Abstract
Developing a biomaterial that promotes regeneration of both respiratory epithelium (RE) and olfactory neuroepithelium (ON) improves the surgical outcome of endoscopic sinus surgery. Although chitosan (CS) inhibits mucociliary differentiation of RE, it has been reported to regenerate ON. In addition, hyaluronic acid (HA) has been demonstrated to promote regeneration of RE. Whether the composite CS + HA would simultaneously benefit RE and ON remains unexplored. Human nasal respiratory epithelial cells (RECs) and olfactory neuroepithelial cells (ONCs) are respectively obtained from the RE and the ON. They are cultured in vitro and divided into groups undergoing four treatments, control, CS, HA, and CS + HA and assessed by scanning electron microscope, immunocytochemistry, and Western blots following indicated growth conditions. RECs keep polygonal morphology with mucociliary differentiation in the CS + HA group. The levels of E-cadherin, zonula occludens-1, mucin 5AC, and forkhead box protein J1 are significantly higher in the CS + HA group than in the CS alone group. In addition, ONCs express lower cytokeratin 18 (CK18) and higher olfactory marker protein (OMP) in the CS + HA group than in HA alone group. ONCs express more signal transduction apparatuses, adenylate cyclase 3, in CS and CS + HA groups than in HA and controls. Chitosan-hyaluronan plays a part in promoting differentiation of ORNs and facilitating mucociliary differentiation of RECs. This composite is a promising biomaterial for the sinonasal application.
Introduction
Chronic rhinosinusitis (CRS) is a highly prevalent inflammatory disease, affecting approximately 11.3% of the people in developed countries [Citation1–3]. This disease not only causes physical symptoms, including mucopurulent drainage, nasal congestion, facial pain-pressure fullness and olfactory dysfunction but also interrupts social functioning as well as the quality of life [Citation4]. Endoscopic sinus surgery (ESS) is the preferred treatment for patients with CRS that is unresponsive to maximal medical therapy [Citation5]. Although ESS has become the mainstay of surgical treatment of CRS, over 15% of the patients require a repeat operation wherein postoperative adhesion formation is one of the main causes of failure [Citation6].
The olfactory neuroepithelium (ON) occupies no more than 10% of the nasal cavity in humans [Citation7,Citation8]. It is thin and patchily scattered from the olfactory cleft to the middle turbinate in the adult human. Metaplasia of respiratory epithelium (RE) occurs within ON after severe damage to this neuroepithelium, resulting in loss of olfactory function [Citation9]. Several materials, such as thrombogenic agents, mitomycin and corticoids, have been used to reduce adhesion formation following ESS but they do not restore RE or ON in many cases [Citation10,Citation11]. Therefore, developing a biomaterial that can facilitate re-epithelization of both RE and ON following ESS is an alternative strategy to improve surgical prognosis.
Chitosan (CS), a cationic polysaccharide with a variable number of randomly located D-glucosamine and N-acetyl-glucosamine units, has been demonstrated to promote differentiation of olfactory receptor neurons (ORNs) [Citation12]. However, CS interferes with tight junction formation in respiratory epithelial cells (RECs) and impedes their mucociliary differentiation [Citation13]. Researchers have found that hyaluronic acid (HA) facilitates mucociliary differentiation of RECs and prolongs ciliated cell survival in RE [Citation14,Citation15]. Nevertheless, researchers have yet to explore whether the medical composite, CS + HA, can promote mucociliary differentiation of RECs and ORN differentiation of olfactory neuroepithelial cells (ONCs), at the same time. This study investigates the effect of the medical composite on the differentiation of RECs and ONCs in vitro, which has a potential clinical application.
