?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Astaxanthin, a Xanthophyll carotenoid, has strong antioxidant properties. Some studies have shown the effectiveness of this compound on the prevention and treatment of cancer. Therefore, the aim of this study was to evaluate the effects of astaxanthin on induction of apoptosis and antioxidant activity in the LS-180 cell line. In this experimental study, after the treatment of LS-180 50, 100 and 150 μm of Astaxanthin for 24 h, the expression levels of Bax, Bcl2 and Caspase3 genes were investigated by Real-time PCR. Also, the level of malondialdehyde, as an indicator of oxidative stress and activity of anti-superoxide dismutase enzymes, catalase and glutathione peroxidase was investigated by colorimetric methods. The results showed that astaxanthin increases the expression of Bax and Caspase3 genes and decreases that of Bcl2, thereby, inducing apoptosis and inhibiting growth and proliferation of the cells. Additionally, reduction in the levels of malondialdehyde was evident with a significant elevation in antioxidant activity mediated by the action of superoxide dismutase, catalase and glutathione peroxidase. These results suggest that astaxanthin has the potency to induce apoptosis in LS-180 cells by increasing the expression of apoptotic genes and activity of antioxidant enzymes. Thus, astaxanthin has potential in the prevention and treatment of cancer.
Introduction
Various studies have shown that the use of exogenous antioxidants can be effective in the prevention and treatment of various diseases such as diabetes, hypertension, atherosclerosis, cardiovascular disease, Alzheimer’s, Parkinson’s disease and cancer. Astaxanthin, belonging to the family of carotenoids, has a very strong antioxidant activity, often called the “king of carotenoids”, due to its significant impact on human health, by strengthening the immune system, preventing and treating various types of cancers and ageing process and including the prevention of cardiovascular disease [Citation1–3]. Hematococcus pluvialis (H. pluvialis) is a microalga known to be the main source of natural astaxanthin. Other natural sources of astaxanthin include; Zantophyllumiss dendorhousis yeast, Phaffia rhodozyma, and from marine organisms such as Ghezel Alla, Crab and Shrimp [Citation4,Citation5]. Astaxanthin does not elicit side-effects even at higher concentrations, which adds to its significance [Citation6,Citation7]. Researches have failed to report any adverse effects of astaxanthin consumption in humans or animals [Citation5,Citation8,Citation9]. Apoptosis or cell death is a mechanism for the removal of unwanted or damaged cells. Disturbance in this process eliminates the balance between cell proliferation and cell death and plays a critical role in the development of cancer. Therefore, the induction of apoptosis in pre-cancerous and cancerous cells is one of the most important goals in the prevention and treatment of cancer [Citation10]. Henceforth, the purpose of this study is to evaluate the effect of astaxanthin on induction of apoptosis, expression of the respective genes in this process, and the activity of antioxidant enzymes in the LS-180 cells of human colorectal cancer.
Materials and methods
Cell culture
Human LS-180 colorectal cancer cell line was purchased from the Pasteur Institute and cultured in a DMEM containing 1% antibiotic (penicillin and streptomycin) and 10% FBS in an incubator containing 5% CO2 at 37 °C, When the cells reached their logarithmic phase, they were treated with the drug.
MTT assay
MTT technique was used to determine the time and dose of the drug on colorectal cancer cells. This method is a mitochondrial metabolic competitive test and is based on the fragmentation of tetrazolium salt by mitochondrial Succinate dehydrogenase enzyme in living cells. Herein, we added 100 μl culture medium (containing 104 cells) per well in a 96-well plate. After 24-h incubation of the cells with different concentrations of astaxanthin and determining cell proliferation after 24, 48 and 72 h, based on the protocol of the MTT assay (Jena Bioscience). Cell survival was calculated according to the following formula:
(1)
(1)
Astaxanthin treatment
Depending on the results of MTT, the LS-180 cells were treated with 50, 100 and 150 μm astaxanthin for 24 h.
RNA extraction and cDNA synthesis
RNA extraction was performed using the Jena Bioscience kit based on the relevant protocol (Jena Bioscience, Germany) and the concentration of RNA extracted was determined, followed by cDNA synthesis using a microgram of extracted RNA.
