to the surface of the plasma treated scaffold after washing with PBS two times and stored at 4 °C for 24 h. After that scaffold was sterilized under UV irradiation for 30 min and then rinsed in 70% ethanol for 2 h at room temperature.
Scanning electron microscopy (SEM)
Bioceramic coated PCL (PCL-Bio) scaffold and cell-polymer constructs were characterized by SEM morphologically. For this aim, stem cell seeded scaffolds were inserted in 2.5% glutaraldehyde for fixation and then rinsed in graded series of ethanol for dehydration and then dried with vacuum. Before imaging by SEM, samples were bonded onto aluminium, coated with gold and finally imaged by scanning electron microscope (S-4500; Hitachi, Tokyo, Japan).
Expansion of the stem cells
Previously isolated and characterized BFP-MSCs, BM-MSCs and USSCs [Citation20,Citation21] were thawed in the three culture flasks (SPL, Pocheon-si, South Korea) under Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen Co., Carlsbad, CA) supplemented with 10% foetal bovine serum (FBS, Sigma-Aldrich, St. Louis, MO) and incubated with 95% air and 5% CO2 at 37 °C. When stem cells became confluence (about 80–85%), cells were detached and numbered and seeded on the surface of the prepared scaffolds.
Stem cell seeding on the surface of the scaffolds
Sterilized scaffolds were inserted on the six-well plates (SPL, Pocheon-si, South Korea) and incubated in 95% air and 5% CO2 at 37 °C for 6 h under DMEM with 10% FBS, then stem cells seeded on the scaffolds and tissue culture plate (TCPS) with a cell density of 2 × 104 cells per cm2 in nine groups including three groups for TCPS, three groups for PCL and three groups for PCL-Bio-Oss®. BFP-MSCs, BM-MSCs and USSCs were cultured on TCPS, PCL and PCL-Bio-Oss® under basal medium and 24 h after that medium was exchanged by an osteogenic induction medium (DMEM supplemented with 10% FBS, 3 mM β-glycerophosphate (βGP), 50 μg/mL ascorbic acid (AA) and 10−9 M dexamethasone) and 70% of the medium was exchanged every three days for 21 days.
MTT assay
After biocompatibility qualitative evaluation of the fabricated and coated scaffolds by SEM, MTT assay was applied as a quantitative method for evaluation of the scaffolds biocompatibility. Briefly, 50 µL of MTT solution (5 mg/mL in DMEM) was added to the each well 1, 3, 5 and 7 days after cell seeding and then incubated at 37 °C for 3.5 h for MTT reduction by living cells. After that, supernatant was removed out and 100 µL dimethyl sulphoxide (DMSO) added to the samples and after gentle shaking, their optical density was measured at 570 nm using a micro-plate reader (BioTek Instruments, Winooski, VT).
Deposited calcium staining by Alizarin red
Matrix mineralization as a main osteogenic marker was investigated for samples using Alizarin red staining two weeks after cell seeding. Samples were washed by PBS for two times and then rinsed in 4% paraformaldehyde for 30–45 min at 4˚C. Fixed cells were washed again and then were stained by 2% Alizarin red (pH 7.2, Sigma-Aldrich, St. Louis, MO) for 5 min at room temperature. Samples were washed by PBS and imaged by an inverted light microscopy.
Calcium measurement
Calcium measurement was carried out in all differentiated cells cultured in all substrates by a calcium content kit (Pars Azmoon, Tehran, Iran) at days 7 and 14 after cell seeding. The calcium was extracted from the cells seeded on scaffolds and TCPS using 0.6 N HCL (Merck, Darmstadt, Germany) by shaking during 1 h on ice. Then, kit reagent was added to the solutions and measured at 570 nm using a micro-plate reader (BioTek Instruments, Winooski, VT). Finally, calcium values of the samples were counted via standard curve obtained from optical density of a serial dilution of calcium concentrations at 570 nm.