Methods
Isolation and culture of human nasal RECs
RECs were obtained from the RE of nasal inferior turbinates in patients with chronic rhinitis during septomeatoplasty [Citation14,Citation15], which was approved by an institutional review board of Far Eastern Memorial Hospital (105104-F). All patients gave informed consent. Briefly, specimens were digested with 0.5% Pronase (type XIV protease, Sigma-Aldrich, St Louis, MO) and the cells were suspended in Dulbecco’s Modified Eagle’s Medium and Ham’s nutrient F12 (DMEM/F12; Invitrogen, CA, USA): bronchial epithelial growth medium (CC-3171 and CC-4175, Lonza, Switzerland) (1:1) supplemented with antibiotics and equally seeded on Transwell membrane inserts until confluence. Then, 1.5 ml cell suspension was added to Transwell inserts with 2.5 ml of the medium deposited on the basolateral side. Cultures were maintained at 37 °C in an atmosphere of 5% carbon dioxide in the air. Following confluence, air-liquid interface (ALI) was created by removing the apical medium and incubated with four treatments, namely control (medium only), CS, HA and CS + HA, from the basolateral compartment for 21 days. In addition, the various molecular weight (MW) of HA (5 kDa, 200 kDa, 15MDa; Lifecore Biomedical, MN, USA) were evaluated. The culture medium was changed every other day thereafter. Cell morphology was observed under an inverse phase contrast microscope (TS-100, Nikon, Tokyo, Japan) and scanning electron microscope (SEM) (S-4800; Hitachi, Tokyo, Japan).
Isolation and culture of human ONCs
ONCs were harvested from nasal superior turbinates near the roof of the nasal cavity in patients with chronic rhinitis during septomeatoplasty. Biopsy specimens were washed, minced finely and then digested with 0.125% Trypsin/EDTA for 30 min at 37 °C. Subsequently, the pellets were gathered by centrifuge, resuspended in the Iscove’s Modified Dulbecco’s Media (IMDM; Invitrogen) with 10% fetal bovine serum and 1% antibiotics and equally seeded to six-wells coated with laminin-co-fibronectin. After 21 days, the medium was replaced with induction medium (DMEM/F12; Invitrogen), and they were randomly divided into four groups, namely control (medium only), CS, HA and CS + HA. The duration of treatment was one week. Various concentrations and MW of chitosan, including chitosan oligosaccharides (5 kDa; 523682, Sigma-Aldrich), medium MW chitosan (190 kDa; C3646, Sigma-Aldrich) and high MW chitosan (310 kDa; 419419, Sigma-Aldrich), were examined.
Preparation of CS and HA
1 wt. % chitosan and 2 wt. % HA was dissolved in 0.5 M acetic acid and PBS, respectively. The concentration of CS and HA (200 kDa, Lifecore Biomedical, MN, USA) were set at 0.1 mg/ml and 2 mg/ml, respectively. The induction medium containing CS was adjusted to pH 7.2 with NaOH. The medium in control group was added the same amount of acetic acid, NaOH, and PBS to ensure the consistent conditions.
Histological stains
The specimens received from RE and ON were fixed in 10% paraformaldehyde at 4 °C overnight, decalcified and embedded with paraffin. Sections for characterization were performed with hematoxylin and eosin (H&E) staining and Alcian blue staining (pH 2.5) to assess the thickness of the ON and mucin.
Immunocytochemical stain
The cultured cells were fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 (X100; Sigma-Aldrich) for 10 min at room temperature. The cells were then blocked with 3% bovine serum albumin (BSA) for 20 min and incubated with primary antibodies diluted in 3% BSA at 4 °C overnight. The primary antibodies and their dilutions were anti-zonula occludens-1 (ZO-1) (Ab190085, 1:100; Abcam, Cambridge, UK), anti-mucin 5AC (MUC5AC) (Ab198294, 1:100; Abcam), anti-acetylated tubulin (T7451, 1:1000; Sigma-Aldrich), anti-cytokeratin18 (CK18) (Ab82254, 1:1000; Abcam), anti-olfactory marker protein (OMP) (Ab62144, 1:100; Abcam) and anti-adenylate cyclase 3 (ADCY3, Ab125093, 1:100; Abcam). The DyLight488-conjugated secondary antibody (Abcam) was employed to visualize the signal by reacting with cells for 1.5 h at room temperature. In addition, 4′,6-diamidino-2-phenylindole (DAPI) and rhodamine-conjugated phalloidin were adopted to counterstain nuclear and cytoskeletal markers, respectively. These immunostained cells were visualized using indirect fluorescence under a confocal microscope (LSM510, Carl Zeiss, Jena, Germany).
