Abstract
Oral squamous cell carcinoma (OSCC) is one of the common type of cancer in humans. A combinatorial approach has been done by using paclitaxel (PTX), 5-fluorouracil (5-FU) and ascorbic acid (AA) loaded solid lipid nanoparticles (SLN) for its treatment. SLN were made by high-speed homogenization and ultrasonication technique and they were used alone and in combination to check their efficacy against OSCC induced animal model. Pharmacokinetics and biodistribution study of the optimized formulations for PTX, 5-FU and AA loaded SLN was performed. The SLN shows a biphasic nature of drug release both in the in-vitro and in-vivo system. SLN loaded with PTX in combination with SLN loaded with AA shows a greater potency in the treatment of OSCC in-vivo. The Pharmacokinetic and biodistribution studies of SLN depict a better therapeutic efficacy. The combination of PTX and AA loaded SLN can be a novel approach for the treatment of OSCC.
Graphical Abstract
Background
Oral Cancer is among one of the most common forms of cancer in human and affects mostly the oral cavity and pharynx [Citation1] and 90% of it gets originated in squamous cell [Citation2]. There are a multiple risk factors associated with the cause of oral cancer including an infection of the human papillomavirus, excessive use of tobacco, betel quid and alcohol [Citation3]. Tobacco smoking and alcohol intake play a synergistic role in the cause of oral cancer [Citation4] whereas; the risk of developing oral cancer is three times higher in smokers as compared to non-smokers [Citation5]. The prevalence of oral cancer has shown a higher increase rate in males as compared to that of females [Citation6], moreover, the death rate is approximately 50% worldwide [Citation7], which also represents a poor prognosis in developing countries [Citation8]. High recurrence rates are found in patients undergoing the standard treatment, and delay in initiation of treatment is considered as the most prominent cause for no relevant improvement in the survival rate [Citation9]. Moreover, conventional chemotherapy suffers from numerous drawbacks including poor drug specificity [Citation10], undesired side effects [Citation11] and resistance towards the treatment [Citation12].
Paclitaxel (PTX) is mostly used in the treatment of cancer as it has a wide range of anti-cancer activity against head and neck cancer, breast cancer, non-small-cell lung cancer, ovarian cancer and Kaposi's sarcoma [Citation11,Citation13,Citation14]. PTX promotes its anti-cancer activity by acting on the microtubules during the mitotic phase and thus enhances the polymerization of the tubulin; microtubules thus formed in its presence have unusual stability and thus it resists the depolymerization [Citation14]. 5-Fluorouracil (5-FU) is an anti-metabolite antitumour drug and it interferes with nucleic acid and DNA synthesis resulting in cell apoptosis [Citation15]. It is mostly used for the treatment of several solid tumours including colorectal, breast, liver and pancreatic cancer alone or in combination with other drugs [Citation16]. Ascorbic acid (AA) is reported to have a profound cytotoxic effect on many cell lines and animal models [Citation17,Citation18]. The cytotoxic action of AA is due to its interaction with different metal ions catalyzing its auto-oxidation with concomitant formation hydrogen peroxide and can scavenge free radicals in-vitro [Citation19,Citation20].
Among the various nano-particulate drug delivery system, solid lipid nanoparticle (SLN) can be cited as one of the most suitable ways to deliver anti-cancer drugs as the lipids used for its preparation are well tolerated by the physiological system [Citation21,Citation22]. Moreover, the rigid hydrophobic core and a monolayer of phospholipids of SLN make it one of the most stable forms of colloidal carriers and thus it remains in the solid state both in room and body temperature [Citation23].
The present research study includes preparation of PTX, 5-FU and AA entrapped SLN to ensure a sustained release of drug at the desired concentration and at a specific site for the treatment of oral cancer. The study involves evaluation of each of these SLN and their combination for the effective treatment of oral cancer. SLN entrapping PTX, 5-FU, and AA were evaluated both in-vitro and in-vivo to get the best combination for the effective therapeutic efficacy.
Methods
Materials
PTX was kindly gifted by Cipla LTD, India. 5-FU, AA, 4-Nitroquinoline-1-Oxide was procured from Sigma-Aldrich. HPLC grade Acetonitrile & Methanol, Acetone, Ethanol, Formalin, Benzene, Paraffin wax, Hematoxylin and Eosin was obtained from Merck. Stearic acid (St-A), Egg lecithin (EL), Glycerol and Pluronic F68 (PF68) was purchased from Himedia, India.
Preparation of SLN dispersion
PTX, 5-FU and AA SLN were made by high-speed homogenization and ultrasonication techniques [Citation24–26]. The PTX loaded SLN was prepared as reported earlier [Citation26]. For preparing 5-FU loaded SLN, 10 mg of 5-FU was dissolved in 3 ml of double distilled water and 10 mg of EL was dissolved in 3 ml of methanol. These solutions were mixed together under mechanical stirring to obtain a homogenous solution. 0.5 g of St-A was dissolved in 13 ml of acetone and it was then mixed with the previously made solution. The mixture thus obtained was added dropwise to the aqueous of PF68 kept at 70 °C. An emulsion state is obtained under stirring for 5 min with an Ultra-TurraxT25 (IKA-werke GmBH, Germany), at 12,000 rpm, 15,000 rpm and 18,000 rpm each. The emulsion thus obtained was sonicated for 15 min by using Ultrasonic processor (6 mm diameter, 20W), to inhibit the crystallization. The o/w nanoemulsion obtained was cooled down to room temperature by continuously stirring it for a time period of 8 h in a magnetic stirrer to form the SLN and for complete evaporation of the organic solvent. The aforementioned procedure is also followed for preparation of AA loaded SLN. SLN obtained thus as mentioned in were used for further studies after the Lyophilization [Citation27]. The concentration of each excipient and drug used for the preparation of nanoformulation are given in . During the preparation of SLN, the concentration of PTX, 5-FU and AA were kept same except for the blank, while in every formulation the concentration of lipid, emulsifier and surfactant were gradually increased to check their effect on the nanoparticles.
