?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Metformin (MET) has received considerable attention in recent years for its anticancer potential activities. However, short half-life and weak bioavailability of MET limited its use as a chemotherapeutic agent. The present study is intended to evaluate the efficiency of PLGA-PEG as a nano-carrier for MET to increase anticancer effects on SKOV3 ovarian carcinoma cells. MET-loaded PLGA-PEG nanoparticles (NPs) were characterized through Dynamic Light Scattering (DLS), Fourier-transform infrared spectroscopy (FTIR) and field emission scanning electron microscopy (FE-SEM). Anti-proliferative and apoptotic effects of nanoformulated MET were evaluated using MTT and flow-cytometric assays, respectively. Also, real-time polymerase chain reaction (Real-Time PCR) was used to determine the gene expression levels of apoptotic genes, p53 and hTERT. Evaluation of cytotoxicity showed that MET-NPs had more cytotoxicity than free MET in a time-and dose-dependent manner. The nuclei fragmentation and the percentage of apoptotic cells induced by MET-NPs were significantly higher than free MET. Also, it was found that MET-NPs triggered more cell cycle arrest at sub-G1 checkpoint than free MET. Compared to MET treated cells, the mRNA expression levels of apoptotic genes, as well as p53 and hTERT were significantly altered in MET-NPs treated cells. In conclusion, it is supposed that nano-encapsulation of MET into polymeric PLGA-PEG NPs may be a convenient drug delivery system to enhance its anticancer effects for ovarian cancer therapy.
Introduction
According to the American Cancer Society’s estimations in the U.S. for 2017, about 22,440 women will be diagnosed for ovarian cancer and about 14,080 women will lose life due to cancer [Citation1]. Ovarian cancer ranks fifth in cancer mortalities among women and a risk of getting ovarian cancer throughout the lifetime is around 1 in 75 [Citation2].
Traditional ovarian cancer therapies including surgery, chemotherapy, radiation, hormonal therapy and immunotherapy are, more or less, defective in treating ovarian cancer. But some of these therapeutic strategies have no considerable improvement on survival rate and the metastasized form of cancer. They may even have some irritating and undesirable side effects, especially chemotherapy, that leads to nausea, thrombocytopenia, neutropenia, anemia and hair loss [Citation3], so developing novel treatment approaches for ovarian cancer is crucial.
Metformin (MET) (1,1-hydrochloride) is a biguanide originated from French lilac or goat’s rue (Galega officinalis), which was most widely prescribed as an oral antihyperglycemic agent particularly for managing type 2 diabetes mellitus [Citation4]. Recently, several observational and laboratory studies have presented considerable interest in its antitumor and antineoplastic activities [Citation5,Citation6].
It has been suggested that the anti-cancer effects of MET rise from both direct and in indirect effects. The direct effects of MET are principally mediated by stimulation of AMP-activated protein kinase (AMPK), causing a downstream inhibition of mammalian target of rapamycin (mTOR) signalling. Inhibition of mTOR results in the reduction of protein synthesis and tumor cell growth and proliferation through downstream targets, 4E-BP1 and p70S6K1 [Citation7,Citation8]. The indirect effects of MET occur through inhibition of gluconeogenesis in the liver. Stimulation of hepatic AMPK results in arresting the transcription of crucial gluconeogenesis genes, and activates glucose uptake in muscle, therefore, it decreases fasting blood glucose and insulin levels and enhances insulin sensitivity [Citation9–11].
Despite its widespread clinical utility, MET has low intestinal absorption and exhibits poor bioavailability owing to excessive aqueous solubility and weak permeability through the cell membranes [Citation5,Citation12]. Use of polymeric nanoparticles (NPs) such as polylactic acid-co-glycolic acid-polyethylene glycol (PLGA-PEG) to the delivery of anti-cancer agents may have a great potential to overcome these limitations. As a drug delivery system, polymeric NPs exhibit some advantages including elevated drug stability, high delivery capability, the feasibility of delivering both hydrophilic and hydrophobic drugs, improved shelf life, and the possibility of different administration routes [Citation13–15].
Thus, this study was designed to fabricate and characterize a PLGA-PEG encapsulated MET based drug delivery system and evaluate its anti-cancer and pro-apoptotic effects against SKOV3 human ovarian carcinoma cells.
Materials and methods
Materials
Human ovarian SKOV3 was obtained from American Type Culture Collection (ATCC), Fetal Bovine Serum (FBS) and penicillin/streptomycin used for cell culture were purchased from Gibco-Invitrogen, USA. MTT [3–(4,5-dimethylthiazole- 2-yl)-2,5-diphenyl tetrazolium] were purchased from Sigma-Aldrich.
Synthesis of PLGA-PEG copolymer
Ring-opening polymerization method was applied to the synthesis of PLGA-PEG copolymer according to previous studies [Citation16,Citation17]. Briefly, glycolide (0.570 g), DL-lactide (2.882 g) and PEG4000 (1.54 g) were melted by stirring in a round-bottom flask at 140 °C under nitrogen. Then, 0.05% (w/w) stannous octoate [Sn(oct)2], as a catalyst, was added and the temperature was increased to 180 °C and maintained for 4 h. After the polymerization was completed, the copolymer was recovered by dissolving in DCM and precipitating in ice-cold diethyl ether.
Preparation of MET-Loaded PLGA-PEG NPs
Double emulsion (w/o/w) process was used to fabrication of MET-loaded PLGA-PEG NPs. In brief, 200 mg of PLGA-PEG copolymer were dissolved in 5 ml of DCM. 20 ml of PVA (0.5% w/v) containing MET HCl 20 mg (the w/o primary mixture) was added to the organic phase and emulsified applying an ultrasonic probe (with 55% power) (Sonoplus, HD 2070; Bandelin Electronics, Berlin, Germany) for 20 min to generate w/o/w emulsion. Next, a rotary evaporator (Hei-VAP series, Heidolph Instruments, Schwabach, Germany) was used to evaporate DCM at 40 °C and the remaining solution was accumulated by centrifugation for 40 min at 15,000 rpm.