Western blot analyses
Western blot analyses for protein expression were carried out in the standard protocol. Briefly, the samples were denatured, separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto polyvinylidene fluoride membranes (Millipore, Billerica, MA). The membranes were blocked with a CIS blocking buffer (CIS-Biotechnology, Taichung City, Taiwan) at room temperature for 60 s and then probed with various primary antibodies at 4 °C overnight. The primary antibodies employed in this work were anti-ZO-1 (Ab190085, 1:1000; Abcam), anti-E-cadherin (Ab15148, 1:1000; Abcam), anti-MUC5AC (Ab198294, 1:1000; Abcam), anti-forkhead box protein J1 (FOXJ1) (05–837; Millipore), anti-CK18 (Ab82254, 1:1000; Abcam), anti-OMP (Ab98124, 1:1000; Abcam) and anti-ADCY3, (Ab125093, 1:1000; Abcam). The membranes were incubated with the horseradish peroxidase-conjugated secondary antibody for 1.5 h and finally visualized utilizing enhanced chemiluminescence (ECL; Millipore). Previous primary antibodies were detached from the membranes, which were also probed with GAPDH antibodies (Ab22555, 1:5000; Abcam), which acted as an internal control. The Western blotting images were acquired with UVP BioSpectrum 810 and analyzed by Vision Works LS software (UVP, LLC, CA).
Statistical analysis
The results were observed from at least three independent experiments among 20 subjects, with the data expressed as mean ± standard deviation (SD). Statistical significance was calculated using one-way ANOVA followed by Fisher Least Significant Difference post-hoc test, with p < .05 being considered significant.
Results
Examination of RE and ON
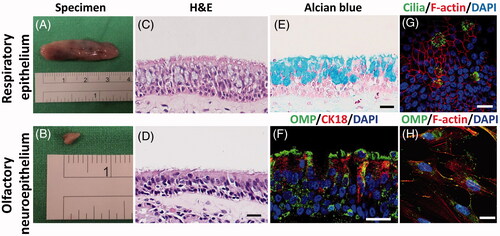
The specimens of RE and ON were obtained from nasal inferior turbinates and superior turbinates near the roof of the nasal cavity during septomeatoplasty, respectively (). The RE thickness was roughly 50 μm ± 2.9 μm, incorporating columnar cells, ciliated cells and mucous cells with a large amount of interspersing mucin, whereas the ON thickness was around 42 μm ± 4.5 μm with condense structure (). To characterize the human ON, histology analysis was performed randomly on selected specimens, which were raised against OMP and CK18. OMP is a representative marker of mature ORNs in adulthood and CK18 is a marker of SCs [Citation16]. In the native region, ORNs and SCs were identified in the ON (). Furthermore, the typical markers of RECs and ORNs, cilia and OMP, were expressed in these culture systems ().
Figure 1. Tissue specimens of human RE (A) and ON (B). Sections through the human RE and ON stained with hematoxylin and eosin (H&E) (C–D) and Alcian blue (E). Paraffin-section of representative ON is immunostained for OMP and CK18 . Nuclei are labeled with DAPI (F). Immunofluorescence images of cultured RECs and ONCs. Cilia (G) and OMP (H) are identified with DyLight488. Cellular cytoskeletal shape (F-actin) is outlined by rhodamine phalloidin. Scale bar= 20 μm.
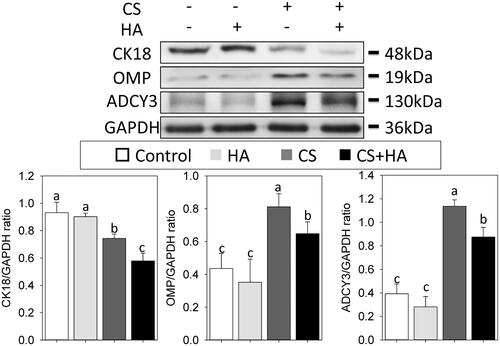
Determination of the concentration and MW of CS and HA
Western blots revealed that ONCs in 0.1 mg/ml chitosan apparently expressed the highest OMP among these four conditions (). Moreover, various MW of 0.1 mg/ml chitosan were further analyzed. As shown in , there were no significant differences between 5 kDa, 190 kDa, and 310 kDa. As to the concentration of HA, the expression level of FOXJ1, the marker of ciliated cells, significantly increased when the concentration of HA was more than 2 mg/ml according to our previous report [Citation15]. Western blots revealed the expression level of FOXJ1 in RECs rose as the MW increased, but those treated with 15MDa of HA showed no difference to the control (). Therefore, 0.1 mg/ml chitosan (190 kDa) and 2 mg/ml HA (200 kDa) were adopted for experiments.
