?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Targeted liposomes have high potentials in the specific and effective delivery of their loaded therapeutic agents to the tumour site. Once at the tumour site, it is important that these liposomes are triggered to release their load in a controlled and effective manner. In this study, pegylated (stealth) liposomes conjugated to human serum albumin (HSA) were investigated for the delivery of a model drug (calcein) to breast cancer cells. The fluorescent results showed that calcein uptake by the two breast cancer cell lines (MDA-MB-231 and MCF-7) was significantly higher with the HSA-PEG liposomes compared to the non-targeted control liposomes. Furthermore, the exposure to low-frequency ultrasound (LFUS) resulted in a statistically significant uptake of calcein compared to the uptake without ultrasound. The described drug delivery (DD) system, which involves combining the targeted liposomal formulation with ultrasonic triggering techniques, promises a safe, effective and site-specific breast cancer therapy.
Introduction
Chemotherapy is an effective method for breast cancer treatment showing many advantages in prolonging patients’ survival time. However, the high toxicity of this treatment limits the drug dosage that can be administrated [Citation1]. A promising solution to overcome these unwanted effects is to use smart nanocarriers that are biocompatible, biodegradable and stimuli-responsive. These nanocarriers are able to encapsulate the drugs efficiently. Thus, ensuring the safe delivery of these drugs to the tumour site. When triggered, nanocarriers release their load destroying the cancer cells while limiting the exposure of the healthy cells to these toxic drugs.
Liposomes are nanoparticles composed of phospholipids which self-assemble to form bilayer spherical shapes. These ideal nanocarriers are highly stable and biocompatible with a high encapsulating capacity for both hydrophilic and hydrophobic drugs. In addition, liposomes can be easily modified to increase their circulation time by coating them with polymers, such as polyethylene glycol (PEG), also known as stealth liposomes [Citation2]. The drugs encapsulated inside these nanocarriers circulate in the body without affecting the healthy tissues due to the inability of these drug delivery (DD) vehicles to cross the endothelial barrier. On the other hand, the nanocarriers easily accumulate and are retained at the tumour site by penetrating through the leaky blood vessels formed as a result of the aberrant angiogenesis in tumours [Citation3]. This is known as the “enhanced permeability and retention effect” (EPR) which is the mechanism behind the “passive targeting” of tumour tissues [Citation4,Citation5]. “Active targeting” of specific receptors on the surfaces of the tumour cells can be achieved by coating these liposomes with targeting ligands which actively target specific receptors that are overly expressed on the membranes of the cancer cells [Citation6]. This will ensure the specific delivery of the liposomal anticancer agents to the tumour site.
Human serum albumin (HSA) is a multi-functional protein that is able to bind and transport numerous endogenous and exogenous molecules [Citation7]. When the cells are stressed, as is the case with the fast-growing cancer cells, albumin is taken up by cells as a source of amino acids and energy needed for cell proliferation [Citation8]. Albumin represents a high percentage of the total amount of extracellular protein in tumour cytosol for patients suffering from breast cancer [Citation9]. A number of studies have reported that albumin receptors (heterogeneous nuclear ribonucleoproteins – hnRNP) are localized on the surface of the cancer cells [Citation10,Citation11]. In experimental animals bearing solid tumours, radioactively- or fluorescently labelled albumin was highly taken up by the tumour cells compared to the healthy cells [Citation12] while Germain et al. [Citation13] reported that albumin is internalized by human breast cancer cells (MDA-MB 231 and MCF-7) in culture, by using confocal laser scanning microscopy. Therefore, pegylated liposomes labelled with HSA are suitable for targeting carriers to deliver therapeutic drugs to HSA receptors’ overexpressing cancer cells due to their targeting capabilities, their colloidal stability and long blood-circulation time [Citation14].
Following the accumulation at the tumour site, liposomes show a slow rate of release of the loaded drug [Citation15,Citation16]. Therefore, it is important to apply smart triggering mechanisms that are strong enough to quickly and completely release the drug-loaded inside theses liposomes. There are a number of drug release triggering mechanisms which have been reported in the literature, such as pH, temperature, enzymes and UV-light stimuli [Citation17–20]. In recent years, ultrasound has been reported as one of the best drug release triggering techniques [Citation21,Citation22]. This is due to the fact that it is a cost-limited non-invasive technique that allows focusing the beam precisely on the tumour. Ultrasound exposure of tumour tissue comprising sonosensitive liposomes may, not only, induce drug release from these carriers, but also increase intracellular drug uptake. Ultrasound consists of sound waves (acoustic waves) with a frequency higher than 20 kHz [Citation23]. When the ultrasound waves are traveling through a medium, a series of compression and rarefaction events occurs creating areas of high and low pressure, respectively. As the waves propagate in the medium, the particles of the medium oscillate in place forward and backward which mediates the progression of the waves [Citation24]. Depending on frequency, intensity (or power density) and length of exposure time, the US waves can be focused on and absorbed by tissues in several ways, so as to achieve different effects for a specific purpose. The therapeutic effects of US are divided into thermal and non-thermal. The non-thermal or mechanical effect is known as the “acoustic cavitation” [Citation22]. While the thermal effect is generally generated by high-intensity focused US (HIFU) in the continuous mode, the mechanical effects of ultrasound (cavitation) are predominantly generated from pulsed low-frequency ultrasound (LFUS).