Drug encapsulation efficiency and drug loading
Following synthesis of MET-loaded PLGA-PEG NPs, the supernatant was collected and compared with the total amount of MET to measure the drug loading efficiency of the NPs. The amount of non-entrapped MET in the aqueous phase was measured at λ-max of MET HCl (237 nm) applying a Lambda 950 Visible-UV spectrophotometer (PerkineElmer Fremont, CA, USA). The percentage of MET encapsulated on the NPs (EE) and drug loading (DL) were assessed by applying the following formula:
(1)
(1)
Characterization of NPs
The average size of the NPs (diameter, nm (was measured by using a Dynamic Light Scattering (DLS) Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, UK) equipped with a helium–neon laser beam at a fixed scattering angle of 90 and a wavelength of 633 nm. For this purpose, NPs were dissolved in deionized water at a concentration of 0.5 mg/mL followed by 10 min sonication at room temperature. Moreover, the surface morphology examination and the size confirmation of the NPs were carried out by field emission scanning electron microscopy (FE-SEM) (Hitachi S-4800 FE-SEM, Hitachi, Chiyoda, Japan) and transmission electron microscopy (TEM). Before imaging, the NPs were coated with gold under vacuum.
A Perkin-Elmer Spectrum One model FT-IR was used to record the IR spectra of free MET, PLGA-PEG and MET-Loaded PLGA-PEG NPs prepared in KBr disks in the region of 4000–400 cm−1.
In vitro drug release
Dialysis method was used to determine in vitro drug release of MET from PLGA-PEG NPs as explained in previous studies [Citation18,Citation19]. In brief, 25 mg of MET-loaded PLGA-PEG NPs were dispersed in 5 ml PBS (pH = 4.4 and 7.4) and transmitted to dialysis membrane tubing (MW cut off: 3000) placed in 30 ml of PBS with stirring at 120 rpm at 37 °C. The buffer solution was substituted with fresh PBS at selected time intervals, and the concentration of the free MET in the removed PBS was measured using a calibration curve at 237 nm (wavelength of maximum absorption). Then, the accumulative ratios of the released MET were measured as a function of time. All of the measurements were carried out in triplicate.
Cytotoxicity assay
The cytotoxicity effects of free MET and MET-loaded PLGA-PEG NPs on SKOV-3 cells were studied using MTT (3-[4, 5-dimethylthiazol-2yl]-2, 5-diphenyl tetrazolium bromide) assay. Briefly, 2 × 104 SKOV-3 cells/well were seeded into 96-well plates and incubated for 24 h to facilitate the cell attachment. Next, the cells were treated with different concentration of free and encapsulated forms of MET (5–60 mM). After 24, 48 and 72 h of treatment, the cells were incubated for 4 h with 0.5 mg/mL of MTT and the content of the wells was replaced with 25 μL of Sorensen’s glycine buffer and 200 μL of DMSO. The number of living cells following the respective treatments was enumerated by measuring the absorbance of formazan, at 570 nm using Beckmann Coulter ELISA plate reader (BioTek Power Wave XS) with a reference wavelength of 630 nm. All experiments were repeated 3 times.
DAPI staining
The nuclei morphology and presence of apoptotic bodies was assessed by DAPI nuclear staining. DAPI staining was done according to a previously described protocol [Citation20]. SKOV-3 cells were seeded at 2 × 105 cells/mL in 6–well plates. Cells at 70% confluence were treated with free MET and MET-loaded PLGA-PEG NPs for 48 h. After the treatment, the cells were fixed with 4% glutaraldehyde for 15 min and permeabilized with 0.1% Triton X-100 for 15 min and stained with 1 mg/mL DAPI for 10 min at 37 °C. Stained images were captured using a fluorescent microscope with the appropriate excitation filter.
Cell cycle arrest analysis
Cell cycle analysis was done by DNA staining with propidium iodide (PI), followed by flow cytometric measurement of the fluorescence. Around 4 × 104 SKOV-3 cells per well were plated in triplicate in 6-well plates and incubated for 48 h. After incubation with free MET and MET-loaded PLGA-PEG NPs for 24 h, the medium was removed and stored. Cells were washed twice with PBS and trypsinized, then harvested in the stored medium, and centrifuged. The pellet was washed twice with PBS, and fixed in freshly prepared cold 70% ethanol, and kept at −20 °C overnight. For flow cytometry analysis, first, the cells were washed with PBS and stained with 50 μg/mL PI in 0.25 mg/mL RNase A and incubated for 1 h at 37 °C, followed by incubation at 4 °C until flow cytometry analysis. BD FACS Calibur (BD Biosciences) at an excitation wavelength of 488 nm and the emission wavelength of 610 nm was used to flow cytometry analysis, and the data collected for 104 cells were analyzed using Cell Quest software 6.0 (BD Biosciences, MD, USA).