Evaluation of gene expression using Real-Time PCR
Analysis of gene expression was performed using the Jena Bioscience kit based on the relevant protocol according to the following program (Jena Bioscience). The first stage characterized by DNA denaturation and activation of the polymerase enzyme at 95 °C for 2 min proceeded by proliferation reaction which was conducted in 40 cycles at 95 °C for 15 s and 60 °C for 40 s. The primers sequences are mentioned in .
Table1. Forward and reverse sequence of different primers [Citation31].
Measuring the activity of antioxidant enzymes
After treatment of the cells with different concentrations of astaxanthin and washing with PBS, 800 μl of lysis buffer was added, and cells were mixed several times. In the next step, using an ultrasonic device, the solutions were sonicated 2 to 3 times, each time for 30 s. Finally, centrifugation was performed at 20 °C for 10 min. The supernatant was stored at −80 °C until the activity of the enzymes was measured.
Total protein measurement
The Bradford method was used to measure the total protein content in cell lysate samples and BSA was used to plot the standard curve after serial dilutions.
Measuring malondialdehyde
To measure malondialdehyde, spectrophotometric measurements were conducted based on the reaction of a thiobarbituric acid substrate in (nmol/mg) protein [Citation11].
Measuring catalase activity
Catalase enzyme activity was measured using a spectrophotometer based on the methanol-enzyme reaction in the presence of oxygenated water per unit/mg protein catalase [Citation12].
Measuring the activity of superoxide dismutase
The activity of superoxide dismutase enzyme was measured based on Pyrogallol radical superoxide reaction using spectrophotometer per unit/mg protein disosmotase superoxide [Citation13].
Measurement of glutathione peroxidase activity
To evaluate the activity of glutathione peroxidase spectrophotometric measurements were used and production of glutathione oxide in terms of unit/mg protein was measured [Citation14].
Data analysis
The analysis of gene expression was accomplished using Real-time PCR by DDCT method. To determine the effect of astaxanthin on antioxidant indices, one-way ANOVA was used with Tukey’s post hoc test.
Results
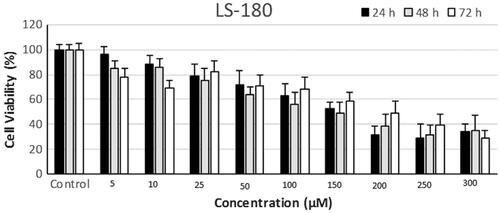
According to the results of MTT assay, 50, 100 and 150 μm of astaxanthin were used for 24 h to treat LS-180 cells (.
Figure 1. LS-180 cell survival at different concentrations of astaxanthin after 24, 48 and 72 h of treatment.
Gene expression results
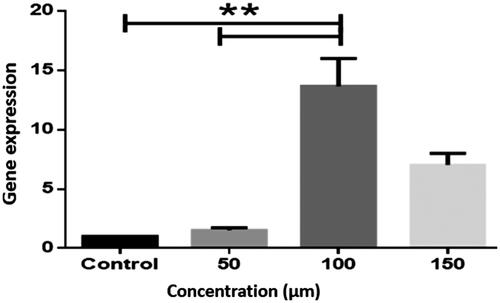
Effect of astaxanthin on caspase 3 gene expression
Expression of caspase 3 at the concentration of 100 μM AST was significantly elevated as compared to control cells (P < .01) as those treated with 50 μM AST (P < .01) (.
Figure 2. Caspase 3 gene expression in the LS-180 cell line. The expression of genes was measured using the Real-Time PCR technique and compared to control cells. The results are repeated three times and tested as Mean ± SEM. The columns that have had a significant difference after the DDCT test are marked with a star sign. The significance level is considered to be P < .01 (**P < .01).
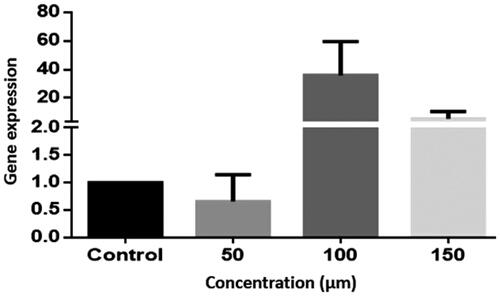
The effect of astaxanthin on the expression of bax gene
The results of the study on LS-180 cells at concentrations of 100 and 150 μm showed an increase in the expression of Bax gene expression in the treated groups in variance with the control one, nonetheless, this increase was not statistically significant (P > .05) (.