In this study, the mechanical effect generated by pulsed LFUS at different densities will be used to trigger the release of the model drug “calcein” encapsulated in non-targeted and HSA-targeted liposomes. Calcein is a water-soluble self-quenching fluorescent dye; the relief of self-quenching is used as an indicator of calcein leakage from the encapsulating liposome. Ultrasound-mediated triggering of calcein release will result in enhancing calcein uptake by both breast cancer cell lines (MDA-MB-231 and MCF-7). In addition, LFUS generates transient or permanent pores in the walls of blood vessels at the tumour site (sonoporation) resulting in a significant enhancement of the extravascular delivery of therapeutics in the tumour site.
Materials and methods
Materials
1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000] (DSPE-PEG(2000)-NH2) were obtained from Avanti Polar Lipids Inc. (Alabaster, AL). HSA (Mw = 68 KD), Calcein disodium salt, bicinchoninic acid (BCA) kit, chloroform, cholesterol, Sephadex® G-100 and 2,4,6 trichloro-1,3,5 triazine (cyanuric chloride [CC]) were obtained from Sigma-Aldrich (St. Louis, MO), supplied through LABCO LLC. (Dubai, UAE). HeLa, MCF-7 and MDA-MB-231 cell lines were obtained from ECACC.
Preparation of non-targeted liposomes
The liposomes were prepared according to the modified lipid film hydration method described by Lasch et al. [Citation25]. The lipids 1,2-DPPC, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000] (DSPE-PEG(2000)-NH2) and cholesterol at a molar ratio of 65:5:30, respectively, were dissolved in chloroform in a round bottom flask. A lipid film was formed by removing the chloroform using a rotatory evaporator at 50 °C for 15 min. The film was then hydrated with 2 ml of 50 mM calcein (dissolved in phosphate buffer saline (PBS) and the pH adjusted to 7.4) using the rotatory evaporator (without applied vacuum) for 50 min at 60 °C followed by sonication at 60 °C using a sonication bath (Agar Scientific, Stansted, UK) for 2 min. The formed liposomes were then extruded at 60 °C through the 0.2 μm polycarbonate membrane using Avanti® mini-extruder (Avanti Polar Lipids, Inc., Alabaster, AL). The liposomes were purified using Sephadex® G-100 gel filtration (size exclusion chromatography) prepared with borate buffer (pH ∼ 8.5). The purified liposomes were collected and stored at 4 °C until used.
Preparation of targeted liposomes
The covalent attachment of the liposomes to the lysine residues of HSA was carried out using CC (2,4,6 trichloro-1,3,5 triazine) as a coupling agent. CC was reacted with the targeted liposomes at a 1:1 molar ratio with DSPE-PEG-NH2 for 3 h at 0 °C. A solution of HSA (50 µl) in Borate (pH ∼ 8.5) was then added dropwise to the liposomes (final concentration of 0.25 mg/ml) and the reaction was left to stir overnight at room temperature to allow the conjugation reaction to proceed. The un-reacted protein was then removed using Sephadex® G-100 gel filtration prepared with PBS buffer (pH ∼ 7.4).
Measuring the size of liposomes by dynamic light scattering
The mean size of the liposomes was determined by dynamic light scattering (DLS) using the DynaProVR NanoStarTM (Wyatt Technology Corp., Santa Barbara, CA). The hydrodynamic radius (Rh) of the diluted liposomes (10 µl in 1 ml PBS) was determined at room temperature.
Estimation of phospholipid content using Stewart assay
The phospholipid content of the liposomes was determined colourimetrically using the Stewart assay. The prepared liposomes were transferred to a round bottom flask and were dried in the rotary evaporator under vacuum. Chloroform (1 ml) was added to the flask followed by sonication for 20 s. Of 200 µl of the liposomes were then transferred a pyrex tube containing 1.8 ml chloroform. Then, 2 ml of ammonium ferrothiocyanate was added, and the mixture was sonicated for 20 s followed by centrifugation 10 min at 1000 rpm. The top dark layer was removed and discarded while the optical density of the bottom clear chloroform layer was measured using UV–Vis spectroscopy at Amax = 485 nm. Three replicates for each sample were used.
Protein quantitation using bicinchoninic acid assay (BCA)
The colorimetric BCA Protein Assay was used to estimate protein content in HSA-PEG liposomes. The BCA reagent was prepared by mixing QuantiProTM QA buffer: QuantiProTM QB: CuSO4 at a ratio of 25:25:1. Of 400 µl of the liposomes were added to Eppendorf tube containing 600 µl PBS buffer, 1 ml of the reagent were added, and the tubes were incubated 60 °C for 1 h. The absorbance of the samples was measured using UV–Vis spectroscopy at Amax = 562 nm. Three replicates for each sample were used.
Cell cultures
The MCF-7 and MDA-MB-231 breast cancer cell lines were cultured in RPMI medium supplemented with 10% heat-inactivated foetal bovine serum (FBS) and 1% penicillin-streptomycin (Sigma-Aldrich, St. Louis, MO). The cultures were maintained at 37 °C in a humidified atmosphere with 5% CO2. Cells were in the logarithmic growth phase by routine passage every 2–3 d and split when reaching confluence. For the cellular uptake of the liposomes studies, exponentially growing cells were harvested with 3 ml of trypsin (0.25% from Sigma-Aldrich, St. Louis, MO) and 3 × 105 cell/ml of growth medium were seeded in 6-well plates to reach confluency at the time of the experiment.