Annexin-V staining apoptosis analysis
Annexin-V staining was done to determine apoptotic and necrotic cell death induced by free MET and MET-loaded PLGA-PEG NPs. The cells (5 × 105) were treated with 15 μM of free MET and MET-loaded PLGA-PEG NPs at 37 °C, for 48 h and then harvested and centrifuged at 200×g for 5 min. Subsequently, the collected cells were washed twice with PBS and re-suspended in PBS at room temperature. Following that step, 10 μl of Annexin-V-FITC labeling solution and 5 μL of PI solution were added to the mixture, and incubated for 5 min at 25 °C and then analyzed with a flow cytometer (FACS Calibur, USA) by the Annexin V-fluorescein isothiocyanate apoptosis kit (Ebioscience, USA). Data were collected for 10,000 gated cells and analyzed using the Flowjo version 7.6.1
Real-time RT-PCR
Real-time PCR assay was used to analyze the relative mRNA of the hTERT gene. SKOV3 cells were treated with different concentrations of free MET and MET-loaded PLGA-PEG NPs for 48 h. After drug exposure time, total RNA was isolated applying the Trizol reagent according to manufactures protocol. Next, the quality and quantity of total RNA were measured according to electrophoresis on 1.5% agarose and OD260/280 ratio measurements, respectively. The equal amount of RNA was taken from all the samples and reverse transcribed applying Revert Aid First-strand cDNA synthesis Kit (Fermentas, St Leon-Rot, Germany) to achieve cDNA. Subsequently, the concentration of 1:20 of synthesized cDNA was amplified by quantitative real-time PCR applying specific primers (Takapou Zist Co., Iran) () and the SYBR Green-I dye (Roche, Germany) by the Rotor-GeneTM 6000 system (Corbett research, Australia). The program for real-time PCR reaction was as follows; Initial denaturation at 95 °C for 10 min, followed by cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s and extension at 72 °C for 30 s. Finally, amplicons were measured by melting curve analysis of 70 °C to 95 °C. The real-time PCR efficiencies were determined for each gene. Relative hTERT expression levels were normalized by housekeeping gene (GAPDH) and relative expression of the genes calculated by this formula: (normalized relative ratio = 2-Ct).
Table 1. List of gene-specific primers, expected product sizes for qRT-PCR.
Statistical analysis
Data are represented as the mean ± SD of three replicates of one representative experiment. The statistical significance was estimated using one-way and two-way analysis of variances. Data with p values <.05 was considered to be significant. All statistical analyses were done by GraphPad Prism v.6.01 software (GraphPad Software, Inc., CA, USA).
Results and discussion
Synthesis of PLGA-PEG copolymer
Recent in vitro and in vivo reports indicate promising findings of the chemopreventive properties of MET in various cancers [Citation7,Citation21]. The epidemiologic data indicate that MET intake significantly enhances the clinical outcome of patients with ovarian cancer [Citation22–24]. However, the short half-life, low bioavailability and side effects of MET impede its use as a chemotherapeutic agent. The present study was intended to evaluate the efficiency of PLGA-PEG as a nano-carrier for MET to increase anticancer effects.
Recently, various materials are progressively applied to comprise biodegradable NPs. Such NPs act as drug reservoirs which cause not only local therapeutic influence but also deliver drugs to target tissues [Citation25,Citation26]. PLGA-PEG copolymer is one of the most commonly used biodegradable polymers for drug encapsulation and has been applied to the administration of various drugs that have absorption problems [Citation27,Citation28]. The copolymer is a free-flowing solution at low temperature and can create a high viscosity gel at body temperature [Citation29]. This temperature-responsive copolymer is a class of block copolymers comprised of hydrophobic PLGA and hydrophilic PEG units. The simplicity of formulation, filtration and filling introduce such copolymers as desirable candidates to develop efficient drug delivery systems in cancer therapy [Citation30,Citation31].
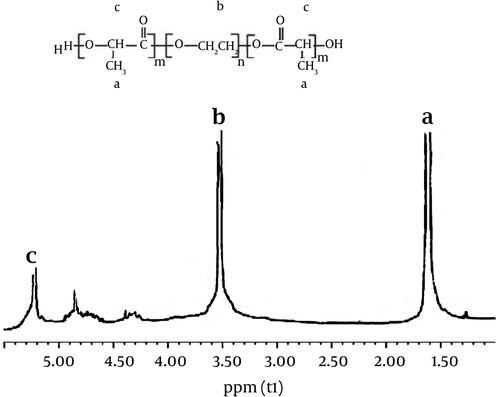
The recorded 1HNMR spectrum verifies the basic chemical structure of the PLGA-PEG copolymer. Tetramethylsilane (TMS) as an internal control was used to determine the chemical shifts in ppm. PLGA-PEG copolymer was produced through direct conjugation of PEG-NH2 and PLGA. shows the basic chemical structure of PLGA-PEG confirmed by 1HNMR. A large peak at 3.65 ppm is one of the striking features that corresponds to the PEG methylene groups. Overlapping doublets at 1.55 ppm are assigned to the methyl groups of the L- and D-Lactide repeat units. The multiple peaks at 4.8 and 5.2 ppm are attributed to the glycolide CH and the Lactide CH, respectively [Citation32]. The effectiveness of coupling reaction measured through 1HNMR showed that about 72% of PLGA were conjugated with PEG. The synthesis yield of PLGA-PEG copolymer was determined to be 92.5%.
Characterization of NPs
LE and EE of PLGA-PEG NPs loaded with MET were acquired to be ≍5.8% and 75%, respectively, and at higher concentrations of MET, the LE and EE were initiated to be decreasing.
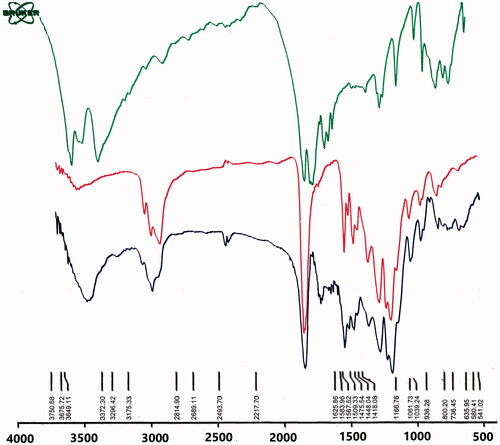
shows the Fourier-transform infrared spectroscopy (FTIR) results of MET, PLGA-PEG and MET-loaded PLGA-PEG NPs. Based on IR spectra of MET, the observed three characteristic peaks, between 3372–3400, 3296 and 3000–3175 cm−1, were relative to the NH primary stretching vibration, NH of imines group and NH secondary stretching. Frequently, the incidence of this vibration is decreased in the presence of the H bond. A weak peak at 1418 cm−1 and the characteristic bands at 1567–1583, 1625 cm−1 are ascribed to NH secondary bending, NH primary bending and C–N stretching vibrations, respectively. The characteristic bands emerged in the area of 1278–1920 cm−1 are defined for C–N stretching of aliphatic amine compounds that are commonly weak. Also, the appeared peak at 1448–1475 cm−1 was assigned to C–H bending vibration mode. It was observed that all the characteristic bands of PLGA-PEG and MET are detectable in MET-loaded PLGA-PEG NPs IRs. OH and NH bands of PLGA-PEG and MET were assigned to the broad peak at 3300–3600 cm−1.