Table 1. Different composition of SLN formulation.
Characterization of SLN dispersion
Particle size and particle size distribution
Particle size and polydispersity index of the SLN were measured by dynamic laser light scattering by dispersing 1 mg of the lyophilized sample in 2 ml of Milli-Q water in Zetasizer NanoZS 90 (Malvern Instruments, Worcestershire, UK). All the measurements were done at 25 °C at a scattering angle of 90°.
Zeta potential (ζ)
Zeta potential of the formulation was performed by diluting 1 mg of the lyophilized SLN in 2 ml of Milli-Q water and were measured by using a disposable Zeta cell for zeta potential analysis by electrophoretic mobility method using Zetasizer NanoZS 90 (Malvern Instruments, Worcestershire, UK).
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM)
Scanning electron microscope (JEOL-JSM-6360, Japan) and Transmission electron microscope (FEI, Tecnai G2 Spirit Bio Twin, Czech Republic) were used to observe the surface morphology of the SLN. The procedures were followed as reported earlier [Citation26].
Drug entrapment efficiency and drug loading determination
For determination of drug loading and entrapment efficacy, a volume of 2 ml of each of the drug-loaded samples was centrifuged at 12,500 rpm for a time period of 45 min to separate the lipid and the aqueous phase. The supernatant after centrifugation was collected then diluted and analyzed using HPLC at the respective wavelength of each drug as reported [Citation28].
In-vitro drug release studies of SLN dispersion
The in-vitro drug release study from the each lyophilized SLN entrapping PTX, 5-FU and AA were performed by using phosphate buffered saline (PBS) pH 7.4. It was performed by dispersing SLN (equivalent to 1 mg of drug) in 2 ml of PBS and which was further placed in a cellulose membrane bag (regenerated seamless cellulose tubing with molecular weight cut-off between 12,000–14,000 kDa, Hi-Media, Mumbai, India) and finally placed in a beaker containing 200 ml of PBS as reported [Citation26,Citation29,Citation30]. The drug content was analyzed by using HPLC.
Drug release kinetic study
The drug release kinetics was studied by fitting the data obtained from the in-vitro release of drug from SLN in various kinetic equations. To investigate the drug release mechanism, mathematical expressions were used in the study [Citation31].
Compatibility studies
The compatibility study was performed for PTX, 5-FU, and AA entrapped SLN by FTIR and DSC analysis.
FTIR Study
In the compatibility study, each of the drugs, excipients, a physical mixture of the drug with an excipient and optimized SLN of each type were analyzed by scanning them over a wavenumber range of 4000–400 cm−1 in FTIR spectrophotometer (Alpha E, Bruker Alpha, Ettlingen, Germany).
DSC Study
To check the compatibility of each drug, excipients, physical mixture of each drug and the optimized SLN of each type, they were heated in a range of 20–300 °C with an increasing temperature rate of 10 °C/minute using (JADE-DSC Perkin-Elmer, Japan).
Stability studies of SLN dispersion
The stability study was done for the optimized formulation of SLN entrapping PTX, 5-FU and AA as per ICH Q1 AR2 guidelines meant for the lyophilized product for a period of 3 months [Citation26,Citation32].
High-performance liquid chromatography analysis (HPLC)
The analysis of PTX, 5-FU and AA was conducted by using a high-performance liquid chromatography (HPLC) with ultraviolet detection. The chromatographic separation was achieved by using a reverse phase C18 column (Phenomenex C18, 150 × 4.6 mm, 5 μm) fitted in Shimadzu prominence (UFLC, LC 20AD, Kyoto, Japan). The HPLC was equipped with Shimadzu LC-20AD PLC pump and SPD-M20A PDA detector and analysis software Shimadzu LC. The mobile phase used for the analysis of PTX was composed of acetonitrile:water (25:75) at a wavelength of 227 nm and the flow rate was 1.0 ml/min. The mobile phase used for the analysis of 5-FU was composed of acetonitrile:water (50:50) at a wavelength of 266 nm with a flow rate of 0.8 ml/min. The mobile phase used for analysis of AA was composed of methanol:water:glacial acetic acid (30:69:1) containing 10g/L of sodium chloride at 279 nm with a flow rate of 0.8 ml/min.
Animal study design
For in-vivo study Swiss albino mice weighing 20–25 g of either sex at a ratio of 1:1 and 3–4 weeks old were used and the in-vivo experimental protocol was approved by Institutional Animal Ethical Committee, Gauhati University with approval no. IAEC/PER/2015–16/02. Animals were kept in individual cages and fed with standard diet and water ad libitum.
Swiss albino mice were divided into 8 respective groups of 12 mice each for the study. Group-1 (Normal control group), Group-2 (Negative control group), and Group-3, Group-4, Group-5, Group-6, Group-7 and Group-8, together were designated as the test group. The treatments were started in the test group after 16 weeks of induction period and it was continued for a period of 4 weeks. The doses were given intravenously at durations of 48 h. The treatments in the respective groups are shown in .
Table 2. In-vivo experimental design.