The physical stability of the control and HSA-PEG liposomes
Both non-targeted and the HSA-PEG liposomes were added to the RPMI medium supplemented with 10% heat-inactivated FBS and were incubated at 37 °C for 24 h. A comparison between calcein released from both types of liposomes was conducted using the QuantaMaster QM 30 Phosphorescence Spectrofluorometer (Photon Technology International, Edison, NJ). Of 50 µl of 1% (v/v) TX-100 was added to the sample cuvette to lyse the liposomes and release all the encapsulated calcein. The corresponding fluorescence intensity is characterized as 100% release.
Low-frequency ultrasound release studies (online experiments)
LFUS (20-kHz) was used to trigger the release of calcein encapsulated in the liposomes. Calcein release was monitored by fluorescence changes using a QuantaMaster QM 30 Spectrofluorometer (Photon Technology International, Edison, NJ). Calcein is a fluorescent molecule with excitation and emission wavelengths of 495 and 515 nm, respectively. To prepare the samples in the test cuvette, 75 µl of the liposomes were diluted with 3 ml of the PBS buffer. The initial fluorescence (Fo) was recorded for the first 60 s without sonication to generate a baseline. The sonication was then applied using a 20-kHz ultrasonic probe (model VCX750, Sonics & Materials Inc., Newtown, CT) on a pulsed mode with 20 s “on” and 10 s “off” cycle for 4 min. This was followed by the addition of 50 µl of 1% (v/v) Triton X-100 into the sample cuvette to lyse liposomes and release all the encapsulated calcein. The corresponding fluorescence intensity is characterized as 100% release. The percentage of calcein release was then calculated at a given time using the fluorescence intensity values obtained experimentally according to the following equation,
(1)
(1)
Where F is the fluorescence intensity at the time (t) of insonation, Fo is the average of the initial fluorescence intensity before exposing the sample to the US, and FTX-100 is the maximum fluorescence achieved after lysing liposomes.
Cellular uptake of the non-targeted and HSA-targeted liposomes
The breast cancer cell lines MDA-MB-231 and MCF-7 were incubated and sonicated with both the non-targeted and the HSA-PEG liposomes at a concentration of 200 µM of DPPC. The liposomes were added to 6-well plates containing the cancer cell lines for 30 min in humidified air at 37 °C and 5% CO2. The plates were then washed with PBS buffer and harvested with a trypsin solution for analysis using flow cytometry measurements (Beckman Coulter FC500). As aforementioned, samples were analyzed to measure calcein fluorescence intensity utilizing an excitation and emission wavelengths of 495 and 515 nm, respectively. At least three independent assays were performed for each treatment.
Application of low-frequency ultrasound on cancer cells incubated with the control and HSA-PEG liposomes
To study the effect of the LFUS on the cellular uptake of liposomes, both the control and the HSA-PEG liposomes were incubated with MDA-MB-231 and MCF-7 breast cancer cell lines at the same previous conditions. After the initial 60 min of incubation, the plates were continuously sonicated floating in a 40-kHz with a density of 1 W/cm2 (Branson 3510-DTH Ultrasonic Cleaner, Ohio, USA) for 5 min. No temperature increase was observed in cell-containing wells during the sonication. After ultrasound exposure, the plates were incubated for an hour in humidified air at 37 °C and 5% CO2. The plates were then washed with PBS buffer and harvested with trypsin solution for calcein uptake quantification using flow cytometry. In each insonation experiment, the sonolysis studies (the effect of US on cell viability) were performed using the Trypan blue exclusion assay, and the cell viabilities were always higher than 90%.
Statistical analysis
The differences between the results were compared using a two-tailed t-test with the assumption of unequal variances. Two values were considered significantly different when p ≤ .05.
Results
Preparation of HSA-PEG-conjugated liposomes
HSA-PEG-conjugated liposomes were prepared by the attachment of HSA to the PEG chain on the outside of the liposome surface. CC was used as a coupling agent linking the amino group (–NH2) of the many accessible primary amino groups (lysine) on the surface of HSA to the amino group on the PEG chain of DSPE-PEG(2000)NH2. Stewart and BCA assays were conducted to measure lipid content and confirm the attachment of the protein to the liposomes. The control sample for the assays was prepared by mixing a sample of the non-targeted liposomes with HSA without coupling agent CC. The mixture was then filtered through the size-exclusion gel, under similar conditions as that of HSA-PEG-conjugated liposomes. The Stewart assay showed that both the control and HSA-PEG-conjugated liposomes had no significant difference in their phospholipid concentration represented by the DPPC content showing values of (5.61 ± 0.18 and 5.24 ± 0.73 mg/ml, respectively, p = .832). The protein content was significantly higher in the HSA-PEG-conjugated liposomes compared to the control liposomes showing on average a 3-fold increase in protein content (0.35 ± 0.006 µg/ml for the control liposomes and 1.05 ± 0.43 µg/ml for the HSA-PEG liposomes, p = .0256) which confirms the conjugation of the HSA to the PEG liposomes.