C=N of imines stretching vibration of MET are related to the peak at 2362 cm−1. A strong peak at 1759 cm−1 was assigned to the characteristic esteric (O–C=O) bands of PLGA unit. The peaks at 1508–16369 cm−1 are characteristic bands of NH primary bending, NH secondary bending and C–N stretching vibrations of MET. The other characteristic bands of PLGA-PEG copolymer, which showed C–O–C, C–O and C–C emerged at 1059–1187 cm−1. Thus, it may conclude the loading and interaction of MET into PLGA-PEG NPs.
Particle size analysis
The particle size analysis via DLS exhibited the uniform dispersion of particles with an average size of 210 nm, a polydispersity index of 0.145 ± 0.054 and zeta potential of −26.5 ± 5.4 mV for PLGA-PEG NPs. Also, it has been revealed that PLGA-PEG NPs loaded with MET exhibited an average diameter of 250 nm, a polydispersity index of 0.105 ± 0.100 and zeta potential of −24.9 ± 4.5 mV ().
Figure 3. Characterization of MET-loaded PLGA-PEG NPs. Dynamic light scattering (DLS) (A), field emission scanning electron microscopy (FE-SEM) (B) and transmission electron microscopy (TEM) (C).
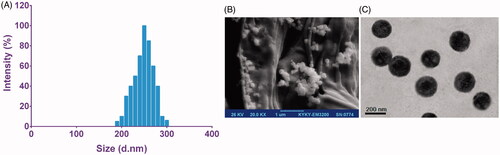
The spherical and uniform shapes with smooth surfaces of the PLGA-PEG and MET-loaded PLGA-PEG NPs were observed through FE-SEM and TEM micrographs (). According to the images, the average size of MET-loaded PLGA-PEG NPs was found to be around 225 nm.
According to particle size analysis, PLGA-PEG NPs loaded with MET had considerably larger sizes than blank PLGA-PEG NPs, representing that the loading MET into the NPs enhanced the volume.
The alterations in the surface conditions of the samples under the applied assay settings is attributable to the modest deviation in diameter measured by FE-SEM and DLS. Indeed, NPs need to be extremely dehydrated for FE-SEM analysis, whereas they are in a fully hydrated condition while using DLS [Citation5].
To escape detection and elimination by the reticuloendothelial system, the average size of NPs should be small enough. Also, it has been proposed that most cells favorably internalize NPs with a diameter smaller than 400 nm. Therefore, it seems that the size range of the PLGA-PEG NPs loaded with MET will be acceptable for achieving longevity during systemic circulation and passive targeting of cancer cells [Citation33].
The surface charge of NPs commonly serves as a key parameter affecting the short- and long-term stability of NP emulsions and the interaction between the cell membrane and NPs [Citation5]. Deprotonation of PLGA carboxyl groups that causes a negatively charged polymer chain resulted in negative zeta potential values for NPs. The NPs with a smaller average size tend to possess a higher zeta potential value in relative to the NPs with a larger size. The NPs with a smaller size have a higher migration rate in a known applied electric field, and hence have higher zeta potential values, causing a better NP stability in the colloidal dispersions.
In vitro drug release
Dialysis method was used to investigate the MET released from the PLGA-PEG copolymer at pHs of 4.4 and 7.4, and shows the release patterns. A burst release in the first 3 h was detected at pH 7.4, which pursued with a controlled release of MET through 7 days and nearly 76% of MET was released within 5 days. The released MET at pH 4.4 was considerably quicker than at pH 7.4 in the mimicked acidic conditions of endosomal/lysosomal compartments, with close to 82% of the total MET content being released from the NPs in the first 2 days.
Figure 4. Drug release patterns of MET from PLGA-PEG NPs in PBS at pH 4.4 and 7.4. The data are presented as mean ± SD (n = 3).
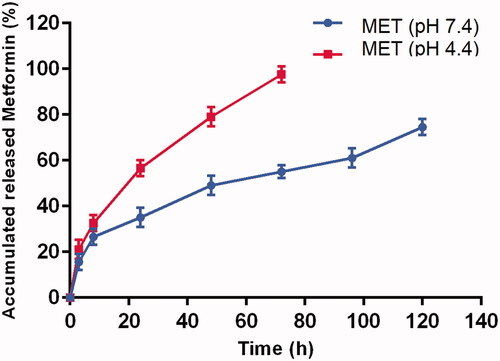
Based on drug release analysis, the absorbed or encapsulated MET close to the surfaces of the NPs might be the reason of initial burst release, due to the higher degradation rate of the PLGA-PEG close to the surfaces of NPs, releasing MET will be elevated.
Endocytosis or possibly pinocytosis are most probably involved in cellular uptake of NPs, in which further release of MET occurs at acidic pHs of lysosomal processing (pH 4–5). Hence, it is expected to evaluate the release pattern of MET from PLGA-PEG NPs at low pH settings.
Cytotoxicity
In this study, the cytotoxic effects of different concentration of free MET and MET-loaded PLGA-PEG NPs on SKOV-3 ovarian cancer cells after 24, 48 and 72 h incubation time were assessed using colorimetric MTT assay. As presented in , the viability of the cancers was substantially reduced by both free and nano-encapsulated MET in dose and time-dependent manner and importantly, MET-loaded PLGA-PEG NPs considerably decreased the cancer cells growth in comparison to free MET in all concentrations (p < .05).