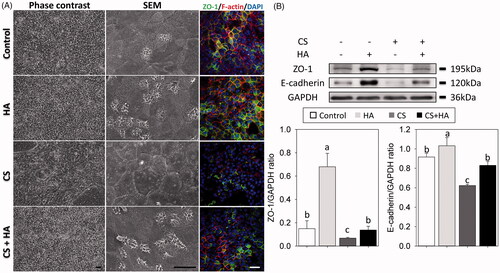
Epithelial integrity in RECs
Airway epithelial integrity plays an important role in preventing foreign agents from stimulating inflammatory responses, while down-regulation of the tight junction signaling decreases cell-to-cell interactions, causing the loss of epithelial integrity [Citation17]. SEM images showed that the morphology of RECs exhibited polygonal morphology with cilia differentiation in controls, compared to irregular morphology without cilia formation in the CS group. However, RECs kept polygonal morphology with cilia differentiation in the HA and CS + HA groups (). Immunofluorescence results revealed that the expression level of ZO-1 was low in the chitosan group. In addition, the expression levels of E-cadherin and ZO-1 were higher in the HA group and lower in the CS group than in controls, which was similar to the CS + HA group () (p < .05).
Figure 3. Morphology and cell-cell boundaries of RECs treated with/without HA (2 mg/ml) and CS (0.1 mg/ml) at day 21 after ALI using light microscope (A, left panels) and scanning electron micrographs (A, middle panel, magnification 1500×). Immunofluorescent confocal images of ZO-1 (A, right panels, 400×). Cellular cytoskeletal shape (F-actin) is outlined by rhodamine-phalloidin. Nuclei are labeled with DAPI. B: Western blot analyses of tight junctions (ZO-1 and E-cadherin) in RECs (top). Densitometric analyses (bottom). Scale bar = 20 μm. Different letters indicate significant differences, while the same letter indicates no significant differences (n = 5, p < .05).
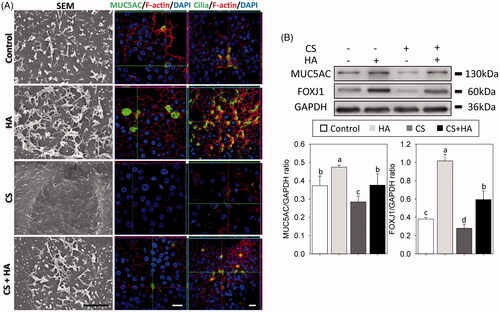
Mucociliary differentiation of RECs
RECs form multiple barriers in the sinonasal system, not only by secreting mucins to block the pathogens access to underlying epithelial cell surfaces but also by removing those with ciliated cells. Mucus and cilia differentiation were evaluated from the expression of MUC5AC and FOXJ1, respectively [Citation18]. SEM and confocal images showed that CS inhibited differentiation of mucous secretory cells and ciliated cells, whereas RECs in the HA and CS + HA groups retained mucociliary phenotypes (). Western blot analyses revealed that the CS group had the lowest expression levels of MUC5AC and FOXJ1. RECs cultured with HA had more expressions of FOXJ1 than those without HA (p < .05) ().
Figure 4. Cilia formation are observed using scanning electron micrographs (Magnification × 10000, Scale bar = 2 μm). Immunofluorescent confocal images of MUC5AC (630×) and cilia (400×) at day 21 after ALI (A). Cellular cytoskeletal shape (F-actin) is outlined by rhodamine-phalloidin. Nuclei are labeled with DAPI. The expression of FOXJ1 and MUC5AC are evaluated using Western blotting assays and densitometric analyses (B). Scale bar = 20 μm. Different letters indicate significant differences, while the same letter indicates no significant differences (n = 5, p < .05).