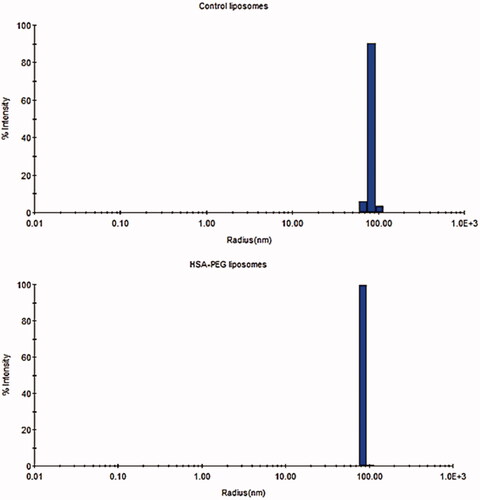
The size of the synthesized liposomes
The radius of the non-targeted (control) liposomes was on average 84.46 ± 0.97 nm with a polydispersity index (PDI) of 7.21 ± 1.42. HSA-PEG liposomes showed an average size of 84.86 ± 1.81 with a PDI of 9.68 ± 0.94 (). Therefore, both types of liposomes were unilamellar structures with no significant difference in size (p = .934).
In vitro release kinetics following insonation with LFUS
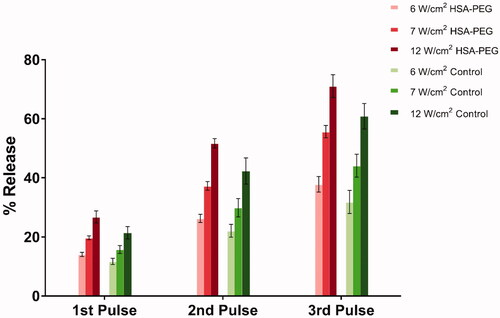
The rate and kinetics of drug release from the non-targeted (control) and HSA-PEG liposomes as a function of LFUS ultrasound exposure were evaluated using a frequency of 20 kHz in a pulsed mode (20 s “on” and 10 s “off”) for 4 min. In addition, the rate and kinetics of drug release were compared at three different power densities (6, 7 and 12 W/cm2, respectively). The normalized-averaged release profiles of the non-targeted (control) and HSA-PEG liposomes are shown in . Lysing the liposomes using Triton-X-100 showed that both the control and HSA-PEG liposomes release most of their encapsulated calcein by 4 min of the pulsed LFUS.
Figure 2. Normalized release profiles for non-targeted (control) and HSA-PEG liposomes triggered by pulsed 20-kHz LFUS for 8 min at three power densities [20% (6 w/cm2), 25% (7 w/cm2) and 30% (12 w/cm2)].
![Figure 2. Normalized release profiles for non-targeted (control) and HSA-PEG liposomes triggered by pulsed 20-kHz LFUS for 8 min at three power densities [20% (6 w/cm2), 25% (7 w/cm2) and 30% (12 w/cm2)].](/cms/asset/a0817301-e170-42b6-9d7b-bd0d6d341528/ianb_a_1573175_f0002_c.jpg)
As shown in , the percentage of calcein release significantly increased with the increasing intensities during the first three pulses for both types of liposomes. Interestingly, the HSA-PEG liposomes showed a higher rate of calcein release compared to the non-targeted liposomes following the first (p = .003), the second (p = .01) and the third (p = .019) pulses at the lowest power density used (6 W/cm2). The same was observed with the higher power density of 7 W/cm2 where HSA-PEG liposomes released more calcein compared to the non-targeted liposomes following the first (p = .00007), second (p = .0002) and the third (p = .00002) pulses. Furthermore, the similar pattern continued following the sonication using the highest power density (12 W/cm2) where HSA-PEG liposomes released more calcein compared to the control following the first (p = .0003), second (p = .0001) and the third (p = .0001) pulses. Overall, the highest percentage of calcein release was recorded with the highest intensity of 12 W/cm2 (30%) following the third pulse releasing 60.86 ± 7.1% of the calcein encapsulated in the control liposomes and 71.09 ± 5.5% of the calcein encapsulated in the HSA-PEG liposomes. No significant change in the size of the liposomes was recorded following the first three pulses in all power densities tested here.
Intracellular uptake and targeting potential of the HSA-PEG liposomes and the effect of low-frequency ultrasound
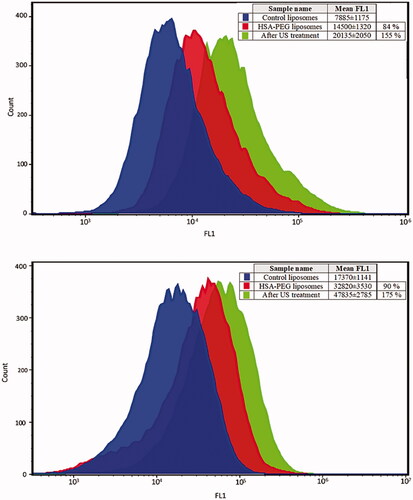
In this study, the subcellular internalization of the model drug calcein was evaluated by flow cytometry as shown in . The mean fluorescent intensity (MFI) of the HSA-PEG liposomes was significantly higher than that of the control liposomes in both the MDA-MB-231 and MCF-7 breast cancer cell lines showing 84% (p = .0002) and 90% (p = .0001) increase in calcein uptake, respectively, when compared to the non-targeted control liposomes. Sonication of both breast cancer cell lines (MDA-MB-231 and MCF-7), previously incubated with the HSA-PEG liposomes, by floating in a 40-kHz water bath significantly increased the intracellular uptake of calcein by the MDA-MB-231 breast cancer cell line by 155% compared to the control liposomes (p = .0001). The same was observed when the MCF-7 breast cancer cell line was sonication exhibiting a 175% increase in calcein uptake (p = .0009).