Alkaline phosphates activity (ALP)
ALP activity of the differentiated cells was measured by ALP activity kit (Pars Azmoon, Tehran, Iran) in all groups at 7 and 14 days after cell seeding. First, samples were washed with PBS two times and their total protein was extracted using 200 μL of RIPA lysis buffer and centrifuged at 15,000 RPM at 4 °C for 15 min. After that ALP substrate (p-nitrophenyl phosphate, pNPP) was added to the supernatant and read at 570 nm using a micro-plate reader (BioTek Instruments, Winooski, VT). Finally, ALP activity level (IU) was normalized versus the total protein.
Gene expression
Quantitative gene expression was carried out using real-time PCR for osteocalcin (BGLAP), Runt-related transcription factor 2 (Runx2) and osteonectin genes at 7 and 14 days after cell seeding in all groups. To do this, RNA content of the cells was isolated using RNeasy kit (Qiagen, Germantown, MD). Then, cDNA was synthesized using cDNA kit (Fermentas, Burlington, Canada) according to the manufactured protocol with this PCR parameters: denaturation at 95 °C for 3 min, then 35 cycles at 95 °C for 15 s, annealing at 60 °C for 30 s, and elongation at 72 °C for 30 s. The primer sequences, which were used at this study, are represented in , where HPRT1 used as an internal control. Real-time PCR was carried out using Rotor Gene 6000 (Corbett, Concorde, Australia) via applying SYBR Premix Ex Taq (Takara, Tokyo, Japan).
Table 1. Primers used in RT-PCR.
Statistics
Experiments were performed for three times and all data were reported as the mean ± standard deviation (SD). The results were compared using one-way analysis of variance (ANOVA) to obtain any significant relation and p values less than .05 were considered statistically significant. Real-time PCR data were analysed using REST2009 software. All statistical analyses were performed with SPSS software (version 11.0, SPSS, Chicago, IL).
Results
Characterization of fabricated nanofibers
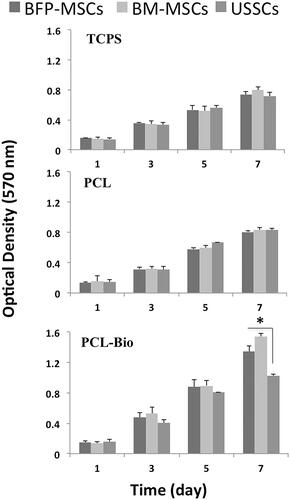
Fabricated scaffolds were characterized quantitatively using SEM and results showed that PCL scaffolds were porous with interconnection pores, smooth and bead free with an average diameter of 730 ± 340 nm and BioOss® micro-particles were distributed homogeneously on the scaffold surface ( with two magnifications). After that, for biocompatibility characterization of the fabricated scaffolds, BFP-MSCs, BM-MSCs and USSCs were cultured and expanded to seeding on the surface of the scaffolds, which is demonstrated in respectively in low and high confluency. SEM results showed that BFP-MSCs (), BM-MSCs () and USSCs () were attached, proliferated and expanded on the surface of the scaffolds and monolayer formatted during the period of study. For quantitative biocompatibility evaluation of the fabricated scaffolds, MTT assay was performed and results showed that scaffolds can support stem cells growth and proliferation and an increasing growth pattern was detected during a week, although measurements in PCL-Bio were higher than PCL and TCPS groups and on day 7 proliferation rate of the BFP-MSCs and BM-MSCs was significantly higher than USSCs in PCL-Bio groups (.
Figure 1. SEM photographs from Bio-Oss®-coated polycaprolactone nanofibrous scaffolds fabricated using electrospinning at two magnifications (1.00 kx (a), 5.00 kx (b)).
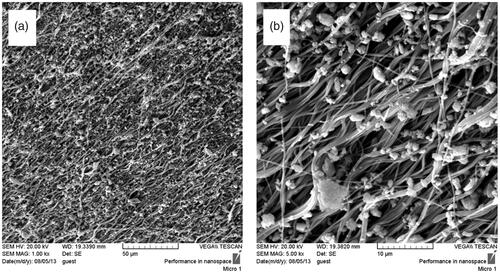
Figure 2. Stem cell images at two magnifications ×10 and ×40, BFP-MSCs (a, b), BM-MSCs (c, d) and USSCs (e, f) at passages two while cultured on tissue culture polystyrene (TCPS) under DMEM supplemented with FBS 10%.