ORN differentiation of ONCs
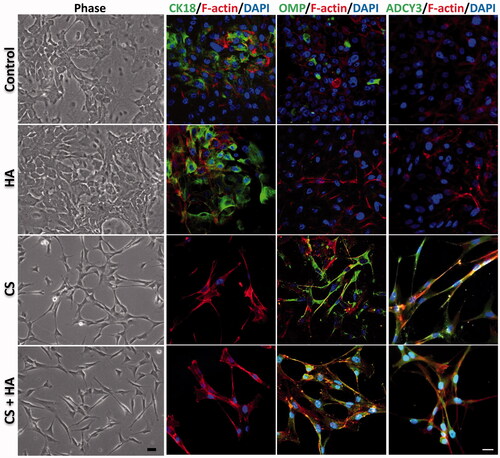
ONCs comprise ORNs and sustentacular cells (SCs). Since CK18 is a marker of SCs and OMP is a representative marker of ORNs, these two markers are used to assess the ONC differentiation [Citation16]. In addition, the essential element and hallmark of functionally mature ORNs, ADCY3, was measured [Citation19]. Light microscopic examination results displayed that ONCs maintained flattened and epithelial-like morphology in the control and HA groups, but exhibited a slender morphology with extended thin processes in CS alone or CS + HA groups. Immunofluorescence results revealed that ONCs treated with HA and controls expressed more CK18 than those with CS and CS + HA. Conversely, the expression levels of OMP and ADCY3 were higher in CS and CS + HA than in HA and controls (. Western blot analyses further confirmed these results. ONCs significantly increased the expressions of OMP and ADCY3, and decreased the CK18 expression as long as CS was present in the culture () (p < .05).
Figure 5. Morphology and differentiation of ONCs treated with/without HA (2 mg/ml) and CS (0.1 mg/ml) for one week. The OMP-, CK18-, ADCY3-positive cells are immunostained with DyLight488 (400×). Cellular cytoskeletal shape (F-actin) is outlined by rhodamine-phalloidin. Nuclei are labeled with DAPI. Scale bar = 20 μm.
Discussion
The diverse epithelia lining the nasal cavity serve different functions. The predominant epithelium is RE (90%), which not only moistens the airways but also prevents infection and tissue injury by the action of mucociliary clearance. Once epithelial damage occurs, the inflammation disrupts tight junction complexes to increase epithelial permeability, decreasing mucociliary clearance and causing CRS-related syndrome [Citation20]. Hyaluronan, a highly hydrated polyanionic macromolecule, exists with MW from 100 kDa in serum to 8000 kDa in the vitreous. The molecular size of hyaluronan plays an important role in its biological activity. Hyaluronan with low MW less than 300 kDa stimulates cell migration, proliferation and differentiation. In contrast, hyaluronan with high MW more than 1000 kDa has no such effect [Citation21]. In the study, the hyaluronan is used as a biomaterial in the form of a viscoelastic solution with MW of 200 kDa, so that HA possesses the biological activity in regulating differentiation of RECs. HA influences the proliferation and differentiation of a wide variety of cells in diverse tissues through the CD44 receptor and receptor for hyaluronan-mediated motility (RHAMM) and organization of microtubules [Citation22]. Previous study demonstrated that HA enhances the ciliogenesis of RECs through regulating RHAMM and organization of microtubules [Citation14,Citation15,Citation21]. Notably, during the cascade of wound healing in the respiratory system, transforming growth factor beta 1 (TGF-β1), an inflammatory cytokine, is an important signal for initiating the healing cascade and inhibiting mucociliary restoration [Citation15]. CD44 interacts physically with TGF-β type I receptor and has a negative regulatory effect on the signaling of TGF-β type I receptor [Citation23]. Therefore, HA is considered to antagonize the TGF-β1 effect on mucociliary differentiation of RECs through down-regulation of TGF-β type I receptor, which is via CD44 [Citation15]
Conversely, CS is known to improve transport of drugs across nasal mucosa membranes. Interaction between positively charged chitosan and the opening of tight junctions causes a decrease in ZO-1 proteins and a change in cytoskeleton protein F-actin from filamentous to a globular structure [Citation24]. Moreover, CS interferes with tight junction formation in RECs and inhibits proliferation, migration and mucociliary differentiation of RECs through increasing TGF-β1 [Citation13]. This study found that HA certainly raises the expression and functionality of intercellular adhesion molecules, but CS impedes cellular integrity and mucociliary differentiation, consistent with previous studies [Citation13,Citation25]. This work also demonstrates that HA antagonizes the inhibitory effect of CS on the epithelial integrity and differentiation of RECs, which is due to the ability of HA to down-regulate TGF-β type I receptor through CD44. It indicates that the composite, CS + HA, is a suitable biomaterial to promote mucociliary differentiation of RECs.