Figure 5. MTT results of in vitro cytotoxicity of free MET and MET-loaded PLGA/PEG NPs against SKOV3 cells incubated for (A) 24, (B) 48 and (C) 72 h. The data are presented as as mean ± SD (n = 3).
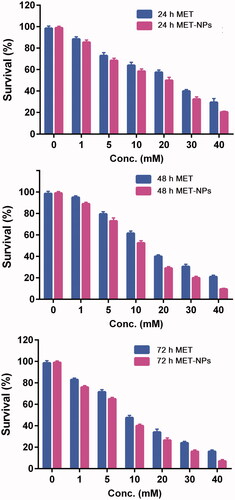
Also, the amount of drug which killed 50% of cancer cells was analyzed through IC50 value estimations. The IC50 values of free MET were 19.54, 14.92 and 9.75 µM and for MET-NPs were 13.81, 10.03 and 6.81 µM, respectively in SKOV-3 ovarian cancer cell line after 24, 48 and 72 h incubation. The IC50s value clearly showed the superior performance of MET-NPs.
The enhanced cytotoxic effect of MET-loaded PLGA-PEG NPs on SKOV-3 ovarian cancer cells was due to the high intracellular concentrations and controlled release of the MET. It has been demonstrated that nano-encapsulated drugs use a certain cellular internalization route and releases the drug in a controlled manner, whereas free drugs simply diffuse through the cellular membrane [Citation34].
Up till now, some works were conducted for nanoencapsulation of MET with the aim of application in type 2 diabetes treatment [Citation35–37]. But using polymeric NPs for the delivery of MET into various cancer cells has not yet been extensively studied. In a study by Snima et al., the efficiency of O-ocarboxymethyl chitosan (O-CMC) NPs as a nanocarrier for delivery of MET into pancreatic cancer cells (MiaPaCa-2) was assessed [Citation38]. Findings of cytotoxicity assay showed that NPs loaded with MET induced substantial toxicity against MiaPaCa-2 cells compared with L929 normal cells. Furthermore, it was found that O-CMC-MET NPs internalized nonspecifically into MiaPaCa-2 and L929 normal cells. In vivo near-infrared (NIR) imaging revealed normal biodistribution profile of the intravenously injected MET-loaded NPs, signifying normal clearance rate of NPs, and no adverse organotoxic effects [Citation39].
Detection of chromosome condensation by DAPI staining
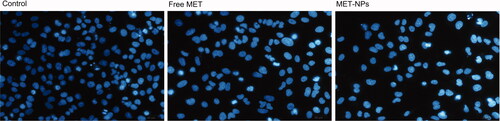
To observe nuclear morphology changes related to apoptosis more precisely, cells were stained by DAPI after treating with free MET and MET-loaded PLGA-PEG NPs (). The nuclei of untreated SKOV3 cells displayed homogenous fluorescence with no sign of segmentation and fragmentation after DAPI staining. Exposure of the cells with IC50 concentration of free MET and MET-loaded PLGA-PEG NPs led to segregation of the cell nuclei into segments, demonstrating a breakdown in the chromatin followed by DNA condensation. As shown in , the percentage of apoptotic cells induced by MET-loaded PLGA-PEG NPs was significantly higher than free MET, denoting that MET-encapsulated in PLGA-PEG NPs effectively induced apoptosis in SKOV3 cells in relation to free MET.
Cell cycle arrest analysis
In order to study the molecular mechanisms of cell cycle arrest by MET, we determined the effects of free MET and MET-loaded PLGA-PEG NPs on various phases of cell cycle. The effects of these agents on cell-cycle progression in SKOV3 cells are shown in . A substantial upsurge in the percentage of SKOV3 cells in sub-G1 phase was found after treatment with MET-loaded PLGA-PEG NPs, compared with free MET. As presented in , the cells exposed to MET-loaded NPs for 48 h showed a striking increase of sub-G1 population (41%) compared with free MET (24.4%) (p < .05). S and G2 phases were reduced accompanied by the upsurge of sub-G1 phase than free MET. DNA damage was suggested to be the main cause of cell cycle arrest [Citation40].
Figure 7. Cell cycle analysis of SKOV3 cells exposed with IC50 concentration of free MET and MET-loaded PLGA/PEG NPs for 48 h. All experiments were carried out in triplicate.
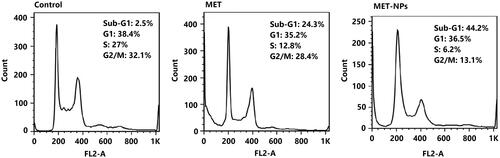
Cells with damaged DNA are collected in the G1, DNA synthesis (S), or in G2/M phase while cells with irreversible damage are accumulated in the sub G1 phase [Citation41]. Our results revealed that the effects of free MET and MET-loaded PLGA-PEG NPs on activation of apoptosis are related to alterations in the cell cycle progression by increased cells entering in sub-G1 phase.
Annexin V/PI staining
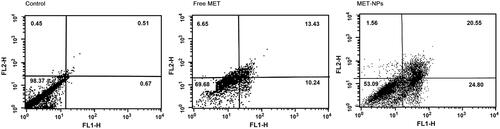
In order to know the molecular basis of the growth inhibitory mechanism caused by free MET and MET-loaded PLGA-PEG NPs, Annexin V-FITC/PI staining was used to quantify the proportion of apoptotic and dead SKOV3 cells (). Our results revealed that MET treatment induced cell death in SKOV3 cells. Compared with the control, free MET and MET-loaded PLGA-PEG NPs increased the percentage of dead cells by 23.67% and 45.35%, respectively. The findings are according to the idea that the cell cycle progression and apoptosis are related to high endocytosis and release profile in response to MET formulation [Citation5,Citation42]. There is increasing evidence that MET could directly decrease cell proliferation in several types of cancers such as liver, prostate and lung [Citation43]. In accordance to these previous reports, it was shown that free MET and MET-loaded PLGA-PEG NPs considerably inhibited cell proliferation in human ovarian cancer cells in our present study. It has been reported that MET induces inhibition of cell growth and cell cycle arrest partially by upregulating miRNA34a in renal cancer cells [Citation43]. Moreover, Queiroz et al. showed that MET inhibits cell proliferation through up-regulation of tumor suppressor genes and cell cycle modulation [Citation44].