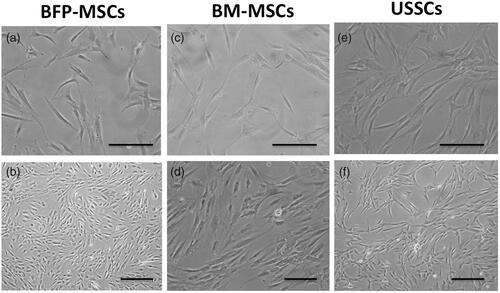
Figure 3. SEM photographs from Bio-Oss®-coated Polycaprolactone nanofibrous scaffolds, two weeks after BFP-MSCs (a, b), BM-MSCs (c, d) and USSC (e, f) seeding under osteogenic differentiation medium at two magnifications.
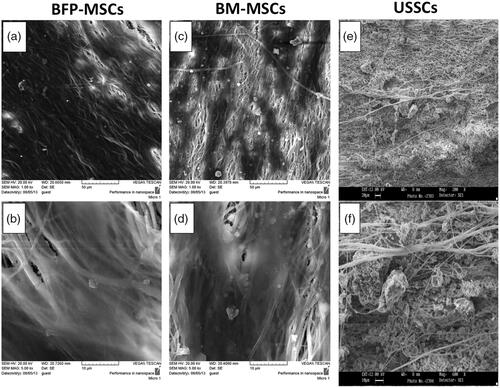
Figure 4. Survival rate of BFP-MSCs, BM-MSCs and USSC while cultured on tissue culture polystyrene (TCPS), polycaprolactone nanofibrous scaffold (PCL) and Bio-Oss®-coated PCL nanofibrous scaffold (PCL-Bio) during days 1, 3, 5 and 7 cultured under DMEM supplemented FBS 10% (asterisks: significant difference between the groups at p < .05).
In vitro osteogenic differentiation assays
Alizarin red staining, calcium content and ALP activity
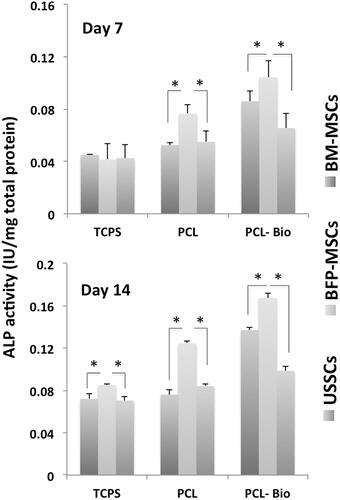
Stem cells calcium deposition during osteogenic differentiation was evaluated using Alizarin red staining for BFP-MSCs, BM-MSCs and USSCs while cultured on TCPS after two weeks (), respectively. As shown, significantly higher mineralization was detected in BM-MSCs, while mineralization was not significantly different between the BFP-MSCs and USSCs. To quantitative measurement of the calcium produced by stem cells during differentiation process, calcium content assay was performed and results showed that at day 7, calcium content in TCPS group was not different among the three stem cells, while in PCL group, it was significantly increased in BM-MSCs and BFP-MSCs compared with USSCs and in PCL-Bio group, calcium content of the BM-MSCs was significantly higher than two others (). At day 14, results of the TCPS group were changed and BM-MSCs showed significantly higher calcium content than BF-MSCs, and its measure in BF-MSCs was also significantly higher than USSCs, and the difference in calcium content of stem cells cultured on PCL and PCL-Bio was the same on day 7 (). ALP activity of the stem cells cultured on TCPS, PCL and PCL-Bio was investigated at days 7 and 14 and results showed that there were no significant differences observed among stem cells cultured on TCPS at day 7, while significantly higher ALP activity was detected in BFP-MSCs that cultured on PCL and PCL-Bio in comparison with other two stem cells (. At day 14, BFP-MSCs also showed significantly higher ALP activity in all three groups compared with BM-MSCs and USSCs; however, in PCL-Bio group BM-MSCs also showed higher ALP activity than USSCs.