Figure 4. Enhanced Calcein uptake by the breast cancer cell lines MDA-MB-231 (top) and MCF-7 (bottom) following the incubation of these cells with HSA-PEG liposomes for 1 h. Exposure to ultrasound (40 kHz for 5 min) further enhanced calcein uptake by both cell lines. Results are average ± standard deviation of three liposome batches (three replicates each).
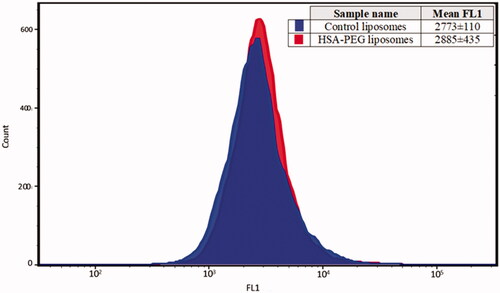
To confirm the specificity of albumin binding to the surface of the two breast cancer cell lines, the same experiment was conducted on the cervical cancer cell line “HeLa”. This cell line does not overexpress albumin receptors on its surface. Following the incubation of both the control and HSA-PEG liposomes with the HeLa cell line, the geometric means of the average cellular calcein uptake for control and the HSA-PEG liposomes were 2773 ± 110 and 2885 ± 435, respectively (). These values were not statistically different (p = .074) which indicated that the uptake of calcein in the HeLa cell line is not affected by the presence of the HSA on the surface of the liposomes. These findings show that the presence of the HSA receptors on the surfaces of the breast cancer cell lines MDA-MB-231 and MCF-7 could be utilized to effectively target cancer cells reducing the cytotoxicity and enhancing the efficacy of the encapsulated therapeutic drugs.
Figure 5. Calcein uptake by the cervical cancer cell line HeLa. No enhancement of calcein uptake was observed following the incubation of the cancer cells with HSA-PEG liposomes compared to the control liposomes. Results are average ± standard deviation of three liposome batches (three replicates each).
Physical stability of the control and HSA-PEG liposomes in foetal bovine serum medium
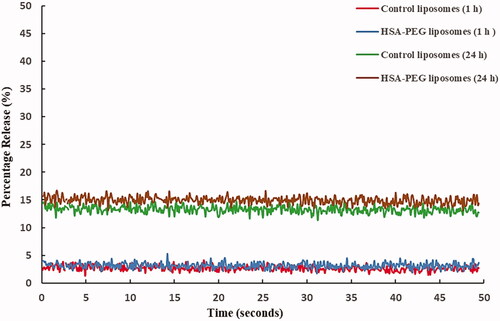
The stability of both the control and HSA-PEG liposomes incubated in FBS medium following 1 and 24 h incubation at 37 °C was analysed by comparing the rate of calcein release from these liposomes. Triton X-100 was used to lyse the cells releasing 100% of the encapsulated calcein. As shown in , both the control and HSA-PEG liposomes showed no significant difference in the rate of releasing the encapsulated calcein following 1 h (control= 2.69% ± 0.264; HSA-PEG= 3.35% ± 0.183, p = .818) and 24 h (control= 13.29% ± 0.303; HSA-PEG = 14.94% ± 0.272, p = .187) incubation. This result indicates that the higher uptake of calcein by the breast cancer cell line from HSA-PEG liposomes, compared to the control liposomes, is not due to the lower stability of the HSA-PEG liposomes.
Figure 6. Control and the HSA-PEG liposomes showed a similar level of stability when incubated in fetal bovine serum medium at 37 °C for 1 h and 24 h. No significant difference was observed in the amount of calcein released during the incubation period. Results are the average of three liposome batches.
Discussion
This study investigated the effect of coupling HSA into the surface of PEG-liposome triggered with LFUS on calcein uptake by the breast cancer cell lines MDA-MB-231 and MCF-7. Our data indicated that the synthesized pegylated liposomes loaded with the model drug calcein and conjugated to the HSA are stable at the physiological temperature in the presence of FBS medium. These targeted liposomes released only 14.94% of their load following 24 h incubation, in FBS medium, at 37 °C making them suitable for medical use as targeted drug carriers. Our results also showed that the synthesized liposomes were around 84.46 ± 0.97 nm in radius; HSA modification had an insignificant effect on the size of these liposomes showing a radius of (84.86 ± 1.81) nm. This is in agreement with Furumoto et al. [Citation14] who reported that serum albumin attached to the surface of the liposomes that were around 200 nm in diameter had no significant effect on their size. Generally, the pore size of tumour microvessels varies from 100 to 1200 nm in diameter. This would allow the extravasation of these targeted liposomes into tumour tissue but not into normal tissue. Thus, increasing the efficiency while reducing the toxicity of the encapsulated drug.
We applied ultrasound as an external stimulus to trigger the release of the encapsulated calcein from both the non-targeted and the HSA-targeted liposomes. The liposomes were sonicated with LFUS at 20-kHz at different power densities. A number of studies have reported the use of LFUS to trigger the release of the drugs encapsulated inside other nanocarriers, such as polymeric micelles [Citation26] and polymeric matrices [Citation27] as well as to enhance the permeability of biological membranes for drug and gene delivery [Citation28].