In-vivo anti-Cancer study
The Oral squamous cell carcinoma in the mice model was induced by mixing 4-NQO in drinking water [Citation33]. Briefly, 4-NQO was mixed in the drinking water in a concentration of 100 µg/ml and given to the mice for a period of 16 weeks for induction of the Oral squamous cell carcinoma. After the induction period, representative mice from each group were confirmed for the formation of oral squamous cell carcinoma and then the treatment were initiated for a period of 4 weeks. The efficacy of the treatment was checked in the respective group during the treatment phase by sacrificing a representative number of mice at a period of 2 weeks and 4 weeks by comparing them with the normal control group.
Histopathological study
Tongue from the sacrificed animals from the respective groups and the time interval of the treatment has been used for the histopathological study to confirm the efficacy of the treatment. The sides were prepared and studied as reported earlier [Citation26].
Pharmacokinetic and biodistribution studies
The Pharmacokinetic and Biodistribution studies of the SLN were performed by using Swiss Albino mice of weights 20–25 g of either sex. Briefly, for the pharmacokinetic study of PTX, 5-FU and AA loaded SLN, the animals were divided into three groups and they were further divided into two sub-groups as test (PTX-T, 5-FU-T and AA-T) and control (PTX-C, 5-FU-C and AA-C). The control group received drugs at a dose of 10 mg/kg whereas, the test group received SLN (at an equivalent dose) through intravenous routes. After the administration of formulation and pure drugs to the respective groups of animals, blood samples were obtained in 0.25, 1, 2, 4, 6, 12, 18 and 24 h. The blood samples were collected in heparinized vials and were followed by separation of plasma which was further stored at −20 °C.
For biodistribution study, separate groups of the animal were made similar to the pharmacokinetic study and they were treated with the developed SLN formulation and pure drug. After 24 h, the animals were sacrificed by using a high dose of diethyl ether and different organs including liver, spleen, kidney, lung, brain and heart were isolated. The isolated organs were stored −80 °C prior to further processing and analysis of the drug content.
Plasma and tissue sample processing
The plasma samples were allowed to thaw at room temperature (20–28 °C), afterwards, to a solution of 100 µL plasma, 150 µL acetonitrile was added to precipitate the protein followed by brief vortex and centrifugation at 14,000 rpm at 4 °C for 10 min. The supernatant was collected and further processed for HPLC analysis. The pharmacokinetic parameters data were analyzed by following non-compartmental method by using Microsoft Excel Add-in PK solver. The data were expressed as mean ± standard deviation (SD) (n = 5).
The tissue samples were similarly thawed at room temperature. The tissues were homogenized with saline and the resultant solution was deproteinized with acetonitrile and further processing was done similar to the plasma samples.
Statistical analysis and graphing
All the results were statistically analyzed and plotted in the graph by using GraphPad Prism v5.01 (GraphPad Software, San Diego, California). The results were expressed as the mean ± the standard deviation. Statistical differences were analyzed by using Student’s t-test and the data were considered significant at p < .05.
Results
Characterization of SLN dispersion
Particle size and particle size distribution
The particle size of SLN entrapping PTX ranges from 78.82–587.8 nm, whereas the particle size of SLN entrapping 5-FU were in the range of 117.5–511.6 nm and the particle size of SLN entrapping AA were in the range 143.1–456.7 nm as shown in .
Table 3. Physicochemical evaluation of solid lipid nanoparticles.
Zeta potential (ζ)
The Zeta potential of SLN entrapping PTX were in the range of −25.2 mV to −35.9 mV, whereas the Zeta potential value of SLN entrapping 5 − FU were in the range of −28.8 mV to −32.9 mV. Similarly, the Zeta potential values of SLN entrapping AA were in the range of −28.1 mV to −31.7 mV as shown in .
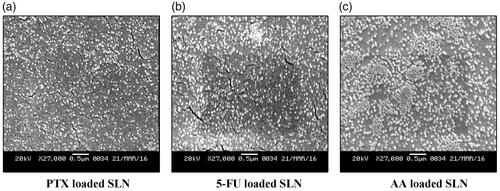
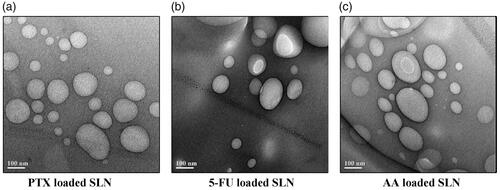
Scanning electron microscopy study and transmission electron microscopy study
The nanoparticles depicted a spherical and disc-like morphology, which were in accordance with the results of particle size analysis. The SEM and TEM micrograph of optimized formulations of SLN entrapping PTX, 5-FU and AA were shown in and respectively.
Percent yield efficiency of SLN formulations
The % yield efficiency of SLN entrapping PTX, 5-FU and AA was found to be in the range 60.97–66.19, 58.74–61.24 and 52.8–61.26 as shown in .
Drug entrapment efficiency and drug loading determination of SLN
The drug entrapment efficiency and drug loading of SLN entrapping PTX, 5-FU and AA was in the range of 61.6–73.1% and 11.25–31.6%, 58.9–68.1% and 11.87–28.9%, 54.4–62.1% and 16.3–30.4% respectively as shown in .
In-vitro drug release studies of SLN dispersion
The optimized formulation of PTX, 5-FU and AA loaded SLN showed 75 ± 5%, 70 ± 3% and 64 ± 3% release of drugs when observed for a period of 24 h. Each of the samples was analyzed in triplicates (n = 3).
Release kinetics study
The results of release are given in and from the table, it could be concluded that the release from the SLN to be anomalous (non-Fickian) diffusion.
Table 4. Release kinetics of SLN.