Figure 5. Alizarin red staining of BFP-MSCs (a), BM-MSCs (b) and USSCs (c) two weeks after cultured on tissue culture polystyrene (TCPS) under osteogenic differentiation medium, magnification ×40. Calcium content of the stem cells while cultured on tissue culture polystyrene (TCPS), Polycaprolactone nanofibrous scaffold (PCL) and Bio-Oss®-coated PCL nanofibrous scaffold (PCL-Bio) under osteogenic differentiation medium on day 7 (d) and day 14 (e) (asterisks: significant difference between the groups at p < .05).
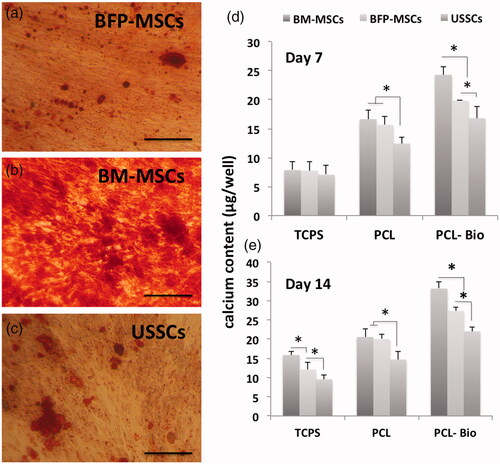
Figure 6. Alkaline phosphatase (ALP) activity of the stem cells while cultured on tissue culture polystyrene (TCPS), polycaprolactone nanofibrous scaffold (PCL) and Bio-Oss®-coated PCL nanofibrous scaffold (PCL-Bio) under osteogenic differentiation medium on day 7 and day 14 (asterisks: significant difference between the groups at p < .05).
Osteogenic gene markers measurement
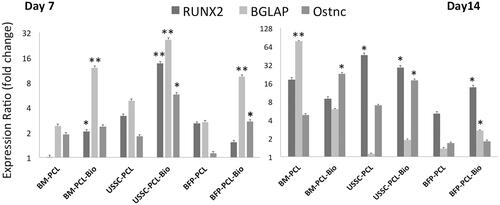
Expression fold change of the Runx-2, BGLPA and osteonectin was detected by RT-PCR on days 7 and 14 in three stem cells during osteogenic differentiation process (), and results showed that at day 7, Runx-2 expression was significantly increased in BM-MSCs and USSCs while cultured on PCL-Bio scaffolds; although, this increase in USSCs was higher than BM-MSCs. BGLAP expression was significantly increased in all three stem cells while cultured on PCL-Bio scaffold; although, this increase in USSCs was higher than two others. Osteonectin expression was also increased significantly in USSCs and BFP-MSCs when cultured on PCL-Bio scaffold and in this case USSCs were also higher than BFP-MSCs. But at day 14, significantly higher Runx-2 expression was detected in USSCs cultured on PCL and PCL-Bio and BFP when cultured on PCL-Bio scaffold. BGLAP expression was also increased significantly in BM-MSCs seeded on PCL and BFP-MSCs when seeded on PCL-Bio scaffold. However, highest osteonectin expression was detected in BM-MSCs and USSCs while cultured on PCL-Bio scaffold.
Figure 7. Quantitative expression changes of the Runx2, osteocalcin (BGLAP) and osteonectin in BFP-MSCs, BM-MSCs and USSCs while cultured on polycaprolactone nanofibrous scaffold (PCL) and Bio-Oss®-coated PCL nanofibrous scaffold (PCL-Bio) under osteogenic differentiation medium on days 7 and 14 (asterisks: significant difference between the groups at p < .05).