Figure 8. Apoptosis induction in SKOV3 cells were treated with IC50 concentrations of free MET and MET-loaded PLGA/PEG NPs for 48 h and analyzed using flow cytometry after staining with Annexin V and PI. The amount of apoptosis was assessed as the percentage of Annexin V+/PI- and Annexin V+/PI + cells.
Gene expression analysis
To further explore the mechanisms involved in MET-NPs -mediated inhibition in SKOV3 ovarian cancer cells, qRT-PCR was used to analyze the mRNA level of apoptotic genes (Bax, bcl-2 and caspase-3 and caspase-7), p53 and hTERT (). Results showed that free and nanoencapsulated MET altered the mRNA expressions levels of these genes in control cells. Compared to MET treated cells, the mRNA expression levels of apoptotic genes Bax, caspase-3 and caspase-7, as well as tumor suppressor p53 gene were significantly up-regulated in MET-NPs treated cells. While it was found that the expression levels of anti-apoptotic gene bcl-2, and hTERT showed a further reduction in MET-NPs treated cells.
Figure 9. The expression levels of bcl-2, Bax, caspase-3 and caspase-7, P53, hTERT genes, relative to reference gene (GAPDH) in SKOV3 cancer cells treated with free MET and MET-loaded PLGA/PEG NPs. *p < .05, **p < .01 and ***p < .001 vs. free MET was considered significant.
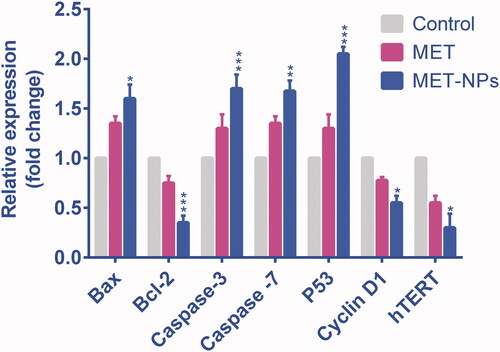
Apoptosis process has a crucial role in cancer pathogenesis, so the genes involved in this process are considered in investigations related to cancer initiation and progression. Bcl-2 impedes apoptosis and extends cell survival while Bax, a homologue of bcl-2, induces apoptosis. Both Bax and Bcl-2 have been reported to be transcriptional targets of tumor-suppressor p53, which induces cell cycle arrest and/or apoptosis in response to a number of cellular stresses, including DNA damage, nucleotide deprivation and hypoxia [Citation45]. The malignant progression principally depends on the expression ratio of anti-apoptotic genes, such as bcl-2, and pro-apoptotic genes, such as Bax. Furthermore, Members of the caspase family of cysteine proteases such as caspase-3 and caspase-7, are well-documented to play a major role in the apoptotic pathways [Citation46].
The maintenance of telomere length is essential for prolonged cell proliferation, and ∼85–90% of human cancers, including ovarian cancer, indicate high activity of telomerase [Citation47]. Telomerase complex includes three major subunits: hTR (human telomerase RNA), TP1 (telomerase-associated protein 1), and hTERT (human telomerase reverse transcriptase) [Citation48]. The hTERT gene is the most important regulator of telomerase activity. hTERT is highly expressed in all tissues regardless of telomerase activity, but in cancer cells usually, have fivefold-higher expression. It was demonstrated that the inhibition of hTERT rapidly induces apoptosis in breast cancer cells. Therefore, targeting hTERT in ovarian cancer may well be a promising step in its treatment.
In this work, the level of apoptosis in SKOV3 ovarian cancer cells treated by MET and MET-loaded PLGA-PEG NPs were evaluated and the findings were confirmed by previous studies. Rogalska et al. showed that MET can induce apoptosis in an AMPK-independent manner and arrest cell cycle in the S and G2/M stages in OVCAR-3 and OVCAR-4 cell lines. In OVCAR-3 and OVCAR-4 cells, apoptosis was induced through activating caspase 3/7, up-regulating Bax and Bad, and down-regulating bcl-2 and bcl-xL [Citation49]. Moreover, it was found that alteration in the BIRC5 mRNA expression was related to apoptosis induction in SKOV-3 ovarian cancer cells treated with MET.
Consistent with our work, Cantrell et al. [Citation50] showed the influence of MET on cell proliferation and possible important targets of cell signaling in endometrial cancer cells, ECC-1 and Ishikawa. Results showed that MET potently arrested cancer cell growth dose-dependently, diminished expression levels of hTERT and promoted apoptosis induction. It was concluded that MET may alter the expression levels of hTERT indirectly, as a result of cell cycle arrest.
Conclusion
In this present study, MET-loaded PLGA-PEG NPs were successfully prepared by applying the w/o/w approach in the presence of PVA as a stabilizer. Particle size and morphological analysis showed that the synthesized MET-loaded PLGA-PEG NPs exhibited approximate spherical shapes with smooth surfaces and an average particle size <250 nm. The developed NPs had an excellent encapsulation efficiency of MET, and the in vitro drug release assay revealed that the NPs may be convenient drug release assay revealed that the NPs may be convenient of sustained drug release purpose. Furthermore, anticancer efficiency of MET-loaded PLGA-PEG NPs treated with reducing cell viability, G2/M arresting, apoptotic morphological changes and alteration in expression levels of apoptotic genes Bax, caspase-3 and caspase-7 as well as tumor suppressor p53 and hTERT in SKOV3 cells. These findings suggest that the nano-encapsulation of MET in PLGA-PEG copolymers can provide an attractive drug delivery approach for the treatment of ovarian cancer. Further in vivo examination should be conducted to clarify the therapeutic efficacy of these MET-loaded PLGA-PEG NPs.