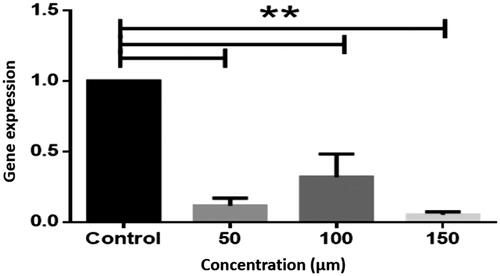
The effect of astaxanthin on the expression of bcl-2 gene
Bcl-2 gene expression was significantly suppressed in the treatment at various concentrations (P < .01) (.
Figure 4. Expression of bcl-2 gene in LS-180 cell line. The expression of genes was measured using the Real-Time PCR technique and compared to control cells. The results are repeated three times and tested as Mean ± SEM. The columns that have had a significant difference after the DDCT test are marked with a star sign. The significance level is considered to be P < .01. (**P < .01).
Effect of astaxanthin on malondialdehyde and antioxidant enzymes activity
The results showed that treatment of the cells at all concentrations of astaxanthin reduced the level of malondialdehyde in the LS-180 cell line. (p < .05). Among different concentrations of the drugs, significant difference was found between the activity of 50 and 150 μM in the reduction of malondialdehyde levels. Similarly, astaxanthin significantly increases the activity of superoxide dismutase in contrast to the control group (p values < .05). This activity was statistically different between the lowest and highest concentration of the drug used in this study. 150 μm AST was more effective to elevate superoxide dismutase activity. ()
The average catalase activity in all treatments was significantly increased compared to the control group (P < 0.05). Furthermore, the increase in mean catalase at 150 μM of astaxanthin was significantly greatest as compared to other concentrations. (). Similarly, increased in glutathione peroxidase activity was statistically evident at all the concentrations (P < .05) as compared to control and significant difference in the activity was also noted when compared at different concentration value ().
Table 2. Results of the effect of astaxanthin on the level of malondialdehyde and the activity of antioxidant enzymes in the LS-180 cell line.
Discussion
Colorectal cancer can be progressive and fatal, however, substantial advancements have been made to prevent and treat it. Studies have shown effects of astaxanthin to eradicate colorectal cancer [Citation15]. Here we present its effects on cell cycle leading to cell death and instigation of anti-oxidation activity.
Various studies indicate the antitumor effects of H. pluvialis extract, which is rich in astaxanthin, being a potent growth inhibitor under laboratory conditions [Citation16]. The results of the present study show anti-proliferative effects of astaxanthin seen by the increase in the expression of caspase 3, Bax, antioxidant activity conversely, decreasing the expression of bcl2, and the level of malondialdehyde in the LS-180 cell line [Citation17,Citation18]. A study reported that daily administration of astaxanthin could be effective in preventing colon cancer [Citation19]. Studies have shown that astaxanthin reduces the proliferation of cancer cells by halting the progression of the cell cycle at the G0/G1 phase, inhibiting the expression of cyclin D1, which in turn increases p53, p21WAF-1/CIP1 and p27 simultaneously, thus controlling the progression of the cell cycle [Citation20]. Studies have shown that cyclin D1 is an oncogene and its expression has been observed in several cancer cell lines [Citation21]. Many studies have shown` that alteration in the expression of caspase 3 leads to tumor formation. Caspase 3 is a protein that works with Caspase 8 and 9 to activate cell death and therefore plays an important role in apoptosis by activating other caspases [Citation22]. Recent studies have shown that astaxanthin alleviates the expression of anti-apoptotic Bcl-2 and p-Bad and increases the expression of a pro-apoptotic protein such as; Bax and Bad, contributing to the release of Smac/Diablo and cytochrome C into the cytosol. Thus, astaxanthin induces mitochondrial apoptosis through caspases and ultimately provides the basis for the death of cancerous cells. The mechanism of inhibition of growth of colon cancer cells by β-carotene appears to involve interfere with the progression of cell cycle and mediate apoptosis. This carotenoid, in a dose-dependent manner, halts cell cycle at the G2/M phase leading to cell death. In the treated cells with effective concentrations of -β-carotene, many biochemical changes are reported such as; transfer of phosphatidylserine to the outer layer of the membrane of the plasma, induction of apoptosis, cellular contraction and alterations in cytoplasmic density and/or nuclear chromatin. Muto et al. suggested that β-carotene induces apoptosis by reducing the expression of EGFR (Epidermal Growth Factor Receptor) and stimulating the production of proinflammatory cytokine [Citation23]. It is worth noting that astaxanthin can reduce the expression of EGFR and interfere with EGF binding to the recipient, thereby inducing apoptosis in cancer cells. It is also seen that the treatment of astaxanthin can decrease the levels of EGFR in intestinal cancer cells [Citation24]. Likewise, it can also elevate the expression of P53 that can interfere with the cell cycle at G2/M phase, reducing the expression of cyclin B1 and Cdc2 [Citation25,Citation26]. In addition, an increase in p53 levels can lead to an increase in the levels of p21Cip1/Waf1, which in turn results in the cessation of the cell cycle [Citation27,Citation28].