Compatibility studies
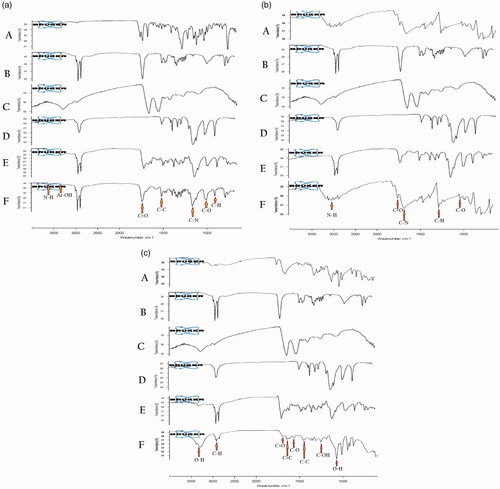
FTIR study
The combined FTIR spectrum of SLN entrapping PTX, 5-FU and AA clearly reveal the presence of a functional group of the drug in the SLN as shown in . In the combined FTIR spectrum, A denotes the drug, B denotes St-A, C denotes EL, D denotes PF68, E denotes physical mixture and F denotes the SLN formulation respectively. The presence of a functional group of the drug in the formulation clearly reveals it inertness.
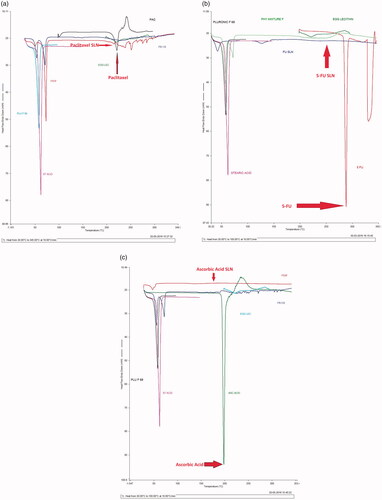
DSC study
DSC analysis of PTX showed a sharp endothermic peak at 217 °C; whereas the corresponding peak of PTX at 217 °C in the PTX loaded SLN was disappearing shown in . DSC analysis of 5-FU showed an endothermic peak at 288.04 °C and the corresponding peak of 5-FU disappeared in the 5-FU loaded SLN as shown in . DSC analysis of AA showed an endothermic peak at 198.01 °C and the corresponding endothermic peak in the AA loaded SLN was disappeared as shown in .
Stability of SLN dispersions
The particle size distribution, particle size and zeta potential of the formulation was observed in the interval of 30, 60 and 90 days are shown in . The formulations were found to be stable as concluded from the studied parameters.
Table 5. Results of stability studies of SLN.
Animal study design
In-vivo efficacy study
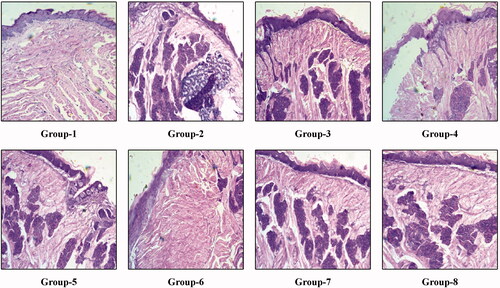
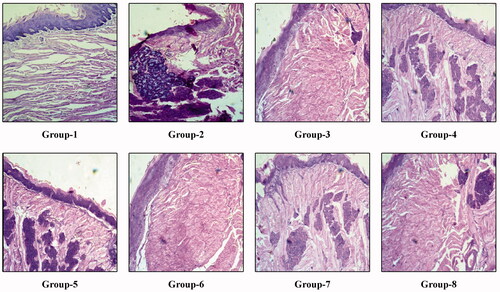
The mice were sacrificed after a period of 2 weeks from the initiation of treatment phase to inspect the efficacy of the treatment. The histopathological changes after 2 weeks of treatment in the control group (Group-1), negative control group (Group-2) and the remaining test group are shown in the . After 2 weeks from the initiation of treatment, we have seen in the representative tongue tissue of Group-3 mice showed moderate dysplasia with in-situ carcinoma formation. Similarly, Group-4 mice showed epithelial hyperplasia with in-situ carcinoma formation. Group-5, Group-7 and Group-8 mice showed moderate dysplasia with in-situ carcinoma formation. Group-6 mice showed mild dysplasia with low in-situ carcinoma formation. Group-2 mice showed moderate dysplasia with well-differentiated squamous cell carcinoma, whereas Group-1 mice showed normal tongue histology with sharp conical projections of filiform papillae with thin smooth keratinized epithelial covering. The treatment was again continued for another period of 2 weeks and again at the end of 4 weeks of treatment, the histological changes observed are shown in . Group-3 mice showed hyperkeratosis with a mild presence of in-situ carcinoma. Group-4 and Group-5 mice showed moderate dysplasia with the presence of in-situ carcinoma. Group-7 showed epithelial hyperplasia with a significant presence of in-situ carcinoma, whereas Group-8 showed moderate dysplasia with the prominent formation of squamous cell carcinoma. Group-6 showed moderate dysplasia with a negligible presence of in-situ carcinoma. Group-2 mice showed the formation of invasive squamous cell carcinoma, whereas Group-1 showed the normal tongue histology with sharp conical projections of filiform papillae with thin smooth keratinized epithelial covering.