In this study, we reported that LFUS significantly enhanced calcein release from both the control and HSA-PEG liposomes. The highest power density used (12 W/cm2) was the most sufficient to cause cavitation in the solution capable of releasing high percentages of the encapsulated calcein in both types of liposomes. This is in agreement with previous studies which showed that applying LFUS (20 kHz) triggers the release of the drugs encapsulated inside the liposomes in a controlled manner [Citation21,Citation29,Citation30]. In addition, Levi [Citation31] showed that 20 kHz ultrasonic irradiation to be more efficient in drug release from liposomes than high-frequency ultrasound (1 and 3 MHz). This could be due to the fact that the intensity needed to induce transient cavitation is lower at low frequencies [Citation32].
The enhanced calcein release triggered by LFUS reported here is mainly due to the mechanical effect (acoustic cavitation) of the ultrasound. However, the energy released from the cavitation process results in a temperature rise. During sonication of the liposomes using the ultrasound probe, we observed an increase in temperature following the end of the third pulse (from 25 to 32 °C). This rise in temperature is still below the transition temperature (Tm) of the phospholipid DPPC (41.3 °C) but does not eliminate the role of the thermal effect of the temperature rise in enhancing the release. Previous studies have shown that ultrasonic absorbance by the lipid bilayer occurs during lipid phase transition, while the absorbance by the membrane is diminishing below the phase transition. This suggests that liposomal drug release, achieved when working below the phase Tm is attributed to mechanical and possible thermal effects due to the rise in temperature rather than absorbance of ultrasound by the lipid bilayer [Citation33,Citation34].
We reported no change in the size of the liposomes following the first three pulses of ultrasound in all the power densities tested here suggesting a pore-mediated release mechanism rather than a full distraction of the bilayer membrane of the liposomes. This is in agreement with Evjen et al. [Citation35] who reported that liposomes based on phosphatidylcholine showed evidence of pore-mediated release mechanisms. Earlier studies have shown that the presence of PEG on the surfaces of the liposomes was found to enhance the ability of LFUS (20 kHz) to permeabilize these liposomes [Citation21,Citation29]. DSPE-PEG has a lower packing parameter (0.5), and higher critical aggregation concentration (CAC) of (∼10 − 5 M) compared to the DPPC and other membrane lipids which have a higher packing parameter of 0.74–1.0 and a CAC value of ∼10 − 10 M. Therefore, DSPE-PEG is likely to be ejected out of the phospholipids bilayer to form micelles upon the exposure to ultrasound waves [Citation29,Citation32,Citation36]. Our results showed that the conjugation of HSA to the surface of the liposomes resulted in enhancing their sonosensitivity. HSA-PEG liposomes significantly released more calcein in all the power densities used here compared to the control liposomes. A possible explanation for this observation is that the attachment of the HSA molecule to DSPE-PEG has further weakened the packing parameter of the DSPE-PEG. Thus, enhancing the ejection of the DSPE-PEG from the liposomal membrane resulting in the enhanced calcein release.
To internalize molecules from outside the cell, cells use a process called endocytosis. Via this process, cells can take up macromolecules, proteins and ligands [Citation37]. The main endocytic routes are the clathrin-mediated endocytosis and the caveolae-mediated endocytosis. Caveolae are specialized membrane domains enriched in certain lipids cholesterol and proteins [Citation38]. Caveolae can mediate endocytosis through a receptor-dependent or -independent fashion [Citation39]. Caveolin-1 (Cav-1) is one of the main functional components of caveolae and plays an important role in caveolae formation. It expected that to induce tumour formation, rapid proliferation is required, and, therefore, downregulation of caveolin-1 expression may be necessary. The level of caveolin-1 is related to the invasiveness of the tumour [Citation40]. According to Chatterjee et al. [Citation41], nanoparticle conjugate of paclitaxel to HSA exhibits efficacy in pancreatic cancer, non–small cell lung cancer and breast cancer. The study found that Cav-1 protein levels correlated positively with cancer sensitivity to their albumin base nanoparticles and, therefore, caveolae are essential for the cancer uptake of albumin. In general, albumin binds to a cell-surface, 60-kDa glycoprotein (gp60) receptor (albondin). gp60 is localized in the caveolae and binds to caveolin-1 (an intracellular protein) with subsequent formation of the caveolae [Citation42,Citation43].
A study by Voigt et al. [Citation44] compared the uptake of the proteins bovine serum albumin (BSA) and transferrin between the HeLa cells and the human umbilical vein endothelial cells (HUVECs) using fluorescently labelled BSA which is known as markers for caveolae-mediated endocytosis and transferrin for clathrin-mediated endocytosis. These researchers found that although HeLa cells internalize more transferrin than HUVECs by three folds, a much stronger distinction in the uptake behaviour of BSA which was 32-fold higher in HUVECs in comparison with HeLa cells. This is in agreement with the Sharma et al. [Citation45] who reported that HeLa cells have low levels of Cav-1. A similar observation was recorded in this study were only breast cancer cell lines showed increased uptake of calcein from the HSA-PEG liposomes. Thus, we can conclude the coating of the pegylated liposomes with HSA enhances the uptake of the drug encapsulated inside these liposomes. This is due to the presence of the caveolae which is not the main route of endocytosis in the HeLa cells.