Discussion
One of the important issues in cell therapy is the variety of sources available for stem cell production, sources that have no significant difference in growth rate and differentiation to target tissue. Although it maybe, one of the resources has a higher potential, but its production has many problems and difficulties. Three adult stem cells from three different sources were studied in this study, with respect that the preparing method of these three stem cells is different in time and area. One of these was USSC that Kögler et al. at the first time isolated and introduced these cells from umbilical cord blood as a promising embryo-safe pluripotent cell [Citation22]. These stem cells were capable to differentiate into a variety of cells such as cells in the body. Obtaining USSC without cytokine unlimited physical environment and unlimited physical ability to produce blood cells as well in vivo and in vitro are two major differences with MSCs [Citation23]. In addition, the use of USSC in the body of different species has not been led to observe macroscopic or microscopic sign of tumours after transplantation over months or years. These cells also lack HLA-II and non-immunogenicity. Although MSCs derived from BM and BFP can be done at any age and time. However, the preparation of stem cells derived from BFP is much faster in terms of facilities and time, and the source tissue is more accessible and the patient will also suffer less. On the other hand, several studies reported that stem cells derived adipose tissues are very similar to BM-MSCs from the viewpoints of morphology and surface markers [Citation24], which are capable to differentiate various kinds of cell linages including the osteogenic ones [Citation20]. ADSCs are known as a valuable resource of stem cells for regenerative medicine and tissue engineering, due to their differentiation capabilities and non-invasive extraction method [Citation25]. BFP-MSCs are subset of the ADSCs [Citation26]; although, this source is less than those prepared from abdominal site. We and other authors previously reported that BFP-MSCs have a great osteogenic potential solely or in comparison with other stem cells in in vitro and in vivo studies [Citation20,Citation27,Citation28]. Proper scaffolds, which can mimic extracellular matrix (ECM) role in the natural tissue in the body have been attracted more attention of the tissue engineering and regenerative medicine specialists. Since, bioceramics are already present at the bone tissue and on the other hand, ECM has a fibril form with nanometre in scales in body, those scaffolds that have both characteristics can have better simulating natural ECM for stem cells to have growth, proliferation and differentiation. For this reason, to understand more of this concept, PCL-BioOss that has both characteristics was used to investigate proliferation and osteogenic differentiation potential of the stem cells with three different sources. Previously, we showed that PCL and BioOss could improve in vitro osteogenic differentiation [Citation29] and in vivo bone regeneration, respectively [Citation30]. Results obtained from this study showed that in some characteristics BM-MSCs were better than others and in some others BFP-MSCs and/or USSCs were higher than others. In calcium content and mineralization, BM-MSCs demonstrated higher measures in comparison with two other stem cells, and in ALP activity, BFP-MSCs showed significantly higher values in comparison with other stem cells. While in gene expression, USSCs showed approximately higher expression levels totally in three genes,, there were no huge differences among them.
Conclusions
According to the results, it is very difficult to conclude which one of the stem cells is better than others and thus regarding availability, easier preparation and less patient suffering, BFP-MSCs have the highest priority in comparison with BM-MSCs and USSCs for use in regenerative medicine and tissue engineering applications. In addition, PCL-Bio showed that it has a great potential to be used in bone tissue engineering and PCL capability was increased significantly when coated with Bio-Oss micro-particles.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Nguyen DT, Burg KJL. Bone tissue engineering and regenerative medicine: targeting pathological fractures. J Biomed Mater Res. 2015;103:420–429.
- Brooks PM. The burden of musculoskeletal disease—a global perspective. Clin Rheumatol. 2006;25:778–781.
- Blank AT, Riesgo AM, Gitelis S, et al. Bone grafts, substitutes, and augments in benign orthopaedic conditions: current concepts. Bull NYU Hosp Joint Dis. 2017;75:119.
- Egol KA, Nauth A, Lee M, et al. Bone grafting: sourcing, timing, strategies, and alternatives. J Orthopaed Trauma. 2015;29:S10–S14.
- Black CRM, Goriainov V, Gibbs D, et al. Bone tissue engineering. Curr Mol Bio Rep. 2015;1:132–140.
- Moradi SL, Golchin A, Hajishafieeha Z, et al. Bone tissue engineering: adult stem cells in combination with electrospun nanofibrous scaffolds. J Cell Physiol. 2018;233(10):6509–6522.
- Tevlin R, Walmsley GG, Marecic O, et al. Stem and progenitor cells: advancing bone tissue engineering. Drug Deliv Transl Res. 2016;6:159–173.
- Wang P, Liu X, Zhao L, et al. Bone tissue engineering via human induced pluripotent, umbilical cord and bone marrow mesenchymal stem cells in rat cranium. Acta Biomater. 2015;18:236–248.
- Yousefi AM, Hoque ME, Prasad RGSV, et al. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: a review. J Biomed Mater Res. 2015;103:2460–2481.