Pharmacokinetic and biodistribution studies
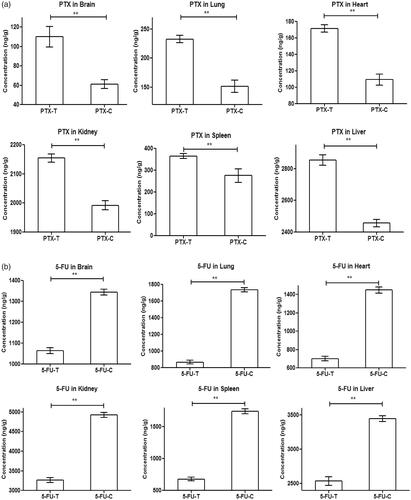
The in-vivo drug release from SLN entrapping PTX, 5-FU and AA were determined for a period of 24 h and the results have been shown in . In the figures, the single asterisk symbol (*) represents statistical significance (p < .05), whereas the double asterisk symbol (**), represents statistical significance (p < .01) of drug concentration at the respective time period between the test and the control group.
Figure 7. Pharmacokinetic profile of (a) PTX loaded SLN, (b) Pharmacokinetic profile of 5-FU loaded SLN, (c) Pharmacokinetic profile of AA loaded SLN.
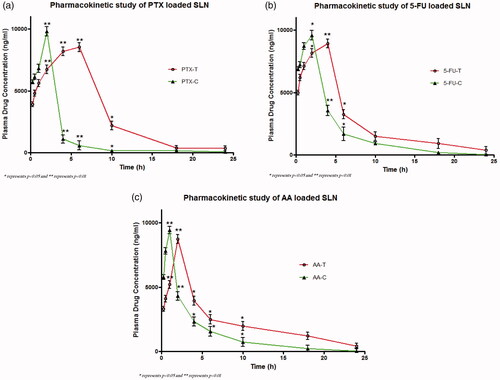
For all the formulations, respective pharmacokinetic parameters including Tmax, Cmax, AUC0–24, AUC0–∞, MRT0–∞, Vd, CL, Kel and t1/2 have been determined and the results have been shown in . In the pharmacokinetic study, we have seen an initial burst release followed by sustained release of drugs from the SLN. The Tmax of PTX, 5-FU and AA loaded SLN in the test group were significantly higher (p < .0001) as compared to their respective control groups. The Cmax of PTX, 5-FU and AA loaded SLN in the test group were higher than that of the control group. Both the AUC0–24 and AUC0–∞ of PTX, 5-FU and AA in the test group were found to be significantly higher (p < .0001) as compared to their respective control groups. The MRT0–∞ of PTX loaded SLN in the test group were higher than the control group (p < .0001) and the MRT0–∞ of AA loaded SLN in the test group were higher than the control group (p < .01) but no statistical significance was observed between the MRT0–∞ 5-FU loaded SLN in the test group and their control group. The pharmacokinetic parameter Vd and CL of PTX, 5-FU and AA loaded SLN in the test group were found to be significantly higher (p < .0001) as compared to their respective control groups. The Kel of PTX loaded SLN in the test group was found to be significantly higher (p < .0001) as compared to control group and the Kel of AA loaded SLN in the test group were higher than the control group (p < .05) but no statistical significance observed between Kel of 5-FU loaded SLN in the test and control group. The t1/2 of PTX loaded SLN in the test group were significantly higher (p < .0001) as compared to their respective control group and similarly the t1/2 of AA loaded SLN were higher (p < .05) as compared to their respective control group but no significant differences in the t1/2 were found among 5-FU loaded SLN and their respective control group.
Table 6. Results of pharmacokinetic studies of SLN.
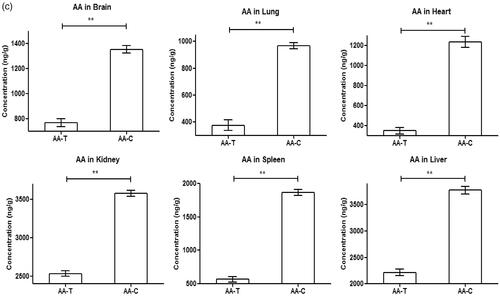
The biodistribution of the drugs released from the SLN in different organs have been shown in . In the figures, the double asterisk symbol (**) represents statistical significance (p < .01) differences. In the biodistribution studies of PTX loaded SLN, highest amount of PTX accumulation was found in liver and it was similar in the control group. The overall magnitude of concentration of PTX is higher in the test group as compared to that of control group shown in . In the biodistribution studies of 5-FU loaded SLN, the highest amount of 5-FU accumulation was found in the kidney and it was similar in the control group except least amount was found in the heart. The overall magnitude of concentration of 5-FU is higher in the control group as compared to that of test group shown in . In the biodistribution studies of the AA loaded SLN, the highest amount of AA accumulation was found in kidney and the highest amount was found in liver in the control group. The overall magnitude of the concentration of AA is higher in the control group as compared to that of test group as shown in .
Figure 8. Biodistribution of (a) PTX loaded SLN, (b) Biodistribution of 5-FU loaded SLN, (c) Biodistribution of AA loaded SLN.