Sonoporation effect of the LFUS is more likely to be the mechanism behind the enhanced calcein uptake by both breast cancer cell lines reported here. Sonoporation will both improve cellular uptake of the liposomes and enhancing the release of the encapsulated calcein by rupturing the liposomes which contain similar membranes to that of the cells. Some studies have investigated the changes in cell morphology immediately after the exposure to acoustic cavitation and reported the formation of pores in the membrane [Citation46,Citation47]. In addition, other studies suggested that, following the exposure to LFUS, the levels of cellular uptake of calcein may be consistent with the levels of the pores formed in the membrane of these cells [Citation48,Citation49]. In a recent work published by our group [Citation50], we reported that the exposure of the estrone-positive breast cancer cell line (MCF-7) incubated with estrone-conjugated liposomes to LFUS revealed a statistically significant uptake of calcein compared to uptake without ultrasound. In this study, we report that no increase in the temperature was recorded during sonication suggesting that the mechanical effect of the LFUS led to the enhanced calcein uptake rather than the thermal effect. Furthermore, both the control and the HSA-PEG liposomes showed similar stability levels indicating that the higher uptake of calcein by the breast cancer cell line from HSA-PEG liposomes, compared to the control liposomes, is not due the lower stability of the HSA-PEG liposomes. While this study is important as a proof of concept since it is an in vitro study in the absence of the circulation with blood, future work should include in vivo breast tumour models to determine the ultimate therapeutic efficacy of this platform.
Conclusion
In summary, this study indicated that the HSA modification of pegylated liposome significantly enhanced their binding to the surface of the breast cancer cell line MDA-MB-231 and MCF-7, resulting in the enhanced uptake of the drug by cancer cells. The mechanical force produced via the ultrasound waves enhanced cellular uptake of the liposomes and triggered calcein release from the nanocarriers, thus further enhancing the calcein uptake by the cancer cells. Thus, our HSA-coated liposome coupled with ultrasound-mediated triggered drug release holds attractive potential in breast cancer chemotherapy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- McKnight JA. Principles of chemotherapy. Clin Tech Small Anim Pract. 2003;18:67–72.
- Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1:297–315.
- Bhushan B, Khanadeev V, Khlebtsov B, et al. Impact of albumin based approaches in nanomedicine: imaging, targeting and drug delivery. Adv Colloid Interface Sci. 2017;246:13–39.
- Maeda H, Wu J, Sawa T, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284.
- Cattel L, Ceruti M, Dosio F. From conventional to stealth liposomes: a new frontier in cancer chemotherapy. Tumori. 2003;89:237–249.
- Hu CM, Kaushal S, Tran Cao HS, et al. Half-antibody functionalized lipid-polymer hybrid nanoparticles for targeted drug delivery to carcinoembryonic antigen presenting pancreatic cancer cells. Mol Pharm. 2010;7:914–920.
- Merlot AM, Kalinowski DS, Richardson DR. Unraveling the mysteries of serum albumin-more than just a serum protein. Front Physiol. 2014;5:299.
- Stehle G, Sinn H, Wunder A, et al. Plasma protein (albumin) catabolism by the tumor itself–implications for tumor metabolism and the genesis of cachexia. Crit Rev Oncol Hematol. 1997;26:77–100.
- Soreide JA, Lea OA, Kvinnsland S. Cytosol protein content and prognosis in operable breast cancer. Breast Cancer Res Tr. 1991;20:25–32.
- Satoh H, Kamma H, Ishikawa H, et al. Expression of hnRNP A2/B1 proteins in human cancer cell lines. Int J Oncol. 2000;16:763–767.
- Sueoka E, Goto Y, Sueoka N, et al. Heterogeneous nuclear ribonucleoprotein B1 as a new marker of early detection for human lung cancers. Cancer Res. 1999;59:1404–1407.
- Stehle G, Sinn H, Wunder A, et al. The loading rate determines tumor targeting properties of methotrexate-albumin conjugates in rats. Anticancer Drugs. 1997;8:677–685.
- Germain P, Metezeau P, Hellio R, et al. Internalization and biological effects of serum albumin in the breast cancer MCF-7 and MDA-MB 231 cells. Cell Mol Biol (Noisy-le-Grand). 1995;41:1119–1129.
- Furumoto K, Yokoe J, Ogawara K, et al. Effect of coupling of albumin onto surface of PEG liposome on its in vivo disposition. Int J Pharm. 2007;329:110.
- Manzoor AA, Lindner LH, Landon CD, et al. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012;72:5566–5575.
- El-Kareh AW, Secomb TW. A mathematical model for comparison of bolus injection, continuous infusion, and liposomal delivery of doxorubicin to tumor cells. Neoplasia. 2000;2:325–338.
- Turk MJ, Reddy JA, Chmielewski JA, et al. Characterization of a novel pH-sensitive peptide that enhances drug release from folate-targeted liposomes at endosomal pHs. Biochim Biophys Acta. 2002;1559:56–68.
- O’Brien DF, Whitesides TH, Klingbiel RT. The photopolymerization of lipid-diacetylenes in bimolecular-layer membranes. J Polym Sci B Polym Lett Ed. 1981;19:95–101.
- Zhu L, Kate P, Torchilin VP. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano. 2012;6:3491–3498.
- Needham D, Anyarambhatla G, Kong G, et al. A new temperature-sensitive liposome for use with mild hyperthermia: characterization and testing in a human tumor xenograft model. Cancer Res. 2000;60:1197–1201.
- Lin HY, Thomas JL. PEG − lipids and oiligo(ethylene glycol) surfactants enhance the ultrasonic permeabilizability of liposomes. Langmuir. 2003;19:1098–1105.