Olfactory dysfunction is a frequent complaint in CRS patients and significantly affects their quality of life [Citation26]. The etiology results from a long-term inflammatory process that causes respiratory metaplasia, airway obstruction and incomplete ORN regeneration [Citation26,Citation27]. Although CS interferes with the formation of tight junction and impedes mucociliary differentiation of RECs, it is a promising agent to facilitate the ORN differentiation of ONCs. Our previous report also demonstrated that CS promotes differentiation of ONCs, based on its role in the ORN differentiation, neurite outgrowth and the transduction apparatus signal expression in rats [Citation12]. HA alone does not affect the ORN differentiation, but additional CS enhances differentiation of ORNs as well as protects ON from differentiating to SCs (). Many reports have demonstrated that CS possesses anti-inflammation and neuroprotection properties, meaning that it may lower the occurrence of olfactory dysfunction through inhibition of rhinosinusitis [Citation28]. In this investigation, although HA promotes the growth and differentiation of RECs, it does not significantly alter the olfactory differentiation. Conversely, CS inhibits the integrity and mucociliary differentiation of RECs but enhances the ORN differentiation. Notably, the composite CS + HA would simultaneously preserve their respective functions in these two epithelia.
Another merit of applying the composite in the sinonasal system is the material characteristics of CS and HA, which provides a moist environment as a realistic imitation of nasal mucus and can thus protect the postoperative wound from dryness and prevent adhesion [Citation29–31]. In addition, the synergistic effect of CS + HA makes the formulation a promising agent that can be adopted in the nasal delivery system [Citation32]. To our knowledge, this is the first report to demonstrate that the composite of CS + HA may simultaneously promote differentiation of both RE and ON in an in vitro system. This investigation is the first step to elucidate the clinical potential use of this medical composite. Whether this composite would restore the RE and ON in vivo needs further investigation.
Conclusion
Experimental results indicate that a medical composite, CS + HA, can combine their respective advantages in promoting differentiation of ORNs and preserve the ability to facilitate mucociliary differentiation of RECs. We believe that this medical composite is a promising biomaterial that can be applied in sinonasal surgery and tissue engineering of the respiratory and olfactory system in the near future.
Acknowledgement
The partial sections of methods were referenced from our previous report in Acta Biomaterialia 2018;68:204–213.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kaliner MA, Osguthorpe JD, Fireman P, et al. Sinusitis: bench to bedside. Current findings, future directions. J Allergy Clin Immunol. 1997;99:S829–S848.
- Ahlroth Pind C, Gunnbjornsdottir M, Bjerg A, et al. Patient-reported signs of dampness at home may be a risk factor for chronic rhinosinusitis: a cross-sectional study. Clin Exp Allergy. 2017;47:1387-1389.
- Shi JB, Fu QL, Zhang H, et al. Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven Chinese cities. Allergy. 2015;70:533–539.
- Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:S1–S31.
- Wormald PJ. Endoscopic sinus surgery: anatomy, three-dimensional reconstruction, and surgical technique/Peter-John Wormald. New York: Thieme; 2005. Available from: https://nla.gov.au/nla.cat-vn3800983).
- Ramadan HH. Surgical causes of failure in endoscopic sinus surgery. Laryngoscope. 1999;109:27–29.
- van Woensel M, Wauthoz N, Rosiere R, et al. Formulations for intranasal delivery of pharmacological agents to combat brain disease: a new opportunity to tackle GBM? Cancers. 2013;5:1020–1048.
- Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64:614–628.
- Konstantinidis I, Witt M, Kaidoglou K, et al. Olfactory mucosa in nasal polyposis: implications for FESS outcome. Rhinology. 2010;48:47–53.