The results of the Jingjing Li study in 2015 on the human hepatoma cell lines LM3 and SMMC-7721 showed that astaxanthin, through NF-κB p65 and Wnt/β-catenin pathways causes apoptosis of tumor cells and inhibits cell proliferation [Citation29]. This result suggests that astaxanthin inhibits PI3K, Akt phosphorylation, ERK, aiding binding of Wnt protein with its receptor, and thus reducing phosphorylation of IKKα/β (Ser176/180) and GSK-3β (Ser9). Low concentrations of β-catenin and p-NFκB reduce Bcl-2 transcription and alter Bax/Bcl-2 ratios. As a result, caspase associated with mitochondrial apoptosis is activated for cell death and ultimately inhibits cancer cell growth. Oxidative stress and the formation of reactive oxygen species (ROS), or disturbance in the production and function of antioxidants chiefly contribute to the development of cancer. ROS, as a secondary messenger, takes part in activating and maintaining some signaling pathways [Citation30]. Endogenous and exogenous antioxidants like astaxanthin appear to function in a similar manner. The results of Li Zhang and Handong Wang’s study in 2015 showed that astaxanthin reduces intracellular production of O2 by activating anti-oxidant SOD and CAT enzymes in U937 cells. Wu et al. showed that astaxanthin, by improving the activity of GPx and SOD, increases GSH content and reduces MDA levels, hence, can reduce the ageing of the brain in rats. Hashimoto, Choi, et al. also obtained that astaxanthin, is a potent antioxidant, due to its ability to increase the activity of superoxide dismutase and other antioxidant enzymes. Increasing the activity of antioxidant enzymes in astaxanthin-treated cells may have altered the antioxidant defense system, thereby inducing anti-proliferative effects and inducing apoptosis in LS-180 cells of colorectal cancer.
Conclusion
This study provides molecular evidence in regard to apoptotic and antioxidant potencies of astaxanthin. Results indicate that astaxanthin can inhibit proliferation and induce apoptosis in LS-180 cell lines by promoting the activity of antioxidant enzymes, reducing the production of malondialdehyde, and increasing the expression of genes that are effective in apoptosis. However, studies with further details, including interrelated molecular pathways can provide greater pieces of evidence.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Pashkow FJ, Watumull DG, Campbell CL. Astaxanthin: a novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am J Cardiol. 2008;101:S58–S68.
- Nikam S, Nikam P, Ahaley SK, et al. Oxidative stress in Parkinson’s disease. Indian J Clin Biochem. 2009;24(1):98–101.
- Sontakke S, Cadenas MB, Maggi RG, et al. Use of broad range16S rDNA PCR in clinical microbiology. J Microbiol Meth. 2009;76:217–225.
- Ranga R, Sarada AR, Baskaran V, et al. Identification of carotenoids from green alga Haematococcus pluvialis by HPLC and LC-MS (APCI) and their antioxidant properties. J Microbiol Biotechnol. 2009;19(11):1333–1341.
- Ravishankar N, Gubbins P, Cooley RJ, et al. Financing of global health: tracking development assistance for health from 1990 to 2007. Lancet. 2009;373:2113–2124.
- Mardani M. Wound antiseptic plants: an overview of the most important medicinal plants in Iran affecting wound infections. J Glob Pharma Technol. 2017;8(8):18–23.