- Shabafrooz V, Mozafari M, Vashaee D, et al. Electrospun nanofibers: from filtration membranes to highly specialized tissue engineering scaffolds. J Nanosci Nanotechnol. 2014;14:522–534.
- Yazdimamaghani M, Razavi M, Vashaee D, et al. Development and degradation behavior of magnesium scaffolds coated with polycaprolactone for bone tissue engineering. Mater Lett. 2014;132:106–110.
- Agrawal CM, Ray RB. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res. 2001;55:141–150.
- Chevalier J, Gremillard L. Ceramics for medical applications: a picture for the next 20 years. J Eur Ceram Soc. 2009;29:1245–1255.
- Gao C, Deng Y, Feng P, et al. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int J Mol Sci. 2014;15:4714–4732.
- Mkhabela VJ, Ray SS. Poly(ε-caprolactone) nanocomposite scaffolds for tissue engineering: a brief overview. J Nanosci Nanotechnol. 2014;14:535–545.
- Lam CXF, Savalani MM, Teoh S-H, et al. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: accelerated versus simulated physiological conditions. Biomed Mater. 2008;3:034108.
- Chouzouri G, Xanthos M. In vitro bioactivity and degradation of polycaprolactone composites containing silicate fillers. Acta Biomater. 2007;3:745–756.
- Dunn AS, Campbell PG, Marra KG. The influence of polymer blend composition on the degradation of polymer/hydroxyapatite biomaterials. J Mater Sci Mater Med. 2001;12:673–677.
- Arjmand M, Ardeshirylajimi A, Maghsoudi H, et al. Osteogenic differentiation potential of mesenchymal stem cells cultured on nanofibrous scaffold improved in the presence of pulsed electromagnetic field. J Cell Physiol. 2018;233:1061–1070.
- Ardeshirylajimi A, Mossahebi‐Mohammadi M, Vakilian S, et al. Comparison of osteogenic differentiation potential of human adult stem cells loaded on bioceramic‐coated electrospun poly (L‐lactide) nanofibres. Cell Prolif. 2015;48:47–58.
- Shafiee A, Seyedjafari E, Soleimani M, et al. A comparison between osteogenic differentiation of human unrestricted somatic stem cells and mesenchymal stem cells from bone marrow and adipose tissue. Biotechnol Lett. 2011;33:1257–1264.
- Kögler G, Sensken S, Airey JA, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–135.
- Kögler G, Radke TF, Lefort A, et al. Cytokine production and hematopoiesis supporting activity of cord blood-derived unrestricted somatic stem cells. Exp Hematol. 2005;33:573–583.
- Salehi-Nik N, Rezai Rad M, Kheiri L, et al. Buccal fat pad as a potential source of stem cells for bone regeneration: a literature review. Stem Cells Int. 2017;2017:1.
- Sterodimas A, de Faria J, Nicaretta B, et al. Tissue engineering with adipose-derived stem cells (ADSCs): current and future applications. J Plast Reconstruct Aesthetic Surg. 2010;63:1886–1892.
- Farré-Guasch E, Martí-Pagès C, Hernández-Alfaro F, et al. Buccal fat pad, an oral access source of human adipose stem cells with potential for osteochondral tissue engineering: an in vitro study. Tissue Eng C Methods. 2010;16:1083–1094.
- Khojasteh A, Mohajerani H, Momen-Heravi F, et al. Sandwich bone graft covered with buccal fat pad in severely atrophied edentulous maxilla: a clinical report. J Oral Implantol. 2011;37:361–366.
- Shiraishi T, Sumita Y, Wakamastu Y, et al. Formation of engineered bone with adipose stromal cells from buccal fat pad. J Dent Res. 2012;91:592–597.
- Mohamadyar-Toupkanlou F, Vasheghani-Farahani E, Bakhshandeh B, et al. In vitro and in vivo investigations on fibronectin coated and hydroxyapatite incorporated scaffolds. Cell Mol Biol (Noisy-le-Grand, France). 2015;61:1–7.
- Daei-farshbaf N, Ardeshirylajimi A, Seyedjafari E, et al. Bioceramic-collagen scaffolds loaded with human adipose-tissue derived stem cells for bone tissue engineering. Mol Biol Rep. 2014;41:741–749.