- Ahmed SE, Awad N, Paul V, et al. Improving the efficacy of anticancer drugs via encapsulation and acoustic release. Curr Top Med Chem. 2018;18:857–880.
- Evjen TJ, Nilssen EA, Rognvaldsson S, et al. Distearoylphosphatidylethanolamine-based liposomes for ultrasound-mediated drug delivery. Eur J Pharm Biopharm. 2010;75:327–333.
- Moussa HG, Martins AM, Husseini GA. Review on triggered liposomal drug delivery with a focus on ultrasound. Curr Cancer Drug Targets. 2015;15:282–313.
- Lasch J, Weissig V, Brandl M. Preparation of liposomes. 2nd ed. In: Torchilin VP, Weissig V, editors. Liposomes—a practical approach. New York (NY): Oxford University Press; 2003. p. 3–30.
- Husseini GA, Christensen DA, Rapoport NY, et al. Ultrasonic release of doxorubicin from Pluronic P105 micelles stabilized with an interpenetrating network of N,N-diethylacrylamide. J Control Release. 2002;83:303–305.
- Kost J, Leong K, Langer R. Ultrasound-enhanced polymer degradation and release of incorporated substances. Proc Natl Acad Sci USA. 1989;86:7663–7666.
- Kinoshita M, Hynynen K. A novel method for the intracellular delivery of siRNA using microbubble-enhanced focused ultrasound. Biochem Biophys Res Commun. 2005;335:393–399.
- Schroeder A, Avnir Y, Weisman S, et al. Controlling liposomal drug release with low frequency ultrasound: mechanism and feasibility. Langmuir. 2007;23:4019–4025.
- Delalande A, Kotopoulis S, Postema M, et al. Sonoporation: mechanistic insights and ongoing challenges for gene transfer. Gene. 2013;525:191–199.
- Levi C. Ultrasound for targeted delivery of cytotoxic drugs from liposomes [M.Sc. thesis]. Beer Sheva, Israel: Ben Gurion University; 2000.
- Schroeder A, Kost J, Barenholz Y. Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chem Phys Lipids. 2009;162:1–16.
- Maynard VM, Magin R, Strom-Jensen P R, et al. Ultrasonic absorption by liposomes. In Ultrasonic Symposium. Vol. 2; 1983. p. 806–809.
- Tata DB, Dunn F. Interaction of ultrasound and model membrane systems: analyses and predictions. J Phys Chem. 1992;96:3548–3555.
- Evjen TJ, Hupfeld S, Barnert S, et al. Physicochemical characterization of liposomes after ultrasound exposure - mechanisms of drug release. J Pharm Biomed Anal. 2013;78–79:118–122.
- Ickenstein LM, Arfvidsson MC, Needham D, et al. Disc formation in cholesterol-free liposomes during phase transition. Biochim Biophys Acta. 2003;1614:135–138.
- Im J, Maiti K K, Kim W, et al. Cellular uptake properties of the complex derived from quantum dots and G8 molecular transporter. Bull Korean Chem Soc. 2011;32:1282–1292.
- Kumari S, Mg S, Mayor S. Endocytosis unplugged: multiple ways to enter the cell. Cell Res. 2010;20:256–275.
- Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194.
- Cokakli M, Erdal E, Nart D, et al. Differential expression of Caveolin-1 in hepatocellular carcinoma: correlation with differentiation state, motility and invasion. BMC Cancer. 2009;9:65–65.
- Chatterjee M, Ben-Josef E, Robb R, et al. Caveolae-mediated endocytosis is critical for albumin cellular uptake and response to albumin-bound chemotherapy. Cancer Res. 2017;77:5925–5937.
- John TA, Vogel SM, Tiruppathi C, et al. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am J Physiol Lung Cell Mol Physiol. 2003;284:L187–L196.
- Jiang Y, Wong S, Chen F, et al. Influencing selectivity to cancer cells with mixed nanoparticles prepared from albumin–polymer conjugates and block copolymers. Bioconjugate Chem. 2017;28:979–985.
- Voigt J, Christensen J, Shastri VP. Differential uptake of nanoparticles by endothelial cells through polyelectrolytes with affinity for caveolae. Proc Natl Acad Sci. 2014;111:2942.
- Sharma DK, Brown JC, Choudhury A, et al. Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol Biol Cell. 2004;15:3114–3122.
- Tachibana K, Uchida T, Ogawa K, et al. Induction of cell-membrane porosity by ultrasound. Lancet. 1999;353:1409.
- Jelenc J, Miklavčič D, Lebar AM, editors. Low-frequency ultrasound in vitro: experimental system and ultrasound-induced changes of cell morphology. 2013 36th international convention on information and communication technology, electronics and microelectronics (MIPRO); 2013 May 20–24; Piscataway, NJ, IEEE.
- Zarnitsyn V, Rostad CA, Prausnitz MR. Modeling transmembrane transport through cell membrane wounds created by acoustic cavitation. Biophys J. 2008;95:4124.
- Zhang JZ, Saggar JK, Zhou ZL, et al. Different effects of sonoporation on cell morphology and viability. Bosn J Basic Med Sci. 2012;12:64–68.
- Salkho NM, Paul V, Kawak P, et al. Ultrasonically controlled estrone-modified liposomes for estrogen-positive breast cancer therapy. Artif Cells Nanomed Biotechnol. 2018;12:1–11.