- Zarei F, Soleimaninejad M. Role of growth factors and biomaterials in wound healing. Artif Cells, Nanomedicine, Biotechnol. 2018;46(supp. 1):906–911.
- Ranga Rao A, Raghunath Reddy RL, Baskaran V, et al. Characterization of microalgal carotenoids by mass spectrometry and their bioavailability and antioxidant properties elucidated in rat model. J Agric Food Chem. 2010;58(15):8553–8559.
- Stewart JS, Lignell Å, Pettersson A, et al. Safety assessment of astaxanthin-rich microalgae biomass: acute and subchronic toxicity studies in rats. Food Chem Toxicol. 2008;46:3030–3036.
- Schoetz DJ. Diverticular disease of the colon: a century-old problem. Dis Colon Rectum. 1999;42:703–709.
- Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278.
- Aebi H. [13] Catalase in vitro. Methods Enzymol. 1984;105:121–126.
- Kuthan H, Haussmann H-J, Werringloer J. A spectrophotometric assay for superoxide dismutase activities in crude tissue fractions. Biochem J. 1986;237:175–180.
- Flohé L, Günzler WA. [12] Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–120.
- Mahdavinia M, Bishehsari F, Ansari R, et al. Family history of colorectal cancer in Iran. BMC Cancer. 2005;5:112.
- Chew BP, Park JS, Wong MW, et al. A comparison of the anticancer activities of dietary beta-carotene, canthaxanthin and astaxanthin in mice in vivo. Anticancer Res. 1999;19:1849–1853.
- Tang G, Yang J, Minemoto Y, et al. Blocking caspase-3-mediated proteolysis of IKKbeta suppresses TNF-alpha-induced apoptosis. Mol Cell. 2001;8:1005–1016.
- Tang X, Liu B, Wang X, et al. Epidermal growth factor, through alleviating oxidative stress, protect IPEC-J2 cells from lipopolysaccharides-induced apoptosis. IJMS. 2018;19:848.
- Tanaka T, Kawamori T, Ohnishi M, et al. Suppression of azoxymethane-induced rat colon carcinogenesis by dietary administration of naturally occurring xanthophylls astaxanthin and canthaxanthin during the postinitiation phase. Carcinogenesis. 1995;16:2957–2963.
- Tanaka T, Makita H, Ohnishi M, et al. Chemoprevention of rat oral carcinogenesis by naturally occurring xanthophylls, astaxanthin and canthaxanthin. Cancer Res. 1995;55:4059–4064.
- Diehl S, Berger S, Ptacnik R, et al. Phytoplankton, light, and nutrients in a gradient of mixing depths: field experiments. Ecology. 2002;83:399–411.
- Parsaei P, Karimi M, Mardani M. A review of treatments for leishmaniasis wound using the prescriptions of traditional medicine. Int J Adv Biotechnol Res. 2017;8:2050–2058.
- Nakamura N, Shidoji Y, Moriwaki H, et al. Apoptosis in human hepatoma cell line induced by 4,5-didehydro geranylgeranoic acid (acyclic retinoid) via down-regulation of transforming growth factor-alpha. Biochem Biophys Res Commun. 1996;219:100–104.
- Muto Y, Fujii J, Shidoji Y, et al. Growth retardation in human cervical dysplasia-derived cell lines by beta-carotene through down-regulation of epidermal growth factor receptor. Am J Clin Nutr. 1995;62:1535S–1540S.
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132.
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677.
- Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803.
- Wang Q, Zhang J, Liu Y, et al. A novel cell cycle-associated lncRNA, HOXA11-AS, is transcribed from the 5-prime end of the HOXA transcript and is a biomarker of progression in glioma. Cancer Lett. 2016;373:251–259.
- Li J, Dai W, Xia Y, et al. Astaxanthin inhibits proliferation and induces apoptosis of human hepatocellular carcinoma cells via Inhibition of NF-κB P65 and Wnt/β-catenin in vitro. Mar Drugs. 2015;13:6064–6081.
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85.
- Karaliotas GI, Mavridis K, Scorilas A, et al. Quantitative analysis of the mRNA expression levels of BCL2 and BAX genes in human osteoarthritis and normal articular cartilage: an investigation into their differential expression. Mol Med Rep. 2015;12:4514–4521.