Acknowledgements
Authors would like to thank the Department of Clinical Biochemistry and Laboratory Medicine, Faculty of Medicine for supporting this project.
Disclosure statement
The authors declare that they have no competing interests.
References
- Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2017: a review of current American Cancer Society guidelines and current issues in cancer screening. CA: A Cancer J for Clinicians. 2017;67:100–121.
- De Souza C. Effect of germline polymorphisms on somatic mutations in High Grade Serous ovarian cancer. Poster session presented at: The University of New Mexico. Shared knowledge Conference; 2017 November 11;. Albuquerque, New Mexico.
- Maasomi ZJ, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. Synergistic anticancer effects of silibinin and chrysin in T47D breast cancer cells. Asian Pac J Cancer Prev. 2017;18:1283.
- Chatran M, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. Synergistic anti-proliferative effects of metformin and silibinin combination on T47D breast cancer cells via hTERT and cyclin D1 inhibition. Drug Res (Stuttg). 2018;68:710–716.
- Farajzadeh R, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. Nano-encapsulated metformin-curcumin in PLGA/PEG inhibits synergistically growth and hTERT gene expression in human breast cancer cells. Artificial Cells, Nanomed Biotechnol. 2017;46(5):1–9.
- Liu B, Fan Z, Edgerton SM, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009;8:2031–2040.
- Shafiei-Irannejad V, Samadi N, Salehi R, et al. Reversion of multidrug resistance by co-encapsulation of doxorubicin and metformin in poly (lactide-co-glycolide)-d-α-tocopheryl polyethylene glycol 1000 succinate nanoparticles. Pharma Res. 2018;35:119.
- Dadashpour M, Pilehvar‐Soltanahmadi Y, Zarghami N, et al. Emerging importance of phytochemicals in regulation of stem cells fate via signaling pathways. Phytother Res. 2017;31:1651–1668.
- Javidfar S, Pilehvar-Soltanahmadi Y, Farajzadeh R, et al. The inhibitory effects of nano-encapsulated metformin on growth and hTERT expression in breast cancer cells. J Drug Del Sci Technol. 2017;43:19–26.
- Jafari-Gharabaghlou D, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. Combination of metformin and phenformin synergistically inhibits proliferation and hTERT expression in human breast cancer cells. Iranian J Basic Med Sci. 2018;21:1167.
- Rasouli S, Zarghami N. Synergistic growth inhibitory effects of chrysin and metformin combination on breast cancer cells through hTERT and cyclin D1 suppression. Asian Pac J Cancer Prev. 2018;19:977–982.
- Ngwuluka NC, Kotak DJ, Devarajan PV. Design and characterization of metformin-loaded solid lipid nanoparticles for colon cancer. AAPS PharmSciTech. 2017;18:358–368.
- Farajzadeh R, Zarghami N, Serati-Nouri H, et al. Macrophage repolarization using CD44-targeting hyaluronic acid–polylactide nanoparticles containing curcumin. Artificial Cells, Nanomed Biotechnol. 2018;46:2013–2021.
- Anari E, Akbarzadeh A, Zarghami N. Chrysin-loaded PLGA-PEG nanoparticles designed for enhanced effect on the breast cancer cell line. Artificial Cells, Nanomed Biotechnol. 2016;44:1410–1416.
- Mohammadian F, Abhari A, Dariushnejad H, et al. Upregulation of Mir-34a in AGS gastric cancer cells by a PLGA-PEG-PLGA chrysin nano formulation. Asian Pac J Cancer Prev. 2016;16:8259–8263.
- Dadashpour M, Pilehvar-Soltanahmadi Y, Mohammadi SA, et al. Watercress-based electrospun nanofibrous scaffolds enhance proliferation and stemness preservation of human adipose-derived stem cells. Artificial Cells, Nanomed Biotechnol. 2018;46:819–830.
- Pilehvar-Soltanahmadi Y, Nouri M, Martino MM, et al. Cytoprotection, proliferation and epidermal differentiation of adipose tissue-derived stem cells on emu oil based electrospun nanofibrous mat. Exp Cell Res. 2017;357:192–201.
- Firouzi-Amandi A, Dadashpour M, Nouri M, et al. Chrysin-nanoencapsulated PLGA-PEG for macrophage repolarization: possible application in tissue regeneration. Biomed Pharmacother. 2018;105:773–780.
- Amirsaadat S, Pilehvar-Soltanahmadi Y, Zarghami F, et al. Silibinin-loaded magnetic nanoparticles inhibit hTERT gene expression and proliferation of lung cancer cells. Artificial Cells, Nanomed Biotechnol. 2013 2017;45:1649–1656.
- Dadashpour M, Firouzi-Amandi A, Pourhassan-Moghaddam M, et al. Biomimetic synthesis of silver nanoparticles using Matricaria chamomilla extract and their potential anticancer activity against human lung cancer cells. Mater Sci Eng C Mater Biol Appl. 2018;92:902–912.
- Schulten H-J. Pleiotropic effects of metformin on cancer. Ijms. 2018;19:2850.
- Rattan R, Graham RP, Maguire JL, et al. Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo. Neoplasia 2011;13:483–491.
- Hart PC, Sheikh S, Lengyel E, et al. Metformin inhibits TGFβ-induced stromal ECM remodeling to impede invasion in ovarian cancer. Paper presented at the 2017 AACR Annual Meeting; 2017 April 1–5; Chicago, USA.