Discussion
In the present research study, we have tried to investigate a novel combination of SLN encapsulating anti-cancer drugs against oral squamous cell carcinoma. Attempts have been made earlier for treating Oral squamous cell carcinoma using PTX and 5-FU alone or in some form of combination [Citation34–36]. Despite having anticancer effects, AA also has been reported to sensitize cancer cells to chemotherapy apart from reducing the toxicity of chemotherapy [Citation37]. SLN have been chosen to be the best mode of anticancer drug delivery since they are generally made up of physiological lipids thus they are well accepted by our physiological system [Citation38]. Due to the presence of drug within the solid core of SLN the drugs achieve greater stability, moreover, their release pattern from it remains sustained [Citation21]. As the SLN has the capacity to entrap the drug within its solid core for a longer time, it thus reduces the toxicity and increases the residence time of the drug within the body [Citation39]. The SLN entrapping PTX, 5-FU and AA alone or in combination thus can be an effective therapeutic approach for the treatment of oral squamous cell carcinoma. Following the preparation of SLN by high-speed homogenization and ultrasonication method, the integrity of drug within the SLN has been checked by using FTIR spectrums. The SLN entrapping PTX, 5-FU and AA showed the specific peak for the respective drug, which reveals the compatibility and integrity of the drug within the SLN. PTX, 5-Fu and AA showed sharp endothermic peaks at 217 °C, 288.04 °C and 198.01 °C, respectively, but these peaks were missing in SLN loaded with PTX, 5-FU and AA during DSC analysis. The absence of peak of the respective drug in the SLN may be due to either conversion of the drug to amorphous form during their process of manufacturing or may be due to their homogenous dispersion within the SLN [Citation40,Citation41]. PTX, 5-FU and AA loaded SLN showed a gradual increase of size with the increase of the lipid concentration used for their formulation. Moreover, it has been observed that for a particular amount of lipid used for the formulation, the size of nanoparticles formed were found to get decreased with increased concentration of the surfactant. The decrease in the size of SLN with increased concentration of surfactant may be due to stabilization of smaller lipid droplets by a high concentration of surfactant preventing the coalescing process [Citation42]. The zeta potential of the SLN entrapping the drug depends on the surface charge on the lipid matrix. It has been observed that the zeta potential value of SLN F3-P is more compared to SLN F6-P and SLN F9-P. The high zeta potential value of SLN F3-P may be due to high concentration of EL, as it may have formed an electric double layer over SLN moreover, it might have stabilized it with its steric properties [Citation28,Citation43]. The zeta potential of SLN F6-5FU is more than SLN F3-5FU and SLN F9-5FU moreover; the zeta potential of SLN F6-AA is more than that of SLN F3-AA and SLN F9-AA. The greater zeta potential value of optimized SLN loaded with PTX, 5-FU and AA depicts their stability over the rest of the formulations. It has been observed that significant differences exist in the value of zeta potential of drug loaded and blank SLN, which may be due to differences in the SLN composition and also may be due to the presence of drugs adsorbed on the peripheral surface of SLN [Citation44,Citation45]. The optimized SLNs were subjected for surface morphology study using a scanning electron microscope and they showed spherical shape with uniform distribution and low agglomeration. Transmission electron microscopy has been used to observe the optimized SLN formulations and the micrograph revealed a regular spherical shape. It has been observed that the percent yield of SLN decreased inversely with increasing concentration of lipid used for formulation, which may be contributed by the poor recovery of SLN due to the sticky nature of lipid used in it. The entrapment efficiency of SLN F3-P, SLN F6-5FU and SLN F6-AA were found to be greater among all the other SLN of their respective composition, which may be due to the high content of emulsifier used in them resulting in increasing lattice density to entrap more amount of drug in them [Citation46]. The high amount of emulsifier used for their formulation might have formed a layer at the surface of the SLN to increase drug entrapment [Citation46,Citation47]. The drug from the SLN got released in a biphasic nature as initially it burst released, followed by a sustained release in in-vitro conditions. The burst released pattern of the drug from the SLN may be contributed by the surface release of poorly entrapped drug within the SLN [Citation48]. The biphasic nature of drug release from SLN is a desirable property as the initial burst release may provide the requisite quantity for initiation of therapy whereas the sustained release property ensures a constant bioavailability of the drug for prolonged time. From the stability study data of the SLN entrapping PTX, 5-FU and AA, the stability of the SLN has been confirmed for a period of 90 days, but storage of SLN at high temperature around 40 °C is not preferable as it may affect the size and zeta potential of the SLN. The kinetic energy of a system increases with an increase in temperature which may result in increased collision of nanoparticles and thus can cause agglomeration [Citation49].
In this study, we have simulated oral cancer pathology in the animal model by using 4-NQO. The in-vivo study was performed to explore the efficacy of the nanoformulation against oral cancer and the best effective combination for the very same. After the induction of oral cancer in the animal model for a period of 16 weeks, we have checked, visually, for the formation of any lesions in the tongue of the animals. After visually inspecting and confirming the formation of oral cancer lesion, the treatment with the prepared nanoformulation was started as per the experimental protocol aforementioned. The treatment period was continued for a period of 4 weeks and the efficacy of the treatment is being checked in the entire representative group at the duration of second and fourth week. At the end of the second week of treatment, we observed that the Group-2 mice showed well-differentiated squamous cell carcinoma but, at the end of the fourth week of treatment they showed invasive squamous cell carcinoma. At the end of the second week, mice in Group-3 treated with SLN entrapping PTX showed moderate dysplasia with in-situ carcinoma formation, which after completion of the fourth week of treatment showed hyperkeratosis with the presence of mild in-situ carcinoma indicating its efficacy in the treatment of oral cancer. In Group-4, the mice were treated with SLN entrapping 5-FU where at the end of the second week of treatment phase they showed epithelial hyperplasia with in-situ carcinoma formation and at the end of the fourth week of treatment, they showed moderate dysplasia with the presence of in-situ carcinoma. In Group-5, the mice were treated with SLN entrapping AA where at the end of the second week of treatment phase they showed moderate dysplasia with the presence of in-situ carcinoma and even at the end of the fourth week of treatment they showed a similar result, which depicts its ineffectiveness in the treatment of oral cancer. The Group-7 mice received SLN entrapping 5-FU and SLN entrapping AA, at the end of second week of treatment phase they showed moderate dysplasia with presence of in-situ carcinoma, whereas at the end of the fourth week of treatment phase they showed epithelial hyperplasia with significant presence of in-situ carcinoma which depicts the ineffectiveness of the combination in the treatment of oral cancer. Group-8 mice received SLN entrapping PTX and SLN entrapping 5-FU, at the end of the second week of treatment phase they showed moderate dysplasia with the in-situ formation of carcinoma and at the end of the fourth week of treatment phase they showed moderate dysplasia with the prominent formation of squamous cell carcinoma depicting a recurrence stage and ineffectiveness of the combination. The Group-6 mice received SLN entrapping PTX and SLN entrapping AA, at the end of second week of the treatment phase they showed mild dysplasia with low in-situ formation of carcinoma, whereas at the end of the fourth week of treatment phase they showed moderate dysplasia with negligible presence of in-situ carcinoma, depicting the optimum combination for the treatment of oral cancer. The combination of SLN entrapping PTX and SLN entrapping AA has shown the best effective combination for the treatment of oral cancer, although the AA was not efficient enough in the treatment of oral cancer when given alone in combination with PTX, it has shown best therapeutic efficacy.
In the pharmacokinetic study of PTX loaded SLN, the magnitude of Cmax for the control group is higher as compared to the test group as the drug was released from that of SLN. We found a high value of AUC0–24 and AUC0–∞ in the test group as compared to that of the control group which depicts a high amount of drug availability in the plasma for a longer duration of time. This may be due to the sustained release behavior of the drug from the SLN. The MRT0–∞ of PTX in the test group was more as compared to that of control group thus revealing the sustained release behavior of the SLN. The sustained release behavior of SLN also contributed to greater t1/2 of PTX in the test group as compared to that of the control group. Similar, results were also obtained in case of 5-FU and AA loaded SLN, thus revealing the efficacy of the SLN in the treatment of oral cancer. The high volume of distribution of drugs in the control groups as compared to that of test groups indicates a greater amount of drug availability in the plasma in the test group. In the biodistribution studies of PTX, we have found a high amount of drug accumulation in the tissue in the test group as compared to that of control. But, in the biodistribution studies of 5-FU and AA loaded SLN, high amount of drug accumulation in the tissue was found in the control group as compared to that of the test group.
Conclusion
The research study conducted on the mice model induced with oral squamous cell carcinoma is to investigate a combination therapy of drugs. From the performed study it may be concluded that PTX, 5-FU and AA can be successfully entrapped within the SLN by using high-speed homogenization and ultrasonication method. The in-vitro results depicted the efficacy of the formed nanoparticles in the treatment of oral squamous cell carcinoma. Considering the H & E staining procedure as the standard diagnostic tool for detection of oral squamous cell carcinoma, we have performed the in-vivo efficacy assay of the developed formulation, which results clearly revealed the efficacy of PTX loaded SLN in combination with AA loaded SLN is quite efficient enough in the treatment of oral cancer. The result outcome of the in-vivo study clearly depicts efficacy of Paclitaxel and Ascorbic acid (1:1) combination in the treatment of the oral squamous cell carcinoma. Thus we can conclude that PTX and AA entrapped in SLN combination can be effectively used for the treatment of oral cancer.
Acknowledgements
The author acknowledges Laboratory of Molecular Virology and Oncology, Department of Bioengineering and Technology, Gauhati University, Guwahati for providing the facilities to conduct the research study.
Disclosure statement
The author confirms that this article content has no conflicts of interest.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/21691401.2020.1843782)
References
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316.
- Lingen MW, Kalmar JR, Karrison T, et al. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008;44:10–22.
- Mashberg A, Boffetta P, Winkelman R, et al. Tobacco smoking, alcohol drinking, and cancer of the oral cavity and oropharynx among U.S. veterans. Cancer. 1993;72:1369–1375.
- Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122:155–164.
- Petti S. Lifestyle Risk Factors for Oral Cancer. Oral Oncol. 2009;45:340–350.
- Kantola S, Parikka M, Jokinen K, et al. Prognostic factors in tongue cancer – relative importance of demographic, clinical and histopathological factors. Br J Cancer. 2000;83:614–619.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
- Shah JP, Lydiatt W. Treatment of cancer of the head and neck. CA Cancer J Clin. 1995;45:352–368.
- Ratain MJ, Mick R. Principles of pharmacokinetics and pharmacodynamics. In: Schilsky RL, Milano GA, Ratain MJ, editors. Principles of antineoplastic drug development and pharmacology. New York (NY): Marcel Dekker; 1996. p. 123–42.
- Lowenthal RM, Eaton K. Toxicity of chemotherapy. Hematol Oncol Clin North Am. 1996;10:967–990.
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265.
- Links M, Brown R. Clinical relevance of the molecular mechanisms of resistance to anti‐cancer drugs. Expert Rev Mol Med. 1999;14:1–21.
- McGuire WP, Rowinsky EK, Rosenshein NB, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989;111:273–279.
- Spencer CM, Faulds D. Paclitaxel: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the treatment of cancer. Drugs. 1994;48:794–847.
- Zhang XQ, Zhang HM, Sun XE, et al. Inhibitory effects and mechanism of 5-fluorouracil combined with celecoxib on human gastric cancer xenografts in nude mice. Exp Ther Med. 2015;9:105–111.
- Schilsky RL, Hohneker J, Ratain MJ, et al. Phase I clinical and pharmacologic study of eniluracil plus fluorouracil in patients with advanced cancer. J Clin Oncol. 1998;16:1450–1457.
- Bram S, Froussard P, Guichard M, et al. Vitamin C preferential toxicity for malignant melanoma cells. Nature. 1980;284:629–631.
- Chen Q, Espey MG, Krishna MC, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA. 2005;102:13604–13609.
- Sestili P, Brandi G, Brambilla L, et al. Hydrogen peroxide mediates the killing of U937 tumor cells elicited by pharmacologically attainable concentrations of ascorbic acid: cell death prevention by extracellular catalase or catalase from cocultured erythrocytes or fibroblasts. J Pharmacol Exp Ther. 1996;277:1719–1725.
- Chen Q, Espey MG, Sun AY, et al. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci USA. 2008;105:11105–11109.
- Müller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur J Pharm Biopharm. 2000;50:61–77.
- Triplett MD. Enabling Solid lipid nanoparticle Drug Delivery Technology by Investigating improved production techniques. Athens (OH): The Ohio State University; 2004.
- Souto EB, Doktorovova S. Solid lipid nanoparticle formulations: pharmacokinetic and biopharmaceutical aspects in drug delivery. Methods Enzymol. 2009;464:105–129.
- Seikmann B, Westesen K. Investigation on solid lipid nanoparticles prepared by precipitation in o/w emulsions. Europen J Pharm. & Biopharm. 1996;43:104–109.
- Ahlin P, Kristl J, Kobar S. Optimization of procedure parameters and physical stability of solid lipid nanoparticles in dispersion. Acta Pharm. 1998;48:257–267.
- Bharadwaj R, Das PJ, Pal P, et al. Topical delivery of paclitaxel for treatment of skin cancer. Drug Dev Ind Pharm. 2016;42:1482–1494.
- Abdelwahed W, Degobert G, Stainmesse S, et al. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv Drug Delivery Rev. 2006;58:1688–1713.
- Souto EB, Wissing SA, Barbosa CM, et al. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm. 2004;278:71–77.
- Kyo YC, Chung JF. Physicochemical properties of nevirapine loaded solid lipid nanoparticles and nano structured lipid carrier. Coll Sur B Biointer. 2011;83:299–306.
- Patel MN, Lakkadwala S, Majrad MS, et al. Characterization and Evaluation of 5-Fluorouracil-Loaded Solid Lipid Nanoparticles Prepared via a Temperature-Modulated Solidification Technique. AAPS PharmSciTech. 2014;15:1498–1508.
- Costa P, Sousa Lobo JM. Evaluation of mathematical model describing Drug release from estradiol transdermal systems. DDIP. 2003;27:89–97.
- Ekambaram P, Abdul Hasan Sathali A. Formulation and Evaluation of Solid Lipid Nanoparticles of Ramipril. J Young Pharma. 2011;3:216–220.
- Schoop RAL, Noteborn MHM, Baatenburg de Jong RJ. A mouse model for oral squamous cell carcinoma. J Mol Hist. 2009;40:177–181.
- Schell A, Ley J, Wu N, et al. Nab-paclitaxel-based compared to docetaxel-based induction chemotherapy regimens for locally advanced squamous cell carcinoma of the head and neck. Cancer Med. 2015;4:481–489.
- Oertel K, Spiegel1 K, Schmalenberg H, et al. Phase I trial of split-dose induction docetaxel, cisplatin, and 5-fluorouracil (TPF) chemotherapy followed by curative surgery combined with postoperative radiotherapy in patients with locally advanced oral and oropharyngeal squamous cell cancer (TISOC-1). BMC Cancer 2012;12:483.
- Hitt R, López-Pousa A, Martínez-Trufero J, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. Jco. 2005;23:8636–8645.
- Ma Y, Chapman J, Levine M, et al. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med. 2014;6:222–318.
- Mandawgade SD, Patravale VB. Development of SLNs from natural lipids: application to topical delivery of tretinoin. Int J Pharmac. 2008;363:132–138.
- Shenoy VS, Vijay IK, Murthy RSR. Tumour targeting: biological factors and formulation advances in injectable lipid nanoparticles. J. Pharm. Pharmcol. 2005;57:411–422.
- Swant KK, Petkar CK, Agrawal Y. Development, evaluation and clinical studies of acitretin loaded nanostructured lipid carriers for topical treatment of psoriasis. Int J Pharm. 2010;401:93–102.
- Hou D, Xie C, Huang K, et al. The production and characteristics of solid lipid nanoparticles (SLNs). Biomaterials 2003;24:1781–1785.
- Shah R, Eldridge D, Palombo E, et al. Optimisation and stability assessment of solid lipid nanoparticles using particle size and zeta potential. J Phys Sci. 2014;25:59–75.
- Fei H, Sanming L, Ran Y, et al. Effect of surfactants on the formation and characterization of a new type of colloidal drug delivery system: nanostructured lipid carriers. Coll and Surf A. A Physicochem Eng Asp. 2008;315:210–216.
- Patel K, Padhye S, Nagarsenker M. Duloxetine HCl lipid nanoparticles: preparation, characterization, and dosage form design. AAPS PharmSciTech. 2012;13:125–133.
- Barratt G. Characterization of the colloidal drug carrier systems with zeta potential measurements. Pharma Tech Eur. 1999;11:25–32.
- Qi C, Chen Y, Jing Q-Z, et al. Preparation and characterization of catalase-loaded solid lipid nanoparticles protecting enzyme against proteolysis. Int J Mol Sci. 2011;12:4282–4293.
- Schubert MA, Harms M, Muller RH, et al. Structural investigations on lipid nanoparticles containing high amounts of lecithin. Eur J Pharm Sci. 2006;27:226–236.
- Casadei MA, Cerreto F, Cesa S, et al. Solid lipid nanoparticles incorporation in dextran hydrogels. Int J Pharm. 2006;325:140–146.
- Hu QF, Jiang S, Du YZ, et al. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Coll and Surf B: Biointerfaces. 2005;45:167–173.