- Chandra RK, Conley DB, Haines GK, et al. Long-term effects of FloSeal packing after endoscopic sinus surgery. Am J Rhinol. 2005;19:240–243.
- Heilmann S, Huettenbrink KB, Hummel T. Local and systemic administration of corticosteroids in the treatment of olfactory loss. Am J Rhinol. 2004;18:29–33.
- Li ST, Young TH, Lin CF, et al. Promotion of olfactory receptor neuron differentiation of olfactory neuroepithelial cells by using chitosan solution. Am J Rhinol Allergy. 2017;131:289–292.
- Huang TW, Wei CK, Su HW, et al. Chitosan promotes aquaporin formation and inhibits mucociliary differentiation of nasal epithelial cells through increased TGF-beta1 production. J Tissue Eng Regen Med. 2017;11:3567–3575.
- Huang TW, Chan YH, Cheng PW, et al. Increased mucociliary differentiation of human respiratory epithelial cells on hyaluronan-derivative membranes. Acta Biomaterialia. 2010;6:1191–1199.
- Huang TW, Li ST, Fang KM, et al. Hyaluronan antagonizes the differentiation effect of TGF-beta1 on nasal epithelial cells through down-regulation of TGF-beta type I receptor. Artif Cells Nanomed Biotechnol. 2018;1–10.
- Holbrook EH, Wu E, Curry WT, et al. Immunohistochemical characterization of human olfactory tissue. Laryngoscope. 2011;121:1687–1701.
- Man Y, Hart VJ, Ring CJ, et al. Loss of epithelial integrity resulting from E-cadherin dysfunction predisposes airway epithelial cells to adenoviral infection. Am J Respir Cell Mol Biol. 2000;23:610–617.
- Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol. 2015;16:27–35.
- Sakano H. Neural map formation in the mouse olfactory system. Neuron. 2010;67:530–542.
- Watelet JB, Bachert C, Gevaert P, et al. Wound healing of the nasal and paranasal mucosa: a review. Am J Rhinol. 2002;16:77–84.
- Huang L, Cheng YY, Koo PL, et al. The effect of hyaluronan on osteoblast proliferation and differentiation in rat calvarial-derived cell cultures. J Biomed Mater Res. 2003;66:880–884.
- Hardwick C, Hoare K, Owens R, et al. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J Cell Biol. 1992;117:1343–1350.
- Porsch H, Mehic M, Olofsson B, et al. Platelet-derived growth factor beta-receptor, transforming growth factor beta type I receptor, and CD44 protein modulate each other's signaling and stability. J Biol Chem. 2014;289:19747–19757.
- Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm Res. 1998;15:1326–1331.
- Zahm JM, Milliot M, Bresin A, et al. The effect of hyaluronan on airway mucus transport and airway epithelial barrier integrity: potential application to the cytoprotection of airway tissue. Matrix Biol. 2011;30:389–395.
- Kohli P, Naik AN, Harruff EE, et al. The prevalence of olfactory dysfunction in chronic rhinosinusitis. Laryngoscope. 2017;127:309–320.
- Kikuta S, Sakamoto T, Nagayama S, et al. Sensory deprivation disrupts homeostatic regeneration of newly generated olfactory sensory neurons after injury in adult mice. J Neurosci. 2015;35:2657–2673.
- Hao P, Duan H, Hao F, et al. Neural repair by NT3-chitosan via enhancement of endogenous neurogenesis after adult focal aspiration brain injury. Biomaterials. 2017;140:88–102.
- Deng Y, Ren J, Chen G, et al. Injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for abdominal tissue regeneration. Sci Rep. 2017;7:2699.
- Sanad RA-B, Abdel-Bar HM. Chitosan–hyaluronic acid composite sponge scaffold enriched with andrographolide-loaded lipid nanoparticles for enhanced wound healing. Carbohydr Polym. 2017;173:441–450.
- Li L, Wang N, Jin X, et al. Biodegradable and injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for postoperative adhesion prevention. Biomaterials. 2014;35:3903–3917.
- Lim ST, Forbes B, Martin GP, et al. In vivo and in vitro characterization of novel microparticulates based on hyaluronan and chitosan hydroglutamate. AAPS PharmSciTech. 2001;2:1.