- Mert I, Chhina J, Allo G, et al. Synergistic effect of MEK inhibitor and metformin combination in low grade serous ovarian cancer. Gynecol Oncol. 2017;146(2):319–326.
- Montazeri M, Pilehvar-Soltanahmadi Y, Mohaghegh M, et al. Antiproliferative and apoptotic effect of dendrosomal curcumin nanoformulation in P53 mutant and wide-type cancer cell lines. Acamc. 2017;17:662–673.
- Anganeh MT, Mirakabad FST, Izadi M, et al. The comparison between effects of free curcumin and curcumin loaded PLGA-PEG on telomerase and TRF1 expressions in calu-6 lung cancer cell line. Int J Biosci. 2014;4:134–145.
- Mohammadinejad S, Akbarzadeh A, Rahmati-Yamchi M, et al. Preparation and evaluation of chrysin encapsulated in PLGA-PEG nanoparticles in the T47-D breast cancer cell line. Asian Pac J Cancer Prev. 2015;16:3753–3758.
- Rezvantalab S, Drude NI, Moraveji MK, et al. PLGA-based nanoparticles in cancer treatment. Front in Pharmacol. 2018;9:1–19.
- Mohammadian F, Pilehvar-Soltanahmadi Y, Mofarrah M, et al. Down regulation of miR-18a, miR-21 and miR-221 genes in gastric cancer cell line by chrysin-loaded PLGA-PEG nanoparticles. Artificial Cells, Nanomed Biotechnol. 2016;44:1972–1978.
- Amirsaadat S, Pilehvar-Soltanahmadi Y, Zarghami F, et al. The effects of nanoencapsulated curcumin-Fe3O4 on proliferation and hTERT gene expression in lung cancer cells. Acamc. 2017;17:1363–1373.
- Mohammadian F, Abhari A, Dariushnejad H, et al. Effects of chrysin-PLGA-PEG nanoparticles on proliferation and gene expression of miRNAs in gastric cancer cell line. Iranian Journal of Cancer Prevention 2016;9:e4190.
- Mohammadian F, Pilehvar-Soltanahmadi Y, Zarghami F, et al. Upregulation of miR-9 and Let-7a by nanoencapsulated chrysin in gastric cancer cells. Artificial Cells, Nanomed Biotechnol. 2017;45:1201–1206.
- Lotfi-Attari J, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. Co-delivery of curcumin and chrysin by polymeric nanoparticles inhibit synergistically growth and hTERT gene expression in human colorectal cancer cells. Nutrition and Cancer. 2017;69:1290–1299.
- Aslan B, Ozpolat B, Sood AK, et al. Nanotechnology in cancer therapy. J Drug Target. 2013;21:904–913.
- Cetin M, Atila A, Sahin S, et al. Preparation and characterization of metformin hydrochloride loaded-Eudragit® RSPO and Eudragit® RSPO/PLGA nanoparticles. Pharma Dev Technol. 2013;18:570–576.
- Sharma N, Rana S, Shivkumar HG, et al. Solid lipid nanoparticles as a carrier of matformin for transdermal delivery. Int J Drug Del. 2013;5:137.
- Lekshmi UMD, Reddy PN. Preliminary toxicological report of metformin hydrochloride loaded polymeric nanoparticles. Toxicol Int. 2012;19:267.
- Snima K, Jayakumar R, Unnikrishnan A, et al. O-Carboxymethyl chitosan nanoparticles for metformin delivery to pancreatic cancer cells. Carbohydr Polym. 2012;89:1003–1007.
- Snima K, Jayakumar R, Lakshmanan V-K. In vitro and in vivo biological evaluation of O-carboxymethyl chitosan encapsulated metformin nanoparticles for pancreatic cancer therapy. Pharm Res. 2014;31:3361–3370.
- Dekanty A, Barrio L, Milan M. Contributions of DNA repair, cell cycle checkpoints and cell death to suppressing the DNA damage-induced tumorigenic behavior of Drosophila epithelial cells. Oncogene 2015;34:978.
- Chunyan W, Valiyaveettil S. Correlation of biocapping agents with cytotoxic effects of silver nanoparticles on human tumor cells. RSC Adv. 2013;3:14329–14338.
- Cai X, Hu X, Tan X, et al. Metformin induced AMPK activation, G0/G1 phase cell cycle arrest and the inhibition of growth of esophageal squamous cell carcinomas in vitro and in vivo. PLoS One. 2015;10:e0133349.
- Xie W, Wang L, Sheng H, et al. Metformin induces growth inhibition and cell cycle arrest by upregulating MicroRNA34a in renal cancer cells. Med Sci Monit. 2017;23:29.
- Queiroz EA, Puukila S, Eichler R, et al. Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PloS One. 2014;9:e98207.
- Montazeri M, Sadeghizadeh M, Pilehvar-Soltanahmadi Y, et al. Dendrosomal curcumin nanoformulation modulate apoptosis-related genes and protein expression in hepatocarcinoma cell lines. Int J Pharma. 2016;509:244–254.
- Hengartner MO. The biochemistry of apoptosis. Nature 2000;407:770.
- Alibakhshi A, Ranjbari J, Pilehvar-Soltanahmadi Y, et al. An update on phytochemicals in molecular target therapy of cancer: potential inhibitory effect on telomerase activity. Cmc. 2016;23:2380–2393.
- Zavari-Nematabad A, Alizadeh-Ghodsi M, Hamishehkar H, et al. Development of quantum-dot-encapsulated liposome-based optical nanobiosensor for detection of telomerase activity without target amplification. Anal Bioanal Chem. 2017;409:1301–1310.
- Rogalska A, Forma E, Ciesielski P, et al. Effect of metformin on apoptosis induction in ovarian cancer cells. pm. 2014;13( 3):155.
- Holysz H, Lipinska N, Paszel-Jaworska A, et al. Telomerase as a useful target in cancer fighting—the breast cancer case. Tumor Biol. 2013;34:1371